Abstract
Objectives
The coronavirus disease 2019 (COVID-19) vaccine represents a cornerstone in tackling the pandemic and with the approval of the BNT162b2 mRNA vaccine in December 2020, it has become a beacon of hope for people around the world, including children. This study aimed to present the data on the humoral response and safety of vaccine in a cohort of patients with paediatric rheumatic diseases receiving immunomodulatory treatments.
Methods
Forty-one children with paediatric rheumatic diseases were included and were vaccinated with the BNT162b2 mRNA vaccine (two doses of 30 µg administered 3–4 weeks apart). To assess the humoral response, IgG antibodies developed against the S1/Receptor-binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein at baseline and 3–4 weeks after the second dose were measured. The possible local and systemic side effects and disease activity scores were evaluated during the study period.
Results
After the second dose of vaccine, markedly elevated anti-RBD IgG titres were observed in all patients with a median titre of 20 474 AU/ml [interquartile range (IQR) 6534–36 151] with a good safety profile. The median disease duration was 4.3 (IQR 3.5–5.6) years. In the cohort, 14 (34.1%) received conventional DMARDs (cDMARDs), 16 (39%) received biologic DMARDs (bDMARDs) and 11 (26.8%) received a combined therapy (cDMARDs and bDMARDs). Patients treated with combined therapy [median 4695 (IQR 2764–26 491)] had significantly lower median titres of anti-RBD IgG than those receiving only cDMARDs.
Conclusion
Paediatric rheumatic diseases patients receiving immunomodulatory treatments were able to mount an effective humoral response after two dose regimens of BNT162b2 mRNA vaccine safely without interrupting their current treatments.
Keywords: paediatric rheumatic disease, coronavirus, BNT162b2 mRNA vaccine, immunogenicity, antibody response
Rheumatology key messages.
Humoral response and safety data of BNT162b2 mRNA vaccine in children with rheumatic diseases is unknown.
Paediatric rheumatic disease patients receiving immunomodulatory treatments were able to mount an effective humoral response.
Postvaccination disease activity remained stable and the vaccine had a good safety profile.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with significant implications for healthcare, social services and economy, is the most crucial public health problem in the world at this time [1, 2]. It is responsible for ∼250 million infections, 5 million of which have been fatal (2.1%), and therefore remains a worldwide hazard [1]. Although the disease course in children is milder than in adults, 0.1–2.0% of all paediatric COVID-19 cases require hospitalization, and approximately one in four of these hospitalized patients require paediatric intensive care [1, 3, 4]. With the declaration of the COVID-19 pandemic on 11 March 2020, scientists started investigations into vaccines and drugs against SARS-CoV-2 [5]. With extraordinary efforts in this area, favourable outcomes in vaccination trials have arisen in far less than a year.
Vaccines have saved humanity from many pandemics with great success by controlling disease transmission and accordingly reduce morbidity and mortality. Likewise, with the emergent approval of the BNT162b2 mRNA vaccine in December 2020, vaccination has again become a beacon of hope for children and adults. Vaccination of children is of great importance for slowing down the pandemic and subsequently restraining multisystem inflammatory syndrome in children (MIS-C) [6]. After the vaccine was determined to be safe for adults, first it was approved for use in children aged ≥15 years and afterwards in June 2021, for children aged ≥12 years, by the World Health Organization.
Children with rheumatic diseases may be at risk of serious infections because of the immunological dysregulation and immunomodulatory drugs they receive. The humoral response to many vaccines may be inadequate and/or their antibody titres may decrease rapidly after vaccination as well [7, 8]. In the literature, insufficient serological response to regular vaccines was reported in children with inflammatory diseases [9–12]. When tested in healthy individuals, the SARS-CoV-2 mRNA vaccine revealed high immunogenicity; however, this study did not involve children with rheumatic diseases [13].
The Centers for Disease Control and Prevention state that ‘Patients with underlying autoimmune/autoinflammatory diseases can be vaccinated with mRNA vaccines, but careful follow-up after vaccination is also recommended for these patient categories’. After this disclosure, the humoral and T cell specific responses to SARS-CoV-2 vaccines were examined in adults with immune dysfunction and the vast majority of patients developed a significant humoral response following administration of the second dose of the BNT162b2 mRNA vaccine [14–16].
In September 2021, children aged 12–18 years with the diagnosis of paediatric rheumatic diseases had the opportunity to voluntarily participate in vaccination campaigns organized by the government. The primary aim of this study was to examine the vaccine antibody response of children and adolescents with rheumatic diseases in relation to confounding factors. The secondary objective was to evaluate the systemic and local side effects of the BNT162b2 mRNA vaccine in children with paediatric rheumatic diseases.
Methods
Study design and participants
This was a single-centre, prospective, observational study conducted in children with paediatric rheumatic diseases receiving conventional DMARDs (cDMARDs) and/or biologic DMARDs (bDMARDs). Patients were invited to the research by phone call, and they were informed about the study. Parents who agreed to give their children the BNT162b2 mRNA vaccine and who were hesitant about the vaccination were welcomed at the hospital and given detailed information about the vaccine and the study protocol by the paediatric rheumatologist. These patients were included in the study provided that their parents gave a written informed consent. Participants were vaccinated with the BNT162b2 mRNA vaccine according to the guidelines provided by the Turkish Ministry of Health between September 2021 and December 2021 (two doses of 30 µg administered 3–4 weeks apart). Patients with a history of COVID-19 and/or known allergic reactions to the components of the BNT162b2 mRNA vaccine were excluded. Participants diagnosed with COVID-19 in the interval between the baseline antibody evaluation and 7 days after the second dose of vaccination were also excluded from the study. SARS-CoV-2 RT-PCR was not tested prior to vaccination.
The demographic and clinical data were abstracted from the patients’ files. Age, gender, current diagnosis, treatment regimens of the last 6 months and the disease activity scores (DAS) were recorded. Approval for the study was obtained from the Ethics Committee of Istanbul University, Istanbul Faculty of Medicine (approved: September 2021–1710).
Study procedures
Patients involved in the study had a routine rheumatologic visit and their disease activities were recorded. The Autoinflammatory Disease Activity Index (AIDAI) was used to determine the disease activity in children with periodic fever syndrome (PFS), SLEDAI in patients with JSLE, the Iranian Behçet’s disease dynamic activity measure (IBDDAM) in patients with Behçet’s disease, juvenile arthritis DAS 27 (JADAS-27) in patients with JIA, childhood myositis assessment scale (CMAS) in patients with JDM and Physician Global Assessment (PGA) in patients with chronic recurrent multifocal osteomyelitis (CRMO) and localized scleroderma.
Peripheral blood was collected before vaccination at baseline (up to 10 days) and, then after the second dose (after 3–4 weeks) from the patients who had no known history of infection or previous laboratory-confirmed SARS-CoV-2 infection. After the first blood sample was taken, the families were told to schedule a vaccination appointment within 10 days via the central appointment system.
To measure antibody levels to SARS-CoV-2 spike subunit 1, the SARS-CoV-2 IgG II Quant kit (Abbott, Sligo, Ireland) based on the chemiluminescent microparticle immune assay method that detects IgG antibodies developed against the S1/Receptor-binding domain (RBD) of the SARS-CoV-2 spike protein were used. Serum samples taken from patients diagnosed with paediatric rheumatic diseases were stored at –20°C until analyses. They were tested in the Architect i2000SR (Abbott, Ireland) device in accordance with the manufacturer’s recommendations. The results of the SARS-CoV-2 IgG quantitative test, the analytical measurement range of which is determined between 21–40 000 AU/ml by the manufacturer, was interpreted according to the cut-off value of 50.0 AU/ml. Patients who had the SARS-CoV-2 IgG antibody ≥50 IU were considered seropositive.
The safety of the vaccine was assessed by using a structured questionnaire addressing local or systemic side effects at the first clinical visit after completion of vaccine regimen. In order to follow-up the side effects accurately, patients were given detailed information about the expected side effects at the pre-vaccine visit. A telephone call was made in the interval between administration of the two vaccine doses to monitor whether relevant safety issues ensued. Parents were advised to keep a diary to record their children’s complaints and to bring their children in for control measures if necessary.
Statistical analysis
Data were analysed using SPSS software (IBM SPSS Statistics) for Windows, V.26, (IBM, Armonk, NY, USA, 2020). All statistical tests were bilateral and statistical significance was described as a P-value <0.05. Continuous variables were appraised for normal distribution using histogram, Kolmogorov-Smirnov or Shapiro–Wilk, Q-Q plots and reported as median and interquartile range (IQR). Categorical variables were summarized as frequency and percentage. Categorical variables were reported as numbers and ratios, while continuous variables including antibody titres, except age, were reported as the median and IQR. The results were evaluated with non-parametric statistical inference tests. Comparisons between groups (>2) were evaluated with the Kruskal–Wallis test, while the Mann–Whitney U test with Bonferroni correction was used for pairwise comparisons. The χ2 test was used for categorical variables.
Results
Demographic and clinical characteristics of the participants
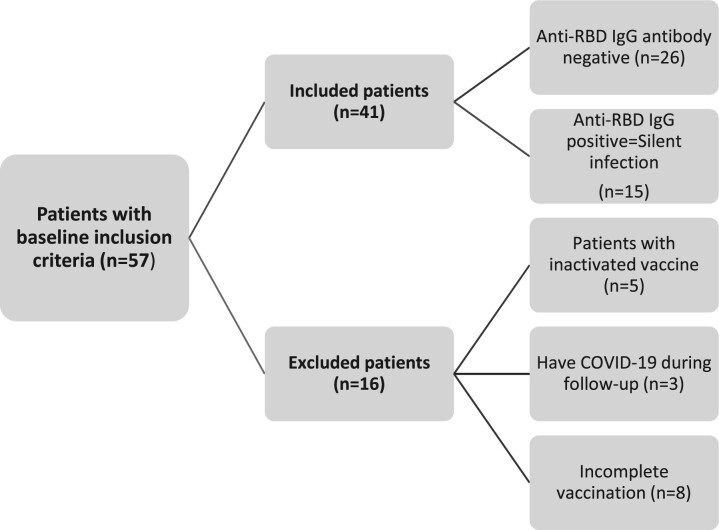
Of 231 patients invited, 57 patients met the baseline inclusion criteria to participate in the study. Eight patients were excluded as they had an incomplete vaccination schedule. Five patients preferring inactive-based vaccines and three patients who had SARS-CoV-2 infection during the study period prior to completion of the vaccine regimen were also excluded. Finally, the study was conducted with 41 patients, as shown in Fig. 1.
Fig. 1.
Patient flowchart
Flowchart indicates patients who met the basic inclusion criteria in our study, the reasons for exclusion, and the pre-vaccine antibody responses of the included patients.
The mean age of the cohort was 15.4 ± 1.5, and 15 (36.6%) were girls. The median disease duration was 4.3 (IQR 3.5–5.6) years.
The diagnoses of the patients included: 25 (60.9%) patients with JIA; 10 (24.4%) patients with PFS, of whom 1 was diagnosed with CRMO, 1 with hyperimmunoglobulinemia D syndrome, while the other 8 were diagnosed with colchicine resistant FMF; and 4 (9.8%) patients with CTD, of whom 2 were diagnosed with JSLE, 1 with JDM, and 1 with localized scleroderma. Two (4.8%) patients diagnosed with vasculitis had Behçet’s disease. The baseline demographic and clinical characteristics of the patients were shown in Table 1.
Table 1.
Assessment of patients according to age, gender, diagnosis, treatment and antibody titres
| Pre-vaccine antibody-positive group (silent infection) (n = 15, 36.6%) | Pre-vaccine antibody-negative group (n = 26, 63.4%) | P-valuea | Data of all patients (n = 41) | P-valueb | |
|---|---|---|---|---|---|
| Age (years), mean ± s.d. | 15.3 ± 1.7 | 15.4 ± 1.5 | P = 0.84c | 15.4 ± 1.5 | P = 0.97d |
| Gender female, n (%) | 1 (6.7%) | 14 (53.8%) | P = 0.003e | 15 (36.6) | P = 0.41d |
| Age at diagnosis (years), median (IQR) | 13.9 (9.4–15.1) | 9.6 (6.8–13.1) | 10.8 (7.4–14.5) | ||
| Diagnosis, n (%) | P = 0.60f | ||||
| Periodic fever syndrome | 5 (33.3) | 5(19.2) | 10 (24.4) | ||
| HIDS | – | 1 (3.8) | 1 (2.4) | ||
| CRMO | 1 (6.7) | – | 1 (2.4) | ||
| Colchicine-resistant FMF | 4 (26.7) | 4 (15.4) | 8 (19.5) | ||
| CTD | – | 4(15.4) | 4 (9.8) | ||
| JSLE | – | 2 (7.7) | 2 (4.8) | ||
| JDM | – | 1 (3.8) | 1 (2.4) | ||
| Localized scleroderma | – | 1 (3.8) | 1 (2.4) | ||
| JIA | 10 (66.7) | 15(57.7) | 25 (61) | ||
| Behçet’s disease | – | 2 (7.7) | 2 (4.9) | ||
| Treatment, n (%) | P = 0.36e | P = 0.04g | |||
| cDMARDs (n = 14) | 4 (26.7) | 10 (38.5) | 14 (34.1) | ||
| MTX | 4 (26.7) | 4 (15.4) | 8 (19.5) | ||
| Salazopyrin | – | 1 (3.8) | 1 (2.4) | ||
| MMF | – | 3 (11.5) | 3 (7.3) | ||
| AZA | – | 2 (7.7) | 2 (4.9) | ||
| bDMARDs (n = 16) | 8 (53.2) | 8 (30.8) | 16 (39.0) | ||
| Etanercept | 4 (26.7) | 1 (3.8) | 5 (12.2) | ||
| Adalimumab | – | 2 (7.7) | 2 (4.9) | ||
| Anakinra | 1 (6.7) | 2 (7.7) | 3 (7.3) | ||
| Canakinumab | 3 (20.0) | 3 (11.5) | 5 (12.2) | ||
| Abatacept | – | 1 (3.8) | 1 (2.4) | ||
| Combined treatment (n = 11) (cDMARDs vs bDMARDs) | 3 (20.0) | 8 (30.8) | 11 (26.8) | ||
| Etanercept + MTX | – | 2 (7.7) | 2 (4.9) | ||
| Etanercept + salazopyrin | 1 (6.7) | 2 (7.7) | 3 (7.3) | ||
| Etanercept + LEF | 1 (6.7) | – | 1 (2.4) | ||
| Adalimumab + MTX | 1 (6.7) | 3 (11.5) | 4 (9.8) | ||
| Canakinumab + MTX | – | 1 (3.8) | 1 (2.4) | ||
| Antibody titres after completing the vaccination schedule, median (IQR) | 25 422h (11 440–36 151) | 15 327.5 (4372.2–33 991.5) | P = 0.2b | 20 474 (6358–34 578) | |
| Patients with a diagnosis period over 48 months, n (%) | 10 (66.7) | 11 (42.3) | P = 0.13e | 21 (51.2) | P = 0.79d |
Comparison between prevaccine anti-S/RBD antibody-negative and -positive groups according to age, gender, antibody titres and diagnosis time.
Comparison of the effect of age, gender, diagnosis, duration of diagnosis and treatment on antibody titres of all patients.
Student’s t-test;
Mann–Whitney U test;
χ2 test;
Kruskal–Wallis test;
Kruskal–Wallis test with Bonferroni adjusted;
AU/ml (unit). cDMARDs: conventional DMARDs; bDMARDs: biologic DMARDs; CRMO: chronic recurrent multifocal osteomyelitis; HIDS: hyperimmunoglobulin D syndrome; IQR: interquartile range; RBD: Receptor-binding domain.
In the cohort, 14 (34.1%) patients were receiving cDMARDs, 16 (39%) were receiving bDMARDs and 11 (26.8%) were receiving combined therapy (cDMARDs and bDMARDs). One patient with JSLE under MMF and HCQ treatment had prednisolone 10 mg/day concomitantly.
Of the 41 patients, 15 (36.5%) had baseline SARS CoV-2 spike protein antibody seropositivity and were considered as having silent infection, and their further analyses were carried out separately.
Antibody response against SARS-CoV-2 vaccine
Markedly elevated anti-RBD IgG titres (median: 20 474 AU/ml, IQR: 6358–34 578 AU/ml) were observed in all patients after the second dose of the vaccine with a median of 24 days. There was no correlation between anti-RBD IgG titres and gender or duration of follow-up (P = 0.4, P = 0.9).
Although not reaching statistical significance, the median anti-RBD IgG titres measured after the completion of the vaccine regimen was higher in patients seropositive before the first vaccine than in the seronegative patients (P = 0.20). The baseline seropositivity was more common in boys (P = 0.003), and there was no difference according to age in terms of baseline seropositivity (P = 0.84) (Table 1).
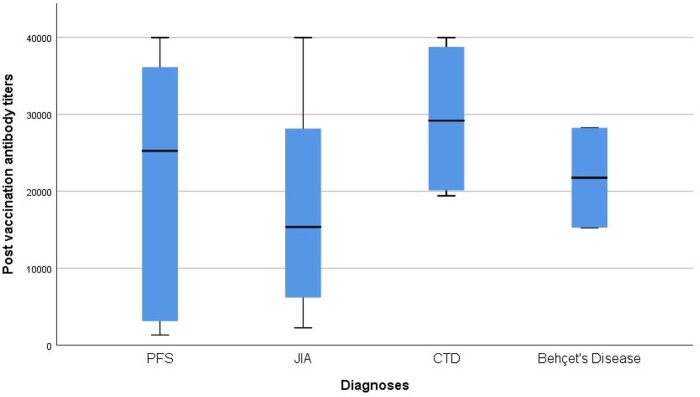
In the cohort, the median anti-RBD IgG titres were measured as 25 272 AU/ml in PFS (IQR 2764–25 272), 15 368 AU/ml in JIA (IQR 5439–30 482), 29 202 AU/ml in CTD (IQR 19 774–39 390) and 21 770 AU/ml in Behçet’s disease (IQR 15 287–28 254) (Fig. 2). The median anti-RBD IgG titres were similar across diagnoses (P = 0.60).
Fig. 2.
Profile of anti-RBD IgG titres after the second dose according to diagnosis
The upper end line of the box is IQR 75, the lower end line of the box is IQR 25, the thick line in the middle of the box indicates the median value, the upper line of the thin horizontal lines indicates the maximum value, and the lower line indicates the minimum value. PFS: periodic fever syndrome; RBD: Receptor-binding domain; IQR: interquartile range.
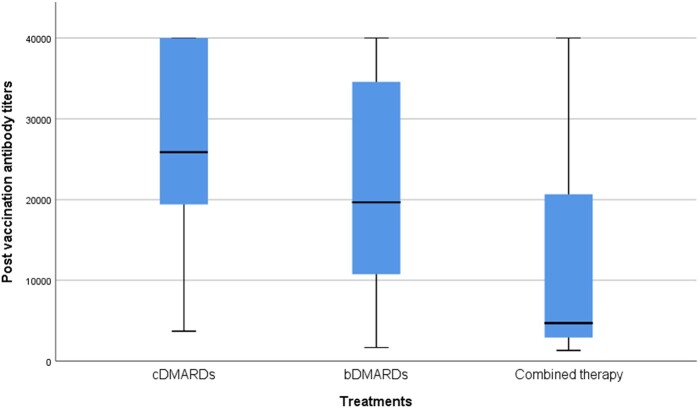
The second dose successfully boosted anti-RBD IgG titres in patients under three specific treatment modalities (cDMARDs, bDMARDs and combined therapy). While the median anti-RBD IgG titres following the second dose of BNT162b2 mRNA vaccine were similar between patients receiving cDMARDs [median 25 876 (IQR 18 385–40 000)] and bDMARDs [median 19 674 (IQR 10 438–35 364), P = 0.35], patients treated with combined therapy [median 4695 (IQR 2764–26 491)] had significantly lower median titres of anti-RBD IgG than those receiving only cDMARDs (P = 0.04) (Fig. 3). While the most commonly received biological therapies were anti-IL-1 [median 17 672 (IQR 2400–38 075)] and anti-TNF agents [median 11 440 (IQR 4647–27 327)] in this cohort, the median antibody titres of patients receiving these therapies were similar (P = 0.38).
Fig. 3.
Profile of anti-RBD IgG titres according to treatments
The upper end line of the box is IQR 75, the lower end line of the box is IQR 25, the thick line in the middle of the box indicates the median value, the upper line of the thin horizontal lines indicates the maximum value, and the lower line indicates the minimum value. cDMARDs: conventional DMARDs; bDMARDs: biologic DMARDs; Combined therapy: both cDMARDs and bDMARDs therapy; RBD: Receptor-binding domain; IQR: interquartile range.
The DAS and side effects
The median DAS of the patients in the initial visit were as follows: SLEDAI of patients with JSLE was 1, AIDAI of PFS patients was 2, JADAS-27 of JIA patients was 2.4 and CMAS of the JDM patient was 46. Median PGA of patients diagnosed with CRMO and localized scleroderma was 2. The follow-up time after completion of the COVID-19 immunization schedule was 62 days (±19.9) and none of the patients had significant increase in the DAS during study or follow-up duration. Disease activity scores at follow-up after two doses of vaccine regimen are given below in order: SLEDAI of patients with JSLE was 2, AIDAI of PFS patients was 2, JADAS-27 of JIA patients was 2.3, CMAS of the JDM patient was 45, and PGA of patients with CRMO and localized scleroderma was 3.
The major local side effect after the first and the second dose of vaccine was pain at the injection site (58.5% vs 48.8%), and the most common systemic side effect was fatigue (31.7% vs 29.3%). Only one patient after the first dose of vaccine and two patients after the second dose received NSAIDs due to headache and myalgia. None of the patients had anaphylaxis or serious side effects requiring hospitalization. Systemic side effects after the first dose of vaccine and both local (Fig. 4) and systemic side effects (Fig. 5) after the second dose were significantly higher in girls (P = 0.013, P = 0.005, P = 0.002, respectively).
Fig. 4.
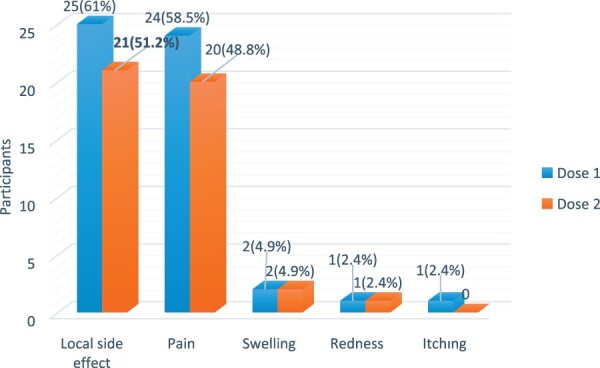
Local side effects after the first and second vaccine doses
Fig. 5.
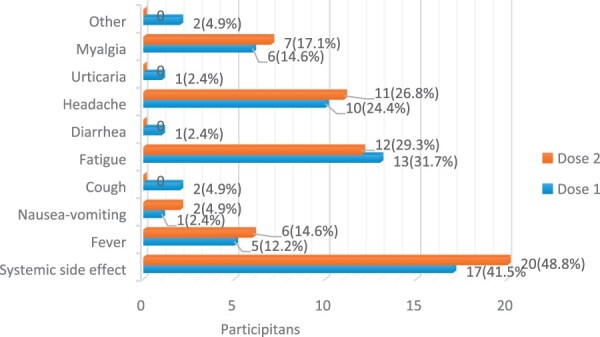
Systemic side effects after the first and second vaccine doses
Discussion
Vaccines play a critical role in controlling the ongoing COVID-19 pandemic and data on the humoral response and safety of novel mRNA vaccines convened expeditiously. However, knowledge about immunogenicity and safety of the vaccines in immunocompromised children is insufficient to draw a conclusion. Exclusion of children followed with the diagnosis of paediatric rheumatic diseases from the phase III clinical vaccine trials necessitated the presentation of vaccine-related real-life data without delay. When we evaluated the results of the humoral immune response to the BNT162b2 mRNA vaccine in children with paediatric rheumatic diseases, it was noticed that all participants achieved an effective immune response after the second dose of vaccine. Our findings indicate that the BNT162b2 mRNA vaccine was successful in inducing antibody response in children receiving cDMARDs and/or bDMARDs. The current results strengthen the safety data of the BNT162b2 mRNA vaccine as it did not cause a disease flare and unmanageable serious side effects.
Even though the frequency of COVID-19 among children remains questionable, the rate of seropositivity in the general paediatric population is reported as 3–5% [17–19]. Walters et al. reported the rate of seropositivity as 13% in children with paediatric rheumatic diseases [20]. In our study, the rate of antibody seropositivity prior to the two-dose vaccine regimen was found to be 36.5%. This difference between seropositivity rates may be attributable to the changing course of the pandemic with the corresponding waves. The absence of evidence of infection in the previous interrogations of children in our cohort may be an indicator of silent carriage. Vaccination of both immunocompetent and immunocompromised children is considered a public health concern, as silent carriers can play a critical role in the spread of infection. As a social issue, during the pandemic, intermittent school closures have both disrupted children’s learning and cut a crucial line of communication for vulnerable children to access help [21]. Vaccination, as the major cost-effective way to prevent infectious diseases, could allow children and adolescents to reintegrate into society, which is an especially important outcome given the severe mental health effects of COVID-19 pandemic [22]. In order to expand the emergency use authorization to include children ≥12 years of age and make a critical step towards achieving herd immunity, and defining the humoral response, immunogenicity and safety profile of BNT162b2 mRNA vaccine, a phase III clinical vaccine trial was designed [23].
The BNT162b2 mRNA vaccine in 12- to 15-year-old children showed a favourable safety profile, produced a greater immune response than in young adults and was highly effective against COVID-19.
Children with paediatric rheumatic diseases are more prone to infections than their healthy peers due to their underlying disease and the immunomodulatory therapy they receive. However, these patients may have lower immunogenicity to the vaccines and in addition, their disease may be activated after vaccinations [24–26]. In a multicentre study of children with paediatric rheumatic diseases receiving bDMARDs, a worsening in the course of both COVID-19 and current disease was not noticed under bDMARDs, but the course of COVID-19 was more severe in children with comorbidities [27].
Studies evaluating the relationship between the BNT162b2 mRNA vaccine immunogenicity and age have shown higher humoral response in younger subjects. According to a phase III clinical vaccine trial, the BNT162b2 mRNA vaccine produced a greater immune response in 12- to 15-year-old recipients than in young adults, and it was found to be highly effective against COVID-19 [23]. With the approval of the BNT162b2 mRNA vaccine in children ≥12 years of age, the question regarding the immunogenicity of vaccine in children with paediatric rheumatic diseases has arisen. While post-vaccine seropositivity after two doses of mRNA vaccine has been reported at 36–47% in adult kidney transplant recipients, a higher seropositivity rate of 63% has been reported in adolescent and young adult kidney transplant recipients after two doses of vaccination [28–30]. Correspondingly, our cohort had high antibody titres after completion of BNT162b2 mRNA vaccine schedule, the relationship between age and antibody titres was not evaluated as adults were not involved in our study.
It was observed that the humoral response was 100% after two doses of vaccination in adults with psoriasis who received immunosuppressive therapy; however, after the first dose none of the patients was seroconverted fully [28]. Similarly, in our patients treated with immunomodulators, a 100% seroconversion rate was observed after completion of two doses of BNT162b2 mRNA vaccine regimen, but we have no data regarding antibody titres after the first dose of vaccine. In another study, it was determined that 98% seropositivity was achieved in adult patients with inflammatory arthritis after two doses of mRNA vaccine, although the median anti-spike IgG titres of these patients were lower than those of healthy controls [29]. During our study period, approval for the vaccination of healthy children between the ages of 12 and 15 years according to the vaccination protocols of the Ministry of Health has not yet been issued and we could not include healthy peers for the comparison. Nevertheless, a direct comparison with phase III clinical vaccine trial data was not possible as different testing systems were used [23].
It is questionable whether low titres could be translated to lower levels of protection [30]. Based on the assumption that high titres of anti-RBD IgG would be protective against new COVID-19 variants, the humoral response of our patients gave us hope that they could be highly protected from SARS-CoV-2.
In our cohort, patients receiving combined therapy had significantly lower anti-RBD IgG titres than patients receiving cDMARDs, and yet non-statistically significantly lower those of than the patients receiving bDMARDs. In a recent study of paediatric patients with inflammatory bowel disease treated with anti-TNF therapy, the group of patients receiving infliximab, the group of patients receiving infliximab combined with MTX or AZA, and the healthy adult control group mounted similar humoral responses 3 months after the first dose of BNT162b2 mRNA vaccine [31]. In our cohort, although not reaching statistical significance, patients diagnosed with PFS had lower antibody responses compared with patients diagnosed with JIA. PFS patients were receiving only biological therapy; however, most of the patients with JIA were under combined therapy. This may be an explanation for the higher antibody titres in PFS patients. The most commonly received biological therapies were anti-IL-1 and anti-TNF agents, and the median antibody titres of patients receiving these therapies were similar; however, the small number of patients limits the ability to a conclusion about the impact of medications.
In a multicentre, observational study evaluating the immunogenicity and safety of two-dose BNT162b2 mRNA vaccine regimen in adult patients with autoimmune inflammatory rheumatic diseases, factors like older age, treatment with glucocorticoids, rituximab, MMF and abatacept were stated to be associated with reduced BNT162b2-induced immunogenicity [32]. In the literature, vaccine-induced humoral response was low in patients receiving rituximab, CYC, MTX, MMF and abatacept; however, in our study, since the cohort was small, comparison of different therapeutic targets was not feasible [33–36]. Even so, post-vaccine anti-RBD IgG titres of one patient receiving abatacept, three patients receiving MMF, and one patient receiving 10 mg/day glucocorticoid were similar to the rest of the patients’ antibody titres.
In our cohort, the BNT162b2 mRNA vaccine showed a good safety profile and patients’ disease activity remained stable without any disease exacerbations, similar to a recent study evaluating BNT162b2 mRNA vaccine safety objectives in patients with paediatric rheumatic diseases [37]. Vaccine safety data can be supported by our study, as there were no unmanageable serious side effects requiring hospitalization in the cohort. While there are recommendations for the COVID-19 vaccine program in adult patients with rheumatic and musculoskeletal diseases, as evidence-based data are lacking, vaccination recommendations for children with paediatric rheumatic diseases have not yet been fully elucidated [38]. Since high antibody titres were achieved without interrupting immunomodulatory treatment of the patients in our cohort, it may be speculated that cDMARDs, bDMARDs and combined therapies do not seem to interfere with the development of vaccine antibody response. These findings strengthen the data on vaccine humoral antibody response and safety, may call attention to the revision of the recommendations for continuing cDMARDs and/or bDMARDs therapy during vaccination in children, and may be a counterargument against the need to discontinue therapy just before vaccination.
Our study has some limitations. Our patients aged 12–15 years with a diagnosis of paediatric rheumatic diseases had the opportunity to participate voluntarily in vaccination campaigns organized by the Ministry of Health, but their healthy peers could not have this opportunity simultaneously, so we do not have a control group in the study design. The low number of patients may limit the impact of the study, especially for comparison of the effects of vaccination among different therapy modalities. The post-vaccine follow-up period may be extended to reach a clear-cut conclusion about vaccine efficacy, immunogenicity and safety in our cohort.
Conclusion
In conclusion, this is the first preliminary study showing that paediatric rheumatic diseases patients receiving immunomodulatory therapies are able to elicit an effective humoral response after two doses of BNT162b2 mRNA vaccine, with a good safety profile. Our results demonstrate that although patients receiving combined therapy had lower antibody titres, they are fully seroconverted. Real-life data with larger series evaluating vaccine safety and immunogenicity in immunocompromised children with paediatric rheumatic diseases are needed to shed light on optimal vaccine strategies for these patients.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. World Health Organization (WHO).org [Internet]. Coronavirus disease (COVID-19) outbreak situation, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (28 December 2021, date last accessed).
- 2. Zhou P, Yang X-L, Wang X-G. et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv 2020.
- 3. Risk for COVID-19 Infection, Hospitalization, and Death by Age Group. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
- 4. Kim L, Whitaker M, O’Halloran A. et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Geneva, Switzerland, 2020.
- 6. Feldstein LR, Tenforde MW, Friedman KG. et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Listing J, Gerhold K, Zink A.. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 8. D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020;26:832–4. [DOI] [PubMed] [Google Scholar]
- 9. Çakmak F, Çakan M, Demir F. et al. Hepatitis B vaccination response of treatment-naive patients with juvenile idiopathic arthritis. Rheumatol Int 2021;doi:10.1007/s00296-021-04833-3. [DOI] [PubMed] [Google Scholar]
- 10. Jensen L, Nielsen S, Christensen AE. et al. Response to influenza vaccination in immunocompromised children with rheumatic disease: a prospective cohort study. Pediatr Rheumatol Online J 2021;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller M, Pittet LF, Zimmermann P.. Immunogenicity and safety of routine vaccines in children and adolescents with rheumatic diseases on immunosuppressive treatment - a systematic review. Eur J Pediatr 2022;181:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanchard-Rohner G. Vaccination in children with autoimmune disorders and treated with various immunosuppressive regimens: a comprehensive review and practical guide. Front Immunol 2021;12:711637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall VG, Ferreira VH, Ierullo M. et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant 2021;21:3980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Picchianti-Diamanti A, Aiello A, Laganà B. et al. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol 2021;12:740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi SG, Moore LW, Eagar T. et al. Risk factors associated with an impaired antibody response in kidney transplant recipients following 2 doses of the SARS-CoV-2 mRNA vaccine. Transpl Direct 2022;8:e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braun-Moscovici Y, Kaplan M, Braun M. et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 17. Bailey LC, Razzaghi H, Burrows EK. et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr 2021;175:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Götzinger F, Santiago-García B, Noguera-Julián A. et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Z, Ren L, Yang J. et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet 2021;397:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walters HM, Mian Z, Thomas L. et al. Seroprevalence and clinical outcomes of SARS-CoV-2 in paediatric patients with rheumatic disease. Rheumatology (Oxford) 2021;keab730. doi: 10.1093/rheumatology/keab730. [DOI] [PubMed] [Google Scholar]
- 21. Sidpra J, Abomeli D, Hameed B, Baker J, Mankad K.. Rise in the incidence of abusive head trauma during the COVID-19 pandemic. Arch Dis Childhood 2021;106:e14. [DOI] [PubMed] [Google Scholar]
- 22. Jones EAK, Mitra AK, Bhuiyan AR.. Impact of COVID-19 on mental health in adolescents: a systematic review. Int J Environ Res Public Health 2021;18:2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frenck RW Jr., Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med 2021;385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maritsi D, Vartzelis G, Soldatou A, Garoufi A, Spyridis N.. Markedly decreased antibody titers against hepatitis B in previously immunised children presenting with juvenile idiopathic arthritis. Clin Exp Rheumatol 2013;31:969–73. [PubMed] [Google Scholar]
- 25. Jaeger VK, Hoffman HM, van der Poll T. et al. Safety of vaccinations in patients with cryopyrin-associated periodic syndromes: a prospective registry based study. Rheumatology (Oxford) 2017;56:1484–91. [DOI] [PubMed] [Google Scholar]
- 26. Jeyaratnam J, Haar NM, Lachmann HJ. et al. The safety of live-attenuated vaccines in patients using IL-1 or IL-6 blockade: an international survey . Pediatr Rheumatol Online J 2018;16:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sozeri B, Ulu K, Kaya-Akça U. et al. The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: a retrospective and multicenter study. Rheumatol Int 2022;42:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahil SK, Bechman K, Raharja A. et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol 2022;4:e42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kearns P, Siebert S, Gaskell C. et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity–the OCTAVE trial. 2021.
- 30. Bergwerk M, Gonen T, Lustig Y. et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shire ZJ, Reicherz F, Lawrence S. et al. Antibody response to the BNT162b2 SARS-CoV-2 vaccine in paediatric patients with inflammatory bowel disease treated with anti-TNF therapy. Gut 2021;doi:10.1136/gutjnl-2021-326196. [DOI] [PubMed] [Google Scholar]
- 32. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 33. Tzioufas AG, Bakasis AD, Goules AV. et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J autoimmunity 2021;125:102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haberman RH, Herati R, Simon D. et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avouac J, Miceli-Richard C, Combier A. et al. Risk factors of impaired humoral response to COVID-19 vaccination in rituximab treated patients. Rheumatology (Oxford) 2021;keab815. doi: 10.1093/rheumatology/keab815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tani C, Pratesi F, Talarico R. et al. Efficacy of anti-SARS-CoV-2 mRNA vaccine in systemic autoimmune disorders: induction of high avidity and neutralising anti-RBD antibodies. RMD Open 2021;7:e001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haslak F, Gunalp A, Cebi MN. et al. Early experience of COVID-19 vaccine-related adverse events among adolescents and young adults with rheumatic diseases: a single-center study. Int J Rheum Dis 2022;25:353–63. [DOI] [PubMed] [Google Scholar]
- 38. Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ et al American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.