Abstract
Background
Transmission of coronavirus disease 2019 (COVID-19) can occur through inhalation of fine droplets or aerosols containing infectious virus. The objective of this study was to identify situations, patient characteristics, environmental parameters, and aerosol-generating procedures (AGPs) associated with airborne severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus.
Methods
Air samples were collected near hospitalized COVID-19 patients and analyzed by RT-qPCR. Results were related to distance to the patient, most recent patient diagnostic PCR cycle threshold (Ct) value, room ventilation, and ongoing potential AGPs.
Results
In total, 310 air samples were collected; of these, 26 (8%) were positive for SARS-CoV-2. Of the 231 samples from patient rooms, 22 (10%) were positive for SARS-CoV-2. Positive air samples were associated with a low patient Ct value (OR, 5.0 for Ct <25 vs >25; P = .01; 95% CI: 1.18–29.5) and a shorter physical distance to the patient (OR, 2.0 for every meter closer to the patient; P = .05; 95% CI: 1.0–3.8). A mobile HEPA-filtration unit in the room decreased the proportion of positive samples (OR, .3; P = .02; 95% CI: .12–.98). No association was observed between SARS-CoV-2–positive air samples and mechanical ventilation, high-flow nasal cannula, nebulizer treatment, or noninvasive ventilation. An association was found with positive expiratory pressure training (P < .01) and a trend towards an association for airway manipulation, including bronchoscopies and in- and extubations.
Conclusions
Our results show that major risk factors for airborne SARS-CoV-2 include short physical distance, high patient viral load, and poor room ventilation. AGPs, as traditionally defined, seem to be of secondary importance.
Keywords: virus transmission, aerosol-generating procedures, infection control, inhalation exposure
Healthcare infection-control guidelines emphasize breathing protection during aerosol-generating procedures with COVID-19 patients. Our study suggests that SARS-CoV-2 in air is mainly determined by distance, patient viral load, and room ventilation. We found weak support for emissions during aerosol-generating procedures.
Transmission of coronavirus disease 2019 (COVID-19) can occur through inhalation of fine droplets or aerosols containing infectious virus. Several factors have been acknowledged that may increase this route of transmission, such as enclosed spaces and poor ventilation [1]. In hospital settings, much debate has concerned aerosol-generating procedures (AGPs), which include different types of respiratory support and airway manipulations [2]. AGPs have been assumed to elevate aerosol concentrations in the surrounding air and thus considered to be of special concern for infection control. Consequently, guidelines have recommended a higher degree of environmental control and personal protective equipment during these procedures [3]. However, the focus on AGPs has been increasingly questioned, as it may divert attention from more important risk factors and possibly lead to an underestimation of exposure for frontline healthcare workers not regularly involved in AGPs [4–7].
The detailed definition of AGP has also been discussed but intubation, noninvasive positive-pressure ventilation, tracheotomy, cardiopulmonary resuscitation, bronchoscopy, and sputum induction are universally included in guidelines [3, 8]. Over time, the concept of AGP has tended to expand and many guidelines also include high-flow oxygen, nebulization, mechanical ventilation, and sometimes other procedures suspected of aerosol production, even though the evidence of increased transmission risk is weak or absent [9]. Also, many studies assess the risk of aerosolization during AGPs based on measurements of the total aerosol concentration or mass, lacking information on the presence of virus [10–12].
The detection of virus present in air is a way to gain understanding of the risk of airborne transmission. Environmental sampling in hospitals has revealed the presence of airborne severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA, but mostly in low concentrations [13–18]. However, only limited focus has been directed towards any connections to specific ongoing medical treatments, such as AGPs. The published observational studies have either collected a limited number of samples or lack associated patient and disease data.
The aim of this work was to identify factors associated with airborne SARS-CoV-2 RNA, including potential AGPs, patient characteristics, and environmental parameters. Air samples were collected from hospital wards where patients with COVID-19 were treated. Samples were linked to patient data and analyzed for the presence of SARS-CoV-2 RNA. The results may help identify situations with increased risk for virus inhalation and thus have implications for infection-control guidelines.
METHOD
Design
An exploratory observational study was performed at wards treating patients with COVID-19 in 2 hospitals in Skåne, southern Sweden, from March 2020 to April 2021, to investigate factors associated with SARS-CoV-2 in the air. Variables investigated included patient characteristics, distance from patient, room ventilation, and supportive treatment with a focus on potential AGPs.
Setting
Air samples were collected at 3 infectious disease wards, 4 intensive care units (ICUs), 3 medical wards converted to COVID-19 units, and 1 emergency department. Ventilation in each room was a nominal 3–4 air changes per hour (ACH). Six rooms at one of the infectious disease wards had an installed higher ventilation rate of 8 ACH. As an addition to regular ventilation, mobile High-Efficiency Particulate Air (HEPA)–filtration units, delivering approximately 200 L filtered air per second, were used at the discretion of the ward staff, but were recommended when AGPs were performed. Rooms with a mobile HEPA-filtration unit or a high ventilation rate (8–9 ACH) were defined as rooms with enhanced ventilation. Rooms were predominantly single or double rooms, but the ICUs also had larger cohort rooms for up to 16 patients, the latter with up to 4 mobile HEPA-filtration units. Most, but not all, rooms were equipped with an anteroom between the patient’s room and the ward corridor.
Patient Characteristics
All patients were laboratory confirmed with COVID-19. Patients with recent admission or ongoing AGPs were preferred. Data regarding any potential AGP of the patient closest to air sampling were collected at sampling. Procedures classified as potential AGPs in this study were: high-flow nasal cannula (HFNC), mechanical ventilation, noninvasive ventilation (NIV), airway manipulation (including bronchoscopy, in- and extubation, deep airway suction, changes of tube sets and tracheotomy). We also included nebulizer treatment and positive expiratory airway pressure (PEP) training, procedures which at the time of our study were not considered AGPs by local infection-control recommendations. PEP training is a part of respiratory physiotherapy that uses a flute-like mouthpiece for the patient to breathe with an active expiration against an expiratory resistance, followed by a forced expiration and, normally, coughing. If airway manipulation or nebulizer treatment was ongoing, the risk from other possible simultaneous APGs was excluded from the analysis. Data were collected from medical records on days since the start of the COVID-19 infection and SARS-CoV-2 polymerase chain reaction (PCR) cycle threshold (Ct) value of the most recent nasal and nasopharyngeal or bronchial specimen. At the ICU COVID-19 cohort rooms, collected patient data were sometimes omitted when air sampling was equally close to more than 1 patient.
Air Sampling
The study team visited the wards several times weekly during high COVID-19 incidence. Air was sampled from patient rooms, anterooms, ward corridors, and hospital public areas. Sampling in patient rooms was performed at a predefined distance of less than 1 m, 1–2 m, or 2–4 m from the patient’s head. A liquid cyclone (Coriolis µ; Bertin Instruments, France) was used to collect air samples. The cyclone operated at 200 L minute−1 for 10 minutes, with 15 mL of phosphate-buffered saline solution as collection liquid, in single-use collection vials (Bertin Instruments). The method has been used previously by our group for sampling norovirus [19]. Samples were transferred to storage at +4°C or −80°C within 2 hours of sampling. Samples were stored for up to 5 months before analysis.
Air Sample Analysis
The collected air samples were concentrated using Amicon Ultra-15 centrifugal filter units (50-kDa cutoff; Merck Millipore KGaA) to a final volume of 140 µL, which was used for RNA extraction using the QIAamp viral RNA mini kit (Qiagen, Germany).
Reverse transcription–quantitative PCR (RT-qPCR) was performed with primers and probes targeting the SARS-CoV-2 N gene, as described [20], using the qPCRBIO Probe 1-Step Virus Detect kit (PCR Biosystems Ltd) (details in the Supplementary Material).
Samples were considered positive if the Ct value was 40 or less in one or both duplicates of the sample. Negative and positive controls were performed for air sampling, sample handling, and RT-qPCR. SARS-CoV-2 RNA stability after storage for longer periods at +4°C was also assessed (details in the Supplementary Material).
Statistical Analysis
Chi square test was used to assess crude differences and odds ratios (ORs) between categorical variables for positive air samples. Chi-square test for heterogeneity and Mantel-Haenszel test were used to test for effect modification and to explore confounding variables. Logistic regression was used to analyze ORs for ordered exposure variables (duration of illness, distance to sampling, patients in the room, patient Ct value) and in multivariate analyses adjusting for confounding factors (Ct value within 5 days, distance to sampling, patients in the room, and room ventilation). STATA SE/15.1 (StataCorp, College Station, TX, USA) was used in all statistical analyses.
Ethical Approval
The study was approved by the National Ethical Review Board in Sweden (project number 2020-01396). An informed written consent from the patients was generally not possible to obtain due to the severity of the disease. The ethical review board approved analysis of limited patient data without consent, including patient Ct values for SARS-CoV-2, age, sex, and medical procedure during sampling.
RESULTS
In total, 310 air samples were collected, of which 26 (8%) were positive for SARS-CoV-2 RNA. Air samples collected from ward corridors were positive for SARS-CoV-2 RNA in 3 of 51 samples (6%) and 1 of 15 (6%) samples from anterooms. The positive corridor and anteroom samples were collected at 2 different wards, both times outside patient rooms in which SARS-CoV-2 was found in the air. None of the 12 samples collected in hospital public areas were positive.
Of the 231 samples collected within patient rooms, 22 (10%) were positive for SARS-CoV-2 (Table 1). Results from 182 samples could be related to data from 88 unique patients nearest to the sampler. For the remaining 49 samples, all collected at ICU cohort rooms, potential AGPs were the only individual data recorded. For each patient, 1–10 samples (median of 2) were collected at 1–4 separate days.
Table 1.
Air Samples Collected and Patient and COVID-19 Characteristics, Patient Rooms, and Related Sampling Factors and Ongoing Potential Aerosol Generating Procedures
| Sampling Related Factors | Total Air Samples (N = 231) | Positive Air Samples (n = 22) | P a |
|---|---|---|---|
| Patient and COVID-19 | |||
| Age | .78 | ||
| <55 years | 49 | 7 (14%) | |
| 55–75 years | 86 | 9 (10%) | |
| >75 years | 47 | 5 (11%) | |
| Missing | 48 | 1 (2%) | |
| Sex | .74 | ||
| Male | 127 | 14 (11%) | |
| Female | 55 | 7 (13%) | |
| Missing | 48 | 1 (2%) | |
| Duration of illness | .33 | ||
| 1–7 days | 33 | 4 (12%) | |
| 8–14 days | 71 | 11 (15%) | |
| >15 days | 78 | 6 (8%) | |
| Missing | 48 | 1 (2%) | |
| Patient Ct values within 5 daysb | <.05 | ||
| <25 | 54 | 10 (19%) | |
| >25 | 69 | 3 (4%) | |
| Missing | 108 | 9 (8%) | |
| Room and sampling | |||
| Sampling distance | .13 | ||
| <1 m | 82 | 11 (13%) | |
| 1–2 m | 88 | 7 (8%) | |
| >2 m | 56 | 2 (4%) | |
| Missing | 5 | 2 (40%) | |
| Room ventilation | <.05 | ||
| Normal | 57 | 10 (18%) | |
| Enhanced ventilation | 174 | 12 (7%) | |
| Patients in room | .10 | ||
| 1 | 116 | 15 (13%) | |
| 2–4 | 36 | 4 (11%) | |
| >4 | 79 | 3 (4%) | |
| Ward type | .25 | ||
| Normal | 120 | 14 (12%) | |
| ICU | 111 | 8 (7%) | |
| Aerosol-generating procedures | |||
| HFNCc | .43 | ||
| Yes | 58 | 4 (7%) | |
| No | 173 | 18 (10%) | |
| Mechanical ventilation3 | .07 | ||
| Yes | 71 | 3 (4%) | |
| No | 160 | 19 (12%) | |
| NIV | .38 | ||
| Yes | 7 | 0 (0%) | |
| No | 224 | 22 (10%) | |
| Airway manipulationd | .28 | ||
| Yes | 26 | 4 (15%) | |
| No | 205 | 18 (9%) | |
| PEP training | <.05 | ||
| Yes | 11 | 4 (36%) | |
| No | 220 | 18 (8%) | |
| Nebulizer treatment | .32 | ||
| Yes | 9 | 0 (0%) | |
| No | 222 | 22 (10%) | |
| Any potential AGP | .41 | ||
| Yes | 174 | 15 (9%) | |
| No | 57 | 7 (12%) |
Abbreviations: AGP, aerosol-generating procedure; COVID-19, coronavirus disease 2019; Ct, cycle threshold; HFNC, high-flow nasal cannula; ICU, intensive care unit; NIV, noninvasive ventilation (bilevel positive airway pressure [BiPAP]/continuous positive airway pressure [CPAP]); PCR, polymerase chain reaction; PEP, positive expiratory pressure training (with a PEP flute); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
P values of difference in positive air samples within category (excluding missing values) by chi-square test.
Ct value of patient respiratory tract sample in a positive PCR for SARS-CoV-2 obtained within 5 days from air sampling in the patient’s room.
During 8 of the air sampling occasions both HFNC and mechanical ventilation were ongoing and these samples occur in both categories.
Airway manipulation = bronchoscopy, in-and extubation, deep airway suction, changes of tube sets (breaking of closed ventilation system), and tracheotomy.
The estimated concentration of SARS-CoV-2 RNA copies in positive air samples collected in patient rooms was a median of 115 (interquartile range [IQR], 31–232) copies/m3.
Patient and COVID-19 Characteristics
The median age of the patients in the rooms sampled was 59 years (IQR, 54–76 years), and a majority were male (Table 1). No association was noted between positive air sample and patient age or gender. Of air samples collected within 2 weeks of symptom onset, 14% were positive, compared with only 8% for those collected after more than 2 weeks (Table 1, Figure 1). The samples were collected a median of 13 days (IQR, 10–20 days) after COVID-19 onset and a median of 5 days after hospital admission (IQR, 2–12 days). Details of the patients in rooms with a positive air sample can be found in Supplementary Table 3.
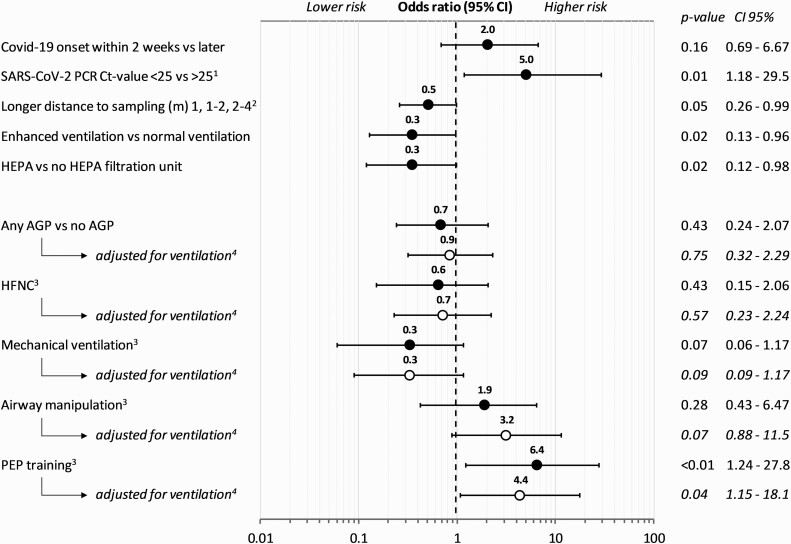
Figure 1.
Odds ratios for characteristics for COVID-19, sampling, room ventilation, and AGPs for positive air samples. 1Ct value of patient diagnostic SARS-CoV-2 PCR test collected within 5 days from air sampling. 2Odds ratio for every category (<1 m, 1–2 m, 2–4 m) away from the patient’s head. 3Ongoing specific category versus no ongoing specific category—for example, HFNC versus no HFNC (all other air samples). 4Logistic regression adjusted for room ventilation; enhanced, including HEPA-filtration unit or normal ventilation. Abbreviations: AGP, aerosol-generating procedure; CI, confidence interval; COVID-19/Covid-19, coronavirus disease 2019; Ct, cycle threshold; HEPA, High-Efficiency Particulate Air; HFNC, high-flow nasal cannula; PCR, polymerase chain reaction; PEP, positive expiratory pressure; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
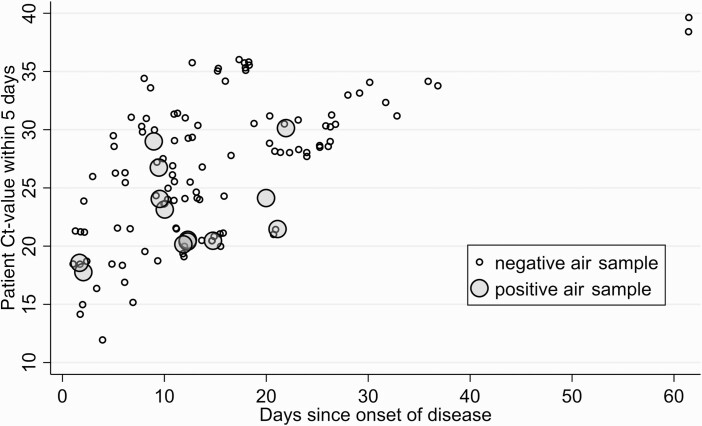
The Ct value of the patient’s most recent PCR test was obtained for 165 patient room-air samples, and 123 were obtained within 5 days of air sampling (Table 1). The median patient Ct value was 27 (IQR, 21–31). A patient sample (nasopharyngeal and tracheal), collected within 5 days of air sampling with a Ct value less than 25 was associated with a positive air sample (Figure 1). Sensitivity analysis including only patient samples collected within 3 days did not change the outcome (Supplementary Table 1). The association between patient Ct values, onset of disease, and positive air samples is shown in Figure 2. A low Ct value of the most recent SARS-CoV-2–positive diagnostic patient test was associated with a positive air sample within the patient room (OR = 1.7; 95% confidence interval [CI]: 1.0–2.8; P < .05 for every 5-cycle decrease in patient Ct value) (Figure 2). Odds ratios were unchanged when adjusting for ventilation and distance, as can be seen in Supplementary Table 1.
Figure 2.
Time since symptom onset, patient Ct value within 5 days, and positive air samples. Abbreviation: Ct, cycle threshold.
Patient Rooms, Distance to Sampling, and Room Ventilation
A shorter distance from air collection to the patient’s head was associated with a SARS-CoV-2–positive air sample. Each step, increasing the distance from more than 1 m, 1–2 m, and further to 2–4 m, reduced the odds of a positive air sample by approximately 50%, respectively (ie, OR = .5 for each step) (Figure 1).
Adding a mobile HEPA-filtration unit to rooms with regular ventilation was associated with a reduction in SARS-CoV-2 RNA in the air (Figure 1). This association was similar for all rooms with an enhanced ventilation, either by a built-in high ventilation rate (35 air samples) or by an added mobile HEPA-filtration unit (139 air samples) (Table 1, Figure 1). Multivariate analyses showed that sampling distance, patient’s most recent Ct values, or number of patients within the room were not considered confounders to the effect of ventilation (Supplementary Table 1).
Potential Aerosol-Generating Procedures
During 174 (75%) of the air sampling occasions, a potential AGP was ongoing within the room. When comparing any ongoing potential AGP as a group with no ongoing AGP, no association was found with SARS-CoV-2–positive air samples (Table 1). The patients not subject to any AGP had a shorter duration of illness, a lower patient Ct value, and were more often treated in a room with regular ventilation (P < .05). Normal oxygen therapy, by mask or nasal cannula, was delivered during 38 of 57 (66%) of samples when no AGP was ongoing. Detailed characteristics of room samples with any or no ongoing AGP are presented in Supplementary Table 2.
Of the air samples collected during HFNC, 4 of 58 (7%) were positive for SARS-CoV-2 (Table 1). No association was seen between HFNC and a positive air sample compared with no HFNC (Figure 1), even when adjusting for sampling distance, patient Ct values, number of patients in the room, or room ventilation (Supplementary Table 1). None of the 7 air samples collected during NIV were positive for SARS-CoV-2.
Mechanical ventilation was ongoing during 71 of the air samples collected, of which 3 (4%) were positive for SARS-CoV-2. No association was found between mechanical ventilation and a positive air sample compared with no mechanical ventilation (Figure 1), or when adjusting for sampling distance, patient Ct values, number of patients in the room, or room ventilation (Supplementary Table 1).
Airway manipulation occurred during, or minutes before, 26 of the air samples, of which 4 (15%) were positive for SARS-CoV-2. No significant statistical support was found for an association between airway manipulation and a positive air sample compared with no airway manipulation (Figure 1). However, a trend towards an association between airway manipulation and a positive air sample was observed when adjusting for room ventilation. At least 1 air sample was positive in 4 (20%) of the, in all, 20 separate airway manipulation occasions sampled. The 4 positive air samples were collected during 1 bronchoscopy, 2 extubations, and 1 change of tube sets of the total of 4 bronchoscopies, 5 extubations, 2 intubations, 2 changes of tube sets, 4 tracheal suctions, and 3 tracheostomies sampled.
Positive expiratory airway pressure training was carried out during 11 of the air samples, involving 7 different patients, of which 4 samples (36%) were positive for SARS-CoV-2 in 3 different patient rooms. There was a significant association between PEP training and a positive air sample, also when adjusting for sampling distance, patient Ct value, number of patients in the room, or room ventilation (Figure 1).
Nebulizer treatment with drug inhalation was ongoing during 9 of the samples collected, involving 6 different patients. None of these samples were positive for SARS-CoV-2.
DISCUSSION
This study shows associations between SARS-CoV-2–positive air samples and decreasing physical distance from the patient, a low patient SARS-CoV-2 Ct value, as well as absence of enhanced room ventilation. No association was observed with AGPs as traditionally defined, but there was a higher proportion of positive samples collected during airway manipulation, such as bronchoscopy and extubation. However, ongoing PEP training, usually not included as an AGP category, was significantly associated with positive air samples.
This is, to our knowledge, the largest investigation of SARS-CoV-2 in hospital and patient room air, with samples collected from 2 hospitals and more than 10 wards including multiple different settings and procedures. A number of previous studies have investigated SARS-CoV-2 RNA in hospital air, although a majority had less than 50 air samples and only limited information about connections to ongoing medical treatments, ventilation, or distance [21]. The proportion of positive air samples varies considerably between studies, but taken together, 10–15% of air samples previously collected in hospitals are reported to be positive for SARS-CoV-2, similar to our findings [18, 21]. The variation may depend on method, volume, location of air collection and the subsequent laboratory analysis [22].
The highest onward transmission and virus shedding have been reported during the first days after onset [23, 24]. Our study shows a direct connection between SARS-CoV-2 in the air and higher viral loads in patient respiratory tract samples. The association between airborne SARS-CoV-2 and the duration of illness was less evident than for the Ct value of the patient, perhaps due to individual variations and prolonged shedding in severe disease [25].
A connection between poor ventilation in enclosed spaces and a higher risk for airborne infections have been noted in numerous outbreak investigations [1]. Mainly based on outbreak reports and modeling, recommendations have been issued to increase room ventilation to reduce transmission risk [3]. In the present study, the fraction of SARS-CoV-2–positive air samples was significantly reduced by increased ventilation or the use of mobile HEPA units. The results also show a higher proportion of positive air samples close to patients, which is in agreement with previous epidemiological and modeling studies showing a higher risk for transmission at shorter distances [26, 27].
For most of the included potential AGPs we did not observe any increased presence of SARS-CoV-2 RNA in the air. With the exception of airway manipulation, a trend towards lower risk was noted during other proposed AGPs, such as mechanical ventilation, HFNC, nebulizer treatment, and NIV. This is in agreement with several recent studies of aerosol emissions from healthcare procedures and previous studies of transmission in healthcare [2, 10, 11, 28]. Although almost none of these studies include analysis of viruses, they show that AGPs generally produce less aerosol than, for instance, coughing or speaking. In addition, particle emissions from AGPs are often limited by masks or tubes around the mouth and nose. However, patients included in this study did not wear face masks. Mechanical ventilators also had filters on the expiratory limb, likely reducing viral emissions. The only procedure that was associated with a higher risk of airborne SARS-CoV-2 was PEP training, which is not usually included as an AGP category. This can be explained by the opening of small, blocked airways, where liquid films may burst into micrometer-sized droplets, generating aerosols to the exhaled breath [29].
One limitation of our study is that the low virus concentrations in the samples did not allow us to determine viability of the collected virus. Successful attempts to isolate infectious virus from air samples have previously been published, despite the difficulties surrounding culturing from low-concentration isolates [15, 30]. Other limitations include a low number of air samples from specific settings, especially for NIV, nebulizer treatment, PEP training, and certain categories of airway manipulation. The number of positive air samples was too few to permit a correct multiple logistic regression of all possible confounders. Air samples were collected during prespecified potential AGPs, but other potentially emitting activities, such as coughing and talking, were not recorded uniformly and could thus not be taken into account. The study was conducted during the first phases of the pandemic, including a short period dominated by the Alpha variant but before the introduction of the Delta variant. We still believe that the main principles for formation of virus-containing aerosols are the same.
The air sampling collection method had a lower particle size limitation of approximately 0.5 µm and, thus, excluded viruses that potentially may be present in very small aerosol particles. Also, large droplets are unlikely to follow the airstream into the sampling inlet. Some samples were stored for a long time before RNA extraction, so RNA degradation might have occurred. Taken together, we expect the number of positive air samples and the calculated virus concentrations to be an underestimation of the true presence of SARS-CoV-2 RNA in the air due to loss at collection and downstream sample analysis. However, we do not consider these limitations to affect the main conclusions of our study.
Albeit with a limited sample size in certain subgroups, this study contributes important additional support for when and where the risk for airborne transmission of SARS-CoV-2 is high in hospital settings, and how it can be reduced. Patients with a high viral load, during the first 1–2 weeks of disease, most likely constitute the highest risk, especially at short distances. Based on our results, this applies irrespectively of any ongoing AGP, with the exception of airway manipulation and PEP training. Increased ventilation, by mobile HEPA-filtration units or similar methods, seems to be an effective way to reduce SARS-CoV-2 in the surrounding air. The expanded concept of AGP as a trigger of increased infection-control measures could thus be questioned in favor of a situation-based approach, where ongoing medical procedures are merely one factor among others. The introduction of new variants could pose an interesting opportunity for future studies. Further studies of airborne transmission are of importance to support and conceptualize a new infection-control model for SARS-CoV-2 and other respiratory infections, where patient, disease, and environmental factors are considered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in design of the study or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Financial support. This work was supported by AFA Insurance (grant numbers 180113, 200109), the Swedish Research Council for Sustainable Development (grant numbers 2017-00383, 2020-01490), the “Sven Olof Jansons livsverk” Foundation, the Swedish Research Council for Health, Working Life and Welfare (grant number 2017-00690), and grants from the SciLifeLab National COVID-19 Research Program, financed by the Knut and Alice Wallenberg Foundation, the Swedish Research Council (grant number 2020-02344), and the Österlund Foundation.
Potential conflicts of interest. C.-J. F. reports being a contributing author to Swedish guidelines for clinical management of COVID-19 and a member of the Board of the Swedish Medical Association of Infection Control (SHLF), both unpaid. J. L. reports receiving payment for lectures by Camfil paid to their institution and received a personal salary as payment for expert advice on protection for airborne disease at Skåne University Hospital in 2020. The other authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Sara Thuresson, Division of Ergonomics and Aerosol Technology, Department of Design Sciences, Lund University, Lund, Sweden.
Carl Johan Fraenkel, Department of Infection Control, Region Skåne, Lund, Sweden; Division of Infection Medicine, Department of Clinical Sciences, Lund University, Lund, Swedenand.
Sviataslau Sasinovich, Department of Translational Medicine, Lund University, Lund, Sweden.
Jonathan Soldemyr, Division of Ergonomics and Aerosol Technology, Department of Design Sciences, Lund University, Lund, Sweden.
Anders Widell, Department of Translational Medicine, Lund University, Lund, Sweden.
Patrik Medstrand, Department of Translational Medicine, Lund University, Lund, Sweden.
Malin Alsved, Division of Ergonomics and Aerosol Technology, Department of Design Sciences, Lund University, Lund, Sweden.
Jakob Löndahl, Division of Ergonomics and Aerosol Technology, Department of Design Sciences, Lund University, Lund, Sweden.
References
- 1. Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y.. Indoor transmission of SARS-CoV-2. Indoor Air 2021; 31:639–45. [DOI] [PubMed] [Google Scholar]
- 2. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J.. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Infection preventio n and control during health care when coronavirus disease (COVID-19) is suspected or confirmed: Interim guidance. Published July 12. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 4. Hamilton F, Arnold D, Bzdek BR, et al. . Aerosol generating procedures: are they of relevance for transmission of SARS-CoV-2? Lancet Respir Med 2021; 9:687–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eyre DW, Lumley SF, O’Donnell D, et al. . Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020; 9. doi: 10.7554/eLife.60675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah ASV, Wood R, Gribben C, et al. . Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ 2020; 371:m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton FW, Gregson FKA, Arnold DT, et al. . Aerosol emission from the respiratory tract: an analysis of aerosol generation from oxygen delivery systems. Thorax 2022; 77:276–82. doi: 10.1136/thoraxjnl-2021-217577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klompas M, Baker M, Rhee C.. What is an aerosol-generating procedure? JAMA Surg 2021; 156:113–4. [DOI] [PubMed] [Google Scholar]
- 9. Jackson T, Deibert D, Wyatt G, et al. . Classification of aerosol-generating procedures: a rapid systematic review. BMJ Open Respir Res 2020; 7:e000730. doi: 10.1136/bmjresp-2020-000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson NM, Marks GB, Eckhardt A, et al. . The effect of respiratory activity, non-invasive respiratory support and facemasks on aerosol generation and its relevance to COVID-19. Anaesthesia 2021; 76:1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown J, Gregson FKA, Shrimpton A, et al. . A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia 2021; 76:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Neil CA, Li J, Leavey A, et al. . Characterization of aerosols generated during patient care activities. Clin Infect Dis 2017; 65:1335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore G, Rickard H, Stevenson D, et al. . Detection of SARS-CoV-2 within the healthcare environment: a multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J Hosp Infect 2021; 108:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ong SWX, Tan YK, Coleman KK, et al. . Lack of viable severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among PCR-positive air samples from hospital rooms and community isolation facilities. Infect Control Hosp Epidemiol 2021; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lednicky JA, Lauzardo M, Fan ZH, et al. . Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 2020; 100:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J, Otter JA, Price JR, et al. . Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis 2021; 73:e1870–e1877. doi: 10.1093/cid/ciaa905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santarpia JL, Rivera DN, Herrera VL, et al. . Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep 2020; 10:12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mallach G, Kasloff SB, Kovesi T, et al. . Aerosol SARS-CoV-2 in hospitals and long-term care homes during the COVID-19 pandemic. PLoS One 2021; 16:e0258151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alsved M, Fraenkel CJ, Bohgard M, et al. . Sources of airborne norovirus in hospital outbreaks. Clin Infect Dis 2020; 70:2023–8. doi: 10.1093/cid/ciz584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petrillo S, Carrà G, Bottino P, et al. . A novel multiplex qRT-PCR assay to detect SARS-CoV-2 infection: high sensitivity and increased testing capacity. Microorganisms 2020; 8:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dinoi A, Feltracco M, Chirizzi D, et al. . A review on measurements of SARS-CoV-2 genetic material in air in outdoor and indoor environments: implication for airborne transmission. Sci Total Environ 2022: 151137. doi: 10.1016/j.scitotenv.2021.151137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubey A, Kotnala G, Mandal TK, et al. . Evidence of the presence of SARS-CoV-2 virus in atmospheric air and surfaces of a dedicated COVID hospital. J Med Virol 2021; 93:5339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 24. Cheng H-Y, Jian S-W, Liu D-P, Ng T-C, Huang W-T, Lin H-H.. High transmissibility of COVID-19 ne ar symptom onset. medRxiv 2020. doi: 10.1101/2020.03.18.20034561 [DOI] [Google Scholar]
- 25. van Kampen JJA, van de Vijver D, Fraaij PLA, et al. . Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021; 12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu DK, Akl EA, Duda S, et al. . Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020; 395:1973–87. doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortellessa G, Stabile L, Arpino F, et al. . Close proximity risk assessment for SARS-CoV-2 infection. Sci Total Environ 2021; 794:148749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson KA, Pappachan JV, Bennett AM, et al. . Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic—the risk of aerosol generation during medical procedures. PLoS One 2013; 8:e56278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson GR, Morawska L, Ristovski ZD, et al. . Modality of human expired aerosol size distributions. J Aerosol Sci 2011; 42:839–51. [Google Scholar]
- 30. Santarpia JL, Herrera VL, Rivera DN, et al. . The size and culturability of patient-generated SARS-CoV-2 aerosol. J Expo Sci Environ Epidemiol 2021. doi: 10.1038/s41370-021-00376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.