ABSTRACT
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is more frequent and severe in patients with chronic kidney disease (CKD) on maintenance haemodialysis (HD). Vaccines are now available, but the protective response rates and determinants of humoral response to the vaccine are poorly described.
Methods
This prospective observational study describes the response rates of detectable and protective antibody titres 1 month after each dose of an mRNA vaccine in a cohort of 851 patients on maintenance HD.
Findings
Among naïve SARS-CoV-2 patients, a vast majority produced detectable (95.2%) or protective levels of antibodies (69.6%) 1 month after the second vaccine dose. In addition, the response rate was significantly higher with the mRNA-1273 than with the BNT162b2 vaccine 1 month after the second dose (79.8 versus 59.1%, respectively; P < 0.001). The main determinants for an inadequate humoral response were older age, treatment with immunosuppressants or oral anticoagulants and low serum albumin. All the patients who encountered coronavirus disease 2019 before vaccination also reached a highly protective humoral response.
Interpretation
We found an acceptable humoral response rate in patients on maintenance HD, much higher than in transplant recipients. Therefore the third dose of vaccine may be justified in those patients with an inadequate humoral response, particularly those with a history of organ transplantation or immunosuppressive treatment.
Keywords: chronic kidney disease, COVID-19 mRNA vaccines, haemodialysis
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is more frequent and severe in patients with chronic kidney disease (CKD) on maintenance haemodialysis (HD). The mortality rate in the general population is ∼1.9% [1] and ranges between 30 and 41% in dialysis patients, depending on the study [2–5]. Therefore these patients have been considered a priority for vaccination against SARS-CoV-2, although they were poorly represented in studies on vaccine efficacy [6]. In addition, CKD patients usually respond poorly to vaccinations, such as for hepatitis B, both in pre-dialysis and the end-stage of the disease [6, 7]. The decreased response to vaccination is restricted to thymo-dependent antigens like hepatitis B, while the immune response to thymo-independent antigens like Streptococcus pneumoniae is normal [8].
Recent studies have shown that the proportion of CKD patients on maintenance dialysis developing anti-SARS-CoV-2 antibodies after vaccination with two doses of mRNA vaccine was significant, ranging from 82 to 96% [9–22]. However, these studies did not consider the protective anti-SARS-CoV-2 antibody titres against the delta variant. In addition, there was no standardization of antibody measurement between different test manufacturers, which obscures the comparisons between studies. Recently the World Health Organization (WHO) has standardized the reporting of antibody level results. A recent study [23] suggests that an antibody level ˃264 BAU/mL provides 80% protection against symptomatic forms of coronavirus disease (COVID) in the general population. Finally, only a few studies compared the mRNA-1273 vaccine (Moderna, Cambridge, MA, USA) and the BNT162b2 vaccine (Pfizer-BioNTech, New York, NY, USA) [23, 24] and there was no formal comparison between the two vaccines according to protective levels.
We conducted a study in a large cohort of patients on maintenance HD administered with one or the other of the two mRNA vaccines available on the European market to quantify the rate of protective humoral response (i.e. >264 BAU/mL) with the two vaccines and analyse the determinants of adequate response to vaccination.
MATERIALS AND METHODS
This was a multicentre prospective study in six dialysis centres within a single French region. Inclusion criteria were patients with CKD on maintenance HD and receiving two doses of mRNA vaccine against SARS-CoV-2. Exclusion criteria were refusal of vaccination, contraindication to vaccination (allergy, COVID-19 within the last 3 months), pregnancy, age ˂18 years, patient unable to give consent and refusal to participate in the study. All included patients gave their consent to participate and the ethics committee of the University Hospital of Strasbourg approved the protocol (CE-2021-45).
We collected the clinical and biological characteristics and the history of the included patients at the time of the first vaccine injection from data available in the computerized dialysis medical record. Biological tests were part of the regular follow-up of the patients and, apart from anti-SARS-CoV-2 serologies, no additional biological samples or complementary examinations were necessary for the study.
According to current recommendations, all patients were vaccinated with two intramuscular injections of the same vaccine 4 weeks apart, either with BNT162b2 or mRNA-1273 on the supply of each centre.
Anti-nucleoplasmid protein (anti-NP) antibody testing was performed at the first vaccination to detect asymptomatic patients who had antibodies before vaccination. Vaccination-specific anti-spike (S) antibodies were tested twice at the second vaccination (1 month after the first dose) and 4 weeks later. Immunoglobulin G (IgG) antibodies to the S1 subunit of the S protein were tested using either the SARS-CoV-2 IgG II Quant Assay (Abbott Laboratories, Abbott Park, IL, USA) or the Elecsys anti-SARS-CoV-2 S kit (Roche, Basel, Switzerland), depending on the centre. According to the manufacturers’ recommendations, the threshold of detection of antibodies was set at 0.8 U/mL for the Roche test and 50 U/mL for the Abbott test. The threshold estimated as protective (>264 BAU/mL) was set at 253 U/mL for the Roche test [23, 24] and 1831 U/mL for the Abbott test [24].
Side effects of the vaccination were assessed by interviewing the patients at each injection and 1 month later.
The primary endpoint was the percentage of patients with protective levels of antibodies 4 weeks after the second vaccination. The secondary endpoints were the percentage of patients who developed antibodies above the detection threshold and above the protection threshold after the first vaccine injection, clinical determinants of protective levels from vaccination and analysis of side effects.
Statistics
The position and dispersion parameters were expressed as median and interquartile range (IQR) for numerical data and number and percentage for categorical and binary variables.
As nearly all numerical variables did not show a normal distribution, these variables were compared with the Mann–Whitney–Wilcoxon test. In addition, the categorial variables were compared with Fisher's exact test. The determinants of humoral response with detectable and protective antibody titres were analysed by logistic regression, first, in univariate analyses and then in a multivariate analysis including parameters with P < 0.20 in the univariate analyses. Missing data were not imputed.
The following variables were used in the univariate analyses: age, gender, weight, body mass index (BMI), dialysis vintage, dialysis modality (haemodialysis or haemodiafiltration), dialysis on polymethylmethacrylate (PMMA) membrane, Kt/V, smoking, coronary artery disease (CAD), peripheral arterial disease (PAD), hypertension, diabetes, history of organ transplantation, flu vaccine, response to hepatitis B virus (HBV) vaccine, treatment with immunosuppressive drug, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), vitamin K antagonist (VKA) or antiplatelet agent (APA), plasma creatinine, potassium, bicarbonate, calcium, phosphate, serum total protein and albumin, C-reactive protein (CRP), intact parathyroid hormone (iPTH), haemoglobin and vaccine brand (Pfizer-BioNTech or Moderna).
All statistical analyses were performed with Stata version 13.1 software (StataCorp, College Station, TX, USA) and P < 0.05 was considered statistically significant.
RESULTS
In our cohort of 1101 dialysis patients (74% of all the patients on maintenance HD in our region), 896 patients agreed to be vaccinated and participate in the study. Of these, five were excluded because of COVID-19 within the last 3 months and two for known allergies. As anti-NP tests may cross-react with previous common cold human coronaviruses, false-positive tests could be expected. In our cohort, 59 patients were found with positive anti-NP antibody tests before vaccination but were considered asymptomatic exposure to SARS-CoV-2. A first dose was given to 889 patients and 862 had the second dose. The second dose was not given to 18 patients because of a history of COVID-19, 8 because of COVID-19 infection after the first dose and 1 patient because of non-COVID-19 infection. In addition, serologies were not collected 1 month after the second dose in 10 patients, 4 of whom had a non-COVID-related death. Thus 852 patients were included in the statistical analyses (Flowchart Figure 1).
FIGURE 1:

Flowchart.
The median age of our patients was 71 years (IQR 61–80), 64% of whom were men, with a BMI of 26.6 kg/m2 (IQR 23.2–31.0). The median dialysis vintage was 3.3 years (IQR 1.5–6.7). Diabetes was present in 45.5% of patients, while 85.7% had hypertension, 34% had coronary artery disease and 27.5% had peripheral arterial disease. Other demographic characteristics of the cohort are shown in Table 1. Before the first vaccination, 3.4% of patients had symptomatic COVID-19 disease, 3.3% had been hospitalized and 0.6% required admission to the intensive care unit. Finally, 420 patients (49.4%) received the BNT162b2 vaccine and 431 (50.6%) received the mRNA-1273 vaccine.
Table 1.
Humoral response rate 1 month after each dose of vaccine in HD patients
| Month 1 | Month 2 | |||||
|---|---|---|---|---|---|---|
| Response | Total | BNT162b2 | mRNA-1273 | Total | BNT162b2 | mRNA-1273 |
| SARS-CoV-2-naïve patients before vaccination, n | 678 | 252 | 426 | 852 | 421 | 431 |
| Detectable anti-S antibodies (%) | 69.1 | 50.9 | 80§ | 95.2 | 94.8 | 95.6a |
| Protective level of anti-S antibodies (%) | 5.4 | 3.1 | 6.7§ | 69.6 | 59.1 | 79.8* |
| Anti-NP-positive patients before vaccination, n | 58 | 26 | 32 | 58 | 26 | 32 |
| Detectable anti-S antibodies (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Protective level of anti-S antibodies (%) | 85.7 | 91.7 | 81.3 | 94.8 | 96.2 | 93.8 |
* P < .001. aP = not significant, mRNA-1273 versus BNT162b2 vaccine.
Serological response after the first dose
In the patients without previous COVID-19 (clinical history or positive serology before vaccination), the response rate detectable/protective was 69.2%/5.4% and non-different between the two mRNA-1273 vaccines (Table 2). In patients with positive anti-NP tests before vaccination, the response rate detectable/protective was much higher (100%/85.7%) and similar for both vaccines.
Table 2.
Main demographic and clinical characteristics of the cohort and univariate analyses of the determinants of humoral response assessed by detectable and protective antibody titres 1 month after each dose of vaccine
| Month 1 | Month 2 | ||||
|---|---|---|---|---|---|
| Protective level of antibodies | Protective level of antibodies | ||||
| Variable | Value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age (years), median (IQR) | 71 (61–80) | 1.00 (0.98–1.01) | 0.82 | 0.97 (0.96–0.98) | <0.001 |
| Male gender (%) | 64 | 0.80 (0.50–1.27) | 0.34 | 0.93 (0.68–1.26) | 0.63 |
| HD vintage (years), median (IQR) | 3.3 (1.5–6.7) | 1.00 (1.00–1.00) | 0.25 | 1.00 (1.00–1.00) | 0.63 |
| HDF (%) | 50.5 | 0.75 (0.46–1.21) | 0.23 | 0.79 (0.59–1.06) | 0.11 |
| PMMA membrane (%) | 8.6 | 1.31 (0.66–2.61) | 0.44 | 2.09 (1.13–3.88) | 0.02 |
| Weight (kg), median (IQR) | 75 (64–88) | 1.00 (0.99–1.01) | 0.91 | 1.01 (1.00–1.02) | 0.01 |
| BMI (kg/m2), median (IQR) | 26.6 (23.2–31.0) | 1.00 (0.97–1.04) | 0.81 | 1.02 (0.99–1.04) | 0.12 |
| Smoking (%) | 53 | 0.66 (0.41–1.06) | 0.08 | 1.02 (0.75–1.39) | 0.91 |
| CAD (%) | 34 | 0.95 (0.58–1.55) | 0.84 | 0.78 (0.58–1.06) | 0.12 |
| Hypertension (%) | 85.7 | 1.61 (0.75–3.45) | 0.22 | 0.93 (0.61–1.41) | 0.72 |
| Diabetes (%) | 45.5 | 1.08 (0.68–1.72) | 0.73 | 0.97 (0.72–1.30) | 0.82 |
| PAD (%) | 27.5 | 0.91 (0.54–1.53) | 0.73 | 0.82 (0.59–1.13) | 0.23 |
| Haemopathy (%) | 2.4 | 0.54 (0.07–4.21) | 0.56 | 0.57 (0.22–1.50) | 0.26 |
| Immunosuppressive drugs (%) | 12.2 | 0.71 (0.31–1.61) | 0.41 | 0.43 (0.27–0.68) | <0.001 |
| Transplant (%) | 15.1 | 0.50 (0.23–1.06) | 0.07 | 0.82 (0.55–1.22) | 0.32 |
| Flu vaccine (%) | 60.3 | 1.43 (0.84–2.43) | 0.18 | 1.18 (0.86–1.61) | 0.31 |
| Response to HBV vaccine (%) | 46.1 | 1.16 (0.68–2.00) | 0.58 | 2.02 (1.44–2.84) | <0.001 |
| ACEI (%) | 11.7 | 0.66 (0.29–1.48) | 0.31 | 0.97 (0.61–1.52) | 0.90 |
| ARB (%) | 11.4 | 1.80 (0.92–3.54) | 0.09 | 1.49 (0.91–2.43) | 0.11 |
| VKA (%) | 26 | 0.79 (0.45–1.38) | 0.41 | 0.53 (0.38–0.73) | <0.001 |
| APA (%) | 53 | 1.45 (0.91–2.32) | 0.12 | 0.96 (0.72–1.29) | 0.80 |
| Kt/V, median (IQR) | 1.59 (1.37–1.86) | 0.71 (0.37–1.37) | 0.31 | 0.78 (0.55–1.11) | 0.18 |
| Creatinine (µmol/L), median (IQR) | 585 (470–723) | 1.00 (1.00–1.00) | 0.61 | 1.002 (1.002–1.003) | <0.001 |
| Potassium (mmol/L), median (IQR) | 4.57 (4.18–4.92) | 0.95 (0.63–1.44) | 0.81 | 1.18 (0.90–1.55) | 0.23 |
| Bicarbonate (mmol/L), median (IQR) | 24.3 (23–25.7) | 1.00 (0.92–1.08) | 0.95 | 0.97 (0.92–1.02) | 0.28 |
| Calcium (mmol/L), median (IQR) | 2.21 (2.12–2.30) | 1.38 (0.31–6.19) | 0.68 | 2.44 (0.93–6.41) | 0.07 |
| Phosphate (mmol/L), median (IQR) | 1.43 (1.18–1.73) | 0.65 (0.37–1.14) | 0.14 | 1.38 (0.97–1.97) | 0.08 |
| Total protein (g/L), median (IQR) | 66.7 (62.8–71.0) | 0.98 (0.95–1.02) | 0.31 | 1.04 (1.01–1.06) | 0.004 |
| Serum albumin (g/L), median (IQR) | 38.7 935.0–41.3) | 1.05 (0.99–1.11) | 0.13 | 1.17 (1.13–1.21) | <0.001 |
| CRP (mg/L), mean ± SD | 7 ± 17.3 | 1.00 (0.98–1.01) | 0.65 | 0.98 (0.98–0.99) | <0.001 |
| iPTH (pg/mL), mean ± SD | 331.2 ± 343.5 | 1.00 (1.00–1.00) | 0.37 | 1.00 (1.00–1.00) | 0.67 |
| Hb (g%), mean ± SD | 11.22 ± 1.12 | 1.14 (0.93–1.40) | 0.19 | 1.17 (1.03–1.34) | 0.02 |
| BNT162b2 vaccine (%) | 49 | 0.95 (0.59–1.53) | 0.84 | 0.37 (0.27–0.50) | <0.001 |
OR, odds ratio; CI, confidence interval.
Serological response after the second dose
One month after the second dose among SARS-CoV-2-naïve patients, 95.2% developed anti-S antibodies at a detectable level and 69.6% had a protective level (Table 2). In addition, patients responded significantly better to the mRNA-1273 than the BNT162b2 vaccine; 79.8% had a protective level with the mRNA-1273 vaccine versus 59.1% with the BNT162b2 vaccine (P < .001). In patients with positive anti-NP tests before vaccination, the response rate detectable/protective was 100%/94.8% for both vaccines (100%/96.2% for BNT162b2, 100%/93.8% for mRNA-1273, not significant).
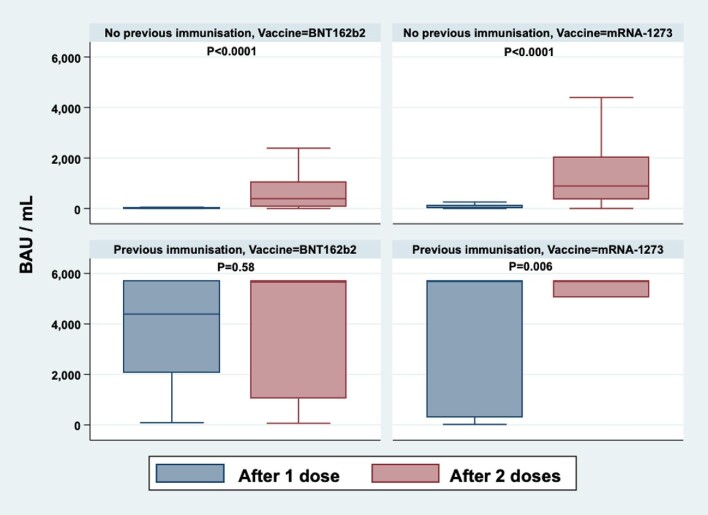
Antibody levels after each dose of vaccine in SARS-CoV-2 patients and those with positive anti-NP tests before vaccination are depicted in Figure 2.
FIGURE 2:
Anti-spike antibody titres 1 month after the first (blue) and second (pink) vaccine doses. Values are represented separately for COVID-naïve patients (upper row) and those with a positive anti-NP test prior to vaccination (lower row). The left column shows the antibody response to the BNT162b2 vaccine and the right column the antibody response to the mRNA-1273 vaccine. Changes in antibody titres before and after each dose of vaccine were compared using a Wilcoxon rank sign test.
In univariate analyses (Table 2), those who did not develop antibodies at protective levels had older age; lower weight; current immunosuppressive treatment; lower plasma creatinine, total protein, serum albumin and haemoglobin and higher CRP. They were also vaccinated with the BNT162b2 vaccine (versus the mRNA-1273 vaccine), were dialysed with PMMA membrane and were treated with VKAs.
All the patients with a history of previous COVID-19 infection or with detectable anti-S antibodies before vaccination reached a protective humoral response after the first dose and kept it after the second dose. Because of the highly predictable response, these patients were not included in the multivariate analysis.
In the multivariate analysis (Table 3), only older age, treatment with VKAs, serum albumin (P = 0.02), immunosuppressive therapy and BNT162b2 vaccine remained significantly associated with a weaker humoral response compared with the mRNA-1273 vaccine.
Table 3.
Multivariate analyses of the determinants of humoral response assessed by protective antibody titres 1 month after each dose of vaccine
| Protective level of antibodies | ||||
|---|---|---|---|---|
| Month 1 | Month 2 | |||
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age (years) | 0.97 (0.95–0.99) | 0.008 | ||
| Haemodiafiltration (%) | 1.02 (0.61–1.71) | 0.93 | ||
| PMMA dialysis membrane | 1.03 (0.42–2.55) | 0.94 | ||
| Weight | 1.01 (0.98–1.04) | 0.42 | ||
| BMI (kg/m2) | 0.99 (0.91–1.07) | 0.78 | ||
| Smoking | 0.60 (0.37–0.98) | 0.04 | ||
| CAD | 0.78 (0.48–1.29) | 0.34 | ||
| Immunosuppressive drugs | 1.14 (0.45–2.85) | 0.78 | 0.37 (0.18–0.73) | 0.005 |
| Transplant | 0.41 (0.18–0.92) | 0.03 | 0.62 (0.32–1.18) | 0.15 |
| Response to HBV vaccine | 1.63 (0.99–2.67) | 0.06 | ||
| ARB | 1.75 (0.84–3.65) | 0.13 | 1.48 (0.59–3.71) | 0.40 |
| VKA | 0.48 (0.29–0.79) | 0.005 | ||
| Kt/V | 0.84 (0.43–1.65) | 0.61 | ||
| Creatinine (µmol/L) | 1.00 (1.00–1.00) | 0.76 | ||
| Calcium (mmol/L) | 0.86 (0.15–5.06) | 0.87 | ||
| Phosphate (mmol/L) | 0.61 (0.34–1.12) | 0.11 | 0.84 (0.46–1.55) | 0.58 |
| Total protein (g/L) | 1.05 (1.004–1.094) | 0.03 | ||
| Serum albumin (g/L) | 1.02 (0.96–1.09) | 0.46 | 1.10 (1.05–1.16) | <0.001 |
| CRP (mg/L) | 1.00 (0.98–1.01) | 0.67 | ||
| Haemoglobin (g%) | 1.13 (0.91–1.40) | 0.28 | 1.05 (0.83–1.33) | 0.66 |
| BNT162b2 vaccine (%) | 0.40 (0.24–0.64) | <0.001 | ||
OR, odds ratio; CI, confidence interval.
Safety and adverse effects
Injection site pain was the most common adverse event (22.1%), followed by fatigue (13.2%), fever (12.1%), myalgia (7.7%), arthralgia and headache (both 3.3%). Apart from pain at the injection site, 23.8% of patients experienced one adverse event, but none were severe.
Of note, patients who exhibited general symptoms (fever, myalgia) following the first or the second dose of the vaccine tended to mount a higher antibody response, with 74.5% reaching a protective level versus 58.1% in patients without side effects. However, the difference was not statistically significant (P = 0.08) because of missing data (n = 176).
DISCUSSION
In a large cohort of 851 patients on maintenance HD, we found that most patients produced detectable (95.2%) or protective levels of antibodies (86%) 1 month after the second vaccine dose. These figures are lower than the general population but much higher than kidney transplant recipients [25]. The most critical determinant to hinder a humoral response was an immunosuppressive treatment in the multivariate analysis.
To the best of our knowledge, this is the first study to report protective antibody levels in dialysis patients (i.e. >264 BAU/mL) according to data from the Oxford Vaccine Group [23]. This threshold is much higher than those given by the manufacturers and may be more realistic, especially with the widespread diffusion of the delta variant. This threshold corresponds to an 80% probability of being protected against a symptomatic form of COVID-19. However, as the study was conducted in healthy subjects, with a viral vector vaccine and before the onset of the delta variant, any extrapolation to present-day viral circulation and dialysis patients should be done with caution.
The French health authorities have made a third dose of the anti-COVID vaccine mandatory for all patients undergoing renal replacement therapy, following the abysmal results of the vaccination in kidney transplant patients. However, at odds with transplant recipients, our results showed that a substantial proportion of patients on HD developed a protective humoral response after two doses of vaccine, which suggests that a booster dose should be offered even more rapidly in those patients with insufficient antibody response after the two first doses.
Half of the patients received the mRNA-1273 vaccine in our cohort and the other half the BNT162b2 vaccine, although the repartition was not randomized. The response rate with the mRNA-1273 vaccine was not different after the first dose but was significantly higher 1 month after the second dose, with a nearly 20% higher response rate. This statistically significant difference may have clinical consequences in a population at increased mortality risk due to SARS-CoV-2 infection. Our results are consistent with those published in the literature. In the general population, the mRNA-1273 vaccine has been shown to achieve more than twice the level of COVID antibodies as the BNT162b2 vaccine [25]. Studies comparing the two vaccines in dialysis patients showed a 10% difference in the response rate favouring the mRNA-1273 vaccine [17]. A proposed explanation relates to the amount of mRNA per vaccine dose, 100 µg for the mRNA-1273 vaccine and 30 µg for the BNT162b2 vaccine.
The main reason for blunted serological response in our cohort was current immunosuppressive treatment. However, even in these groups of patients, the efficacy was acceptable, with a seroconversion rate of 94.8% in immunocompromised patients and 80% in those receiving immunosuppressive drugs. These results were comparable to those of patients treated with immunosuppressive drugs for inflammatory rheumatic diseases, with a response rate of 86% [25]. Unfortunately, the rate of patients with a protective level of antibodies was not evaluated in this study. In contrast, the response rate was significantly higher than the 48% reported in kidney transplant patients [26]. The intensity, length and type of immunosuppression are likely to explain this difference.
Decreased serum total protein and albumin levels were essential factors associated with the lack of antibody production. This profile probably identified malnourished patients who were less likely to respond to vaccination. We cannot exclude that low protein/albumin levels were related to an inflammatory state via the interleukin-6 pathway, but high CRP was not significant in the multivariate analysis.
Unexpectedly, treatment with VKAs was associated with an inadequate response to the vaccine. We initially included this variable to investigate whether it was associated with more frequent adverse events such as haematoma or pain at the injection site. We submit that in patients treated with VKAs, nurses injected the vaccine less deeply for fear of bleeding and that the injection was partly done subcutaneously. Another hypothesis is a subclinical haematoma at the puncture site, which would reduce the diffusion of the vaccine and blunt the production of antibodies. Our reassuring safety data, however, do not support this last hypothesis.
Age had a significant effect on the outcome of vaccination in the logistic regression analysis, as in some previous studies [9–11, 13, 15, 16, 19]. In the general population, age has been previously associated with an impaired ability to mount a robust immune humoral response [23].
Dialysis characteristics or performance indicators (vintage, HD versus haemodiafiltration, Kt/V, dialysis membrane) did not influence the response to vaccination. PMMA dialysis membranes, which have been suggested to improve the vaccine response against hepatitis B through better clearance of soluble CD40s [27], did not positively affect the anti-COVID vaccine response in the multivariate analysis.
The vaccination results against SARS-CoV-2 were significantly better than those against hepatitis B. However, there was no correlation between the response to hepatitis B vaccination and the protective response to the anti-COVID vaccine. The type of antigens and the mRNA vaccine technology probably explains these differences and it would be interesting to evaluate the efficacy of this type of vaccine against hepatitis B.
There are several limitations to our study. First, our study focussed on the humoral response to vaccines, representing a part of the global immune response to vaccination. A study in a small cohort of dialysis patients found a dissociation between humoral and cellular responses [22]. Second, we used anti-NP tests to detect asymptomatic SARS-CoV-2 exposure before vaccination. As these tests may cross-react with previous common cold human coronaviruses, false-positive tests could be expected. Third, the ‘protective’ thresholds we used in this study were based on the Oxford Vaccine Group specifications and may not indicate protection in real-life conditions, especially with the delta and omicron variants. Fourth, the repartition between the mRNA-1273 vaccine and the BNT162b2 vaccine was not randomized at the patient level. Fifth, we used two different assays according to their on-site availability to assess the humoral response. However, the standardization of antibody levels as BAU according to WHO specifications secured our study's comparability of protective levels. Finally, we cannot extrapolate our response rates to other non-mRNA vaccines such as the ChAdOx1-SARS-COV-2 or Ad26.COV2.S.
CONCLUSION
In a large cohort of patients on maintenance HD, we found that most patients produced detectable (95.2%) or protective levels of antibodies (69.6%) 1 month after the second vaccine dose. The most critical determinant to hinder a humoral response was immunosuppressive treatment in the multivariate analysis. We also found that the response rate with the mRNA-1273 vaccine was significantly higher compared with the BNT162b2 vaccine.
A growing number of national guidelines now recommend a third vaccine ‘booster’ dose for all individuals, including at-risk persons, especially in the context of the delta or omicron variants. With the limitation that the humoral response is only a proxy for the global immune response to a vaccine, our results suggest that a third dose may be helpful in the dialysis population but should be offered even more rapidly in those patients with inadequate antibody response after the two first doses.
ACKNOWLEDGEMENTS
We are indebted to all the health professionals involved in this study: Project manager: S. Le Calvez (AURAL); Nephrologists: Dr T. Nussbaumer, Dr V. Betz, Dr H. Sissoko, Dr M. Ismer (Colmar Hospital), D. May, K. Kunz (AURAL); Biologists: Dr V. Camberlein, Dr C. Hess (Saverne Hospital), Dr J. Exinger (Haguenau Hospital), Dr O. Augerau (Colmar Hospital), Prof S. Fafi-Kremer (CHU Strasbourg); Clinical research associate: AC Bertaux; Study nurses: A. Roesslinger, I. Friedmann, N. Richard, H. Mountassir, JM. Daessle, N. Borzer, M. Geisen.
Contributor Information
Yves Dimitrov, Service de Néphrologie, Centre Hospitalier de Haguenau, Haguenau, France; AURAL Alsace, Strasbourg, France.
Thierry Krummel, Service de Néphrologie-Dialyse, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg, France.
François Chantrel, AURAL Alsace, Strasbourg, France; Service de Néphrologie-Dialyse, Groupe Hospitalier de Mulhouse-Sud Alsace, Mulhouse, France.
Anne-Laure Faller, AURAL Alsace, Strasbourg, France; Service de Néphrologie-Dialyse, Groupe Hospitalier Saint-Vincent, Strasbourg, France.
Julien Ott, Service de Néphrologie, Centre Hospitalier de Haguenau, Haguenau, France; AURAL Alsace, Strasbourg, France.
Daniela David, AURAL Alsace, Strasbourg, France.
Dorothée Bazin-Kara, Service de Néphrologie-Dialyse, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg, France.
Thierry Hannedouche, Service de Néphrologie-Dialyse, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg, France; School of Medicine, University of Strasbourg, Strasbourg, France.
Claire Borni, AURAL Alsace, Strasbourg, France.
AUTHORS’ CONTRIBUTIONS
Y.D., F.C., A.L.F., J.O., D.M., D.B.K. and C.B. acquired the data. Y.D. conceived the study and wrote the draft article. T.K. performed the statistical analyses and revised the article. T.H. planned the analyses and finalized the article. C.B. and T.H. acquired the funding. All authors approved the final article.
FUNDING
AURAL Alsace supported part of the cost of serological tests.
DATA AVAILABILITY STATEMENT
No additional data is available.
CONFLICT OF INTEREST STATEMENT
None of the authors declare any conflict of interest.
REFERENCES
- 1. COVID-19 situation update for the EU/EEA, as of 24 June 2021. [Internet]. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea (24 June 2021, date last accessed) [Google Scholar]
- 2. Scarpioni R, Manini A, Valsania Tet al. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol 2020; 37: 2020-vol2 [PubMed] [Google Scholar]
- 3. Alberici F, Delbarba E, Manenti Cet al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 2020; 98: 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goicoechea M, Sánchez Cámara LA, Macías Net al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int 2020; 98: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valeri AM, Robbins-Juarez SY, Stevens JSet al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 2020; 31: 1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimitrov Y, Ducher M, Kribs Met al. Variables linked to hepatitis B vaccination success in non-dialyzed chronic kidney disease patients: use of a bayesian model. Nephrol Ther 2019; 15: 215–219 [DOI] [PubMed] [Google Scholar]
- 7. Soni R, Horowitz B, Unruh M. Immunization in end-stage renal disease: opportunity to improve outcomes. Semin Dial 2013; 26: 416–426 [DOI] [PubMed] [Google Scholar]
- 8. Raskova J, Ghobrial I, Czerwinski DKet al. B-cell activation and immunoregulation in end-stage renal disease patients receiving hemodialysis. Arch Intern Med 1987; 147: 89–93 [PubMed] [Google Scholar]
- 9. Simon B, Rubey H, Treipl Aet al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls. Nephrol Dial Transplant 2021; 36: 1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grupper A, Sharon N, Finn Tet al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 2021; 16: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Attias P, Sakhi H, Rieu Pet al. Antibody response to BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int 2021; 99: 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yanay NB, Freiman S, Shapira Met al. Experience with SARS-COV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int 2021; 99: 1496–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agur T, Ben-Dor N, Goldman Set al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients – a prospective cohort study. Nephrol Dial Transplant 2021; 36: 1347–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lacson E, Argyropoulos CP, Manley HJet al. Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol 2021; 32: 2735–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frantzen L, Cavaille G, Thibeaut Set al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in a hemodialysis cohort. Nephrol Dial Transplant 2021; 36: 1756–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jahn M, Korth J, Dorsch Oet al. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines (Basel) 2021; 9: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anand S, Montez-Rath ME, Han Jet al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol 2021; 32: 2435–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan L, Fuca N, Zeldis Eet al. Antibody response to mRNA-1273 SARS-CoV-2 vaccine in hemodialysis patients with and without prior COVID-19. Clin J Am Soc Nephrol 2021; 16: 1258–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Speer C, Göth D, Benning Let al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol 2021; 16: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longlune N, Nogier MB, Miedougé Met al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant 2021; 36: 1704–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertrand D, Hamzaoui M, Lemée Vet al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol 2021; 32: 2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monzó JJB, Rodríguez-Espinosa D, Soruco Eet al. Weekly seroconversion rate of the mRNA-1273 SARS-CoV-2 vaccine in hemodialysis patients. Nephrol Dial Transplant 2021; 36: 1754–1755 [DOI] [PubMed] [Google Scholar]
- 23. Feng S, Phillips DJ, White Tet al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkmann T, Perkmann-Nagele N, Koller Tet al. Anti-Spike protein assays to determine post-vaccination antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr 2021; 9: e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braun-Moscovici Y, Kaplan M, Braun Met al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021; 80: 1317–1321 [DOI] [PubMed] [Google Scholar]
- 26. Benotmane I, Gautier-Vargas G, Cognard Net al. Low immunization rates among kidney transplant recipients who received two doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 2021; 99: 1498–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Contin-Bordes C, Lacraz A, de Précigout V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: new findings of its amelioration using polymethylmethacrylate (PMMA) membrane. NDT Plus 2010; 3: i20–i27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data is available.