Learning Point for Clinicians
mRNA-based coronavirus disease 2019 vaccines may cause de novo or relapsing glomerulonephritis, including minimal change disease. Clinicians should be aware of the various potential renal complications after vaccination.
Introduction
In the global fight against the coronavirus disease 2019 (COVID-19) pandemic, large-scale vaccinations have been carried out worldwide to prevent infection and progression to severe disease. It has been confirmed that 44 458 206 people have completed the second vaccination dose in the Republic of Korea till March 2022. According to a report by the Korea Disease Control and Prevention Agency on 18 March 2022, 221 cases of acute kidney injury were reported. Recent studies have reported that SARS-CoV-2 vaccination is associated with de novo or relapsing glomerular disease.1–3
Here, we report two clinical cases of minimal change disease (MCD) with nephrotic syndrome after the second dose of Moderna COVID-19 vaccine (mRNA-1273).
Patient 1
A 34-year-old man with dyspnea was admitted to the hospital 3 days after administration of the second dose of the Moderna vaccine. He had periocular edema from the day after vaccination and 2 kg weight gain during the 3 days. Laboratory tests showed serum creatinine, 0.86 mg/dl; albumin, 1.4 g/dl; total cholesterol, 473 mg/dl; and 24-h urine protein, 7.7 g/day. From the fourth day of hospitalization, renal function began to aggravate, and the creatinine level rose to 5.38 mg/dl. Renal biopsy findings were consistent with MCD (Figure 1A and C). Prednisolone 60 mg/day (1 mg/kg) was started. After 2 weeks, the proteinuria and serum creatinine began to decrease. After 4 weeks, spot urine protein-to-creatinine ratio (UPCR) was 1.91 g/g and serum creatinine became normal, implying partial remission. After 11 weeks, creatinine level was 0.89 mg/dl, albumin level was 4.1 g/dl and UPCR was 0.28 g/g.
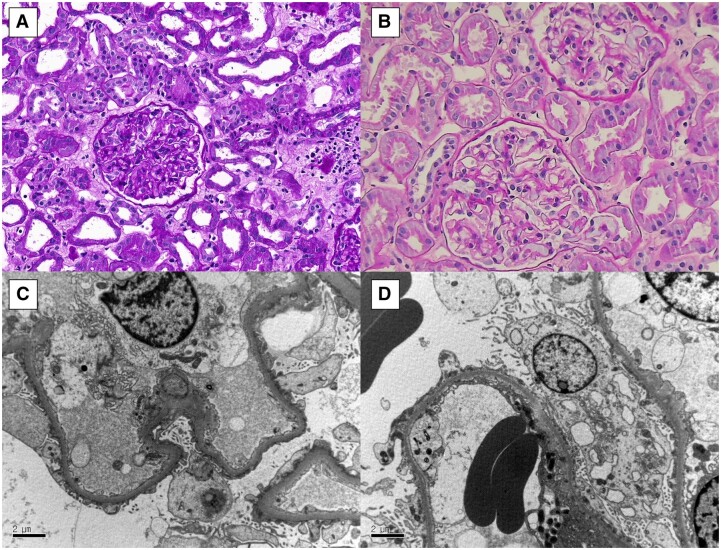
Figure 1.
Renal biopsy findings: (A, B) Light microscopy of sections from Patients 1 and 2 shows relatively normal glomeruli in both (Periodic acid-Schiff stain, 200×). (C, D) Electron microscopy of sections from Patients 1 and 2 shows diffuse effacement of podocyte foot processes without electron-dense deposits (transmission electron microscopy, 7000×).
Patient 2
A 60-year-old Korean man with edema was admitted to the hospital 9 days after administration of the second dose of Moderna vaccine. The patient began to show swelling in both lower extremities on the fifth day after vaccination and 7 kg weight gain during the 9 days. Laboratory tests showed serum creatinine, 0.72 mg/dl; albumin, 2.1 g/dl; total cholesterol, 381 mg/dl; and 24-h urine protein, 16.1 g/day. Renal biopsy showed features consistent with MCD (Figure 1B and D). High-dose oral steroid therapy was initiated. After 1 week, proteinuria began to decrease. After 2 weeks, UPCR was 0.10 g/g, showing complete remission. After 4 weeks, creatinine level was 0.70 mg/dl, albumin level was 3.8 g/dl and UPCR was 0.05 g/g. High-dose oral steroids were maintained for 6 weeks and then tapered off.
Discussion
Here, we report two male cases of de novo MCD following the second dose of Moderna vaccine, presenting with nephrotic syndrome.
After vaccination, vaccine antigens are taken up by dendritic cells and presented to T-cell receptors. This results in the activation of antigen-specific effector T cells, which peak between 7 and 14 days post-vaccination. Thereafter, virus-specific memory B cells, antibodies and memory T cells are produced to create immune memory against the infection.4 In our patients, there were no symptoms after the first vaccine dose, but they occurred after the second dose. It can be inferred that the immune memory created after the first inoculation induced a rapid immune response after the second injection. Whether vaccination alone caused the de novo MCD or the timing of vaccination and MCD onset coincidentally overlapped is unclear. Although there are no specific treatment guidelines for post-vaccination MCD, our cases achieved complete remission with high-dose oral glucocorticoids. This can be adopted as a standard protocol in similar cases. Clinicians should be aware of the various potential renal complications after vaccination. Continued research is required to better understand the pathogenesis of post-vaccination glomerulonephritis and explore better treatment options.
Consent
Consent was obtained from both the patients to publish this report without disclosure of their identities.
Funding
This work was supported by Dong-A University Research Fund.
Conflict of interest: None declared.
References
- 1. Shetty AA, Tawhari I, Safar-Boueri L, Seif N, Alahmadi A, Gargiulo R, et al. COVID-19-associated glomerular disease. J Am Soc Nephrol 2021; 32:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim JH, Han MH, Kim YJ, Kim MS, Jung HY, Choi JY, et al. New-onset nephrotic syndrome after Janssen COVID-19 vaccination: a case report and literature review. J Korean Med Sci 2021; 36:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep 2021; 6:2969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184:861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]