Abstract
Purpose
To report historical patterns of pharmaceutical expenditures, to identify factors that may influence future spending, and to predict growth in drug spending in 2022 in the United States, with a focus on the nonfederal hospital and clinic sectors.
Methods
Historical patterns were assessed by examining data on drug purchases from manufacturers using the IQVIA National Sales Perspectives database. Factors that may influence drug spending in hospitals and clinics in 2022 were reviewed—including new drug approvals, patent expirations, and potential new policies or legislation. Focused analyses were conducted for biosimilars, cancer drugs, generics, COVID-19 pandemic influence, and specialty drugs. For nonfederal hospitals, clinics, and overall (all sectors), estimates of growth of pharmaceutical expenditures in 2022 were based on a combination of quantitative analyses and expert opinion.
Results
In 2021, overall pharmaceutical expenditures in the US grew 7.7% compared to 2020, for a total of $576.9 billion. Utilization (a 4.8% increase), price (a 1.9% increase) and new drugs (a 1.1% increase) drove this increase. Adalimumab was the top drug in terms of overall expenditures in 2021, followed by apixaban and dulaglutide. Drug expenditures were $39.6 billion (a 8.4% increase) and $105.0 billion (a 7.7% increase) in nonfederal hospitals and in clinics, respectively. In clinics and hospitals, new products and increased utilization growth drove growth, with decreasing prices for both sectors acting as an expense restraint. Several new drugs that are likely to influence spending are expected to be approved in 2022. Specialty and cancer drugs will continue to drive expenditures along with the evolution of the COVID-19 pandemic.
Conclusion
For 2022, we expect overall prescription drug spending to rise by 4.0% to 6.0%, whereas in clinics and hospitals we anticipate increases of 7.0% to 9.0% and 3.0% to 5.0%, respectively, compared to 2021. These national estimates of future pharmaceutical expenditure growth may not be representative of any particular health system because of the myriad of local factors that influence actual spending.
Keywords: biosimilars, cancer drugs, COVID-19, drug expenditures, pandemic, public policy
Key Points.
Overall, pharmaceutical expenses in the US grew 7.7% in 2021, for a total of $576.9 billion.
Nonfederal hospitals accounted for $39.6 billion in prescription expenditures in 2020, an increase of 8.4% in 2021 after a historic decrease in 2020, whereas in clinics spending grew 7.7% to $105.0 billion.
For 2022, we expect overall prescription drug spending to rise by 4.0% to 6.0%, whereas in clinics and hospitals we anticipate increases of 7.0% to 9.0% and 3.0% to 5.0%, respectively.
Total annual healthcare expenditures in the United States, and the change from year to year, can be influenced by many different factors. Economic disruptions, population demographics, changes in technology and healthcare practice, and evolving patterns of disease all play a role. Rarely does a single disease have significant impact. An exception is coronavirus disease 2019 (COVID-19), which resulted in a global pandemic that turned our healthcare system upside down.1 Among its many effects, the pandemic increased US healthcare expenditures by a magnitude not experienced since 2002. In 2020, national spending on healthcare services grew by 9.5% to $4.1 trillion, while gross domestic product (GDP) dropped by nearly 20%.2 As data becomes available, similar increases are expected for 2021, and the long-term economic effects are likely to also be enduring.
Annual changes in prescription drug expenditures, the subject of this paper, are also influenced by many different factors. The COVID-19 pandemic has played a leading role in the past 2 years, and it is expected to influence medication expenditures across different sectors of care in 2022 and beyond. In this paper we describe relevant data, public policy direction, and trends in the pharmaceutical marketplace to support healthcare leaders’ planning for drug expenses for their organizations. The goal of this article is to guide healthcare leaders, especially in health-system pharmacy, in understanding and budgeting for drug expenditures. We review historical trends in pharmaceutical expenses with an emphasis on clinics and nonfederal hospitals, and we identify factors that may influence future pharmaceutical spending—including new drugs and newly available biosimilar or generic products. We also forecast drug expenditure growth for 2022 for clinics, nonfederal hospitals, and overall, at the national level.
Methods
Data for spending in 2021 and prior years were acquired from the IQVIA National Sales Perspectives (NSP) database, which tracks purchases of medications by hospitals, clinics, retail pharmacies, mail-service pharmacies, home health facilities, long-term care outlets, and other healthcare entities. The NSP data used here were inclusive through December 31, 2021. Data for drugs nearing approval were obtained from the clinical pipeline database provided by IPD Analytics (available at www.ipdanalytics.com), from which we identified drugs and biologics anticipated to be approved by the Food and Drug Administration (FDA) in 2022. The methods are further described in the eSupplement (“Methods and Limitations of the Annual AJHP Paper on National Trends and Projections of Pharmaceutical Expenditures”; available at www.ajhp.org).
Like previous papers in this series, we conducted focused analyses of drug categories of special interest to clinic and hospital medication expenditures. In this paper, we included (1) biosimilars and generic drugs, (2) cancer drugs, (3) influence of COVID-19, and (4) specialty drugs. We defined biosimilar drugs in the same manner as the FDA and examined expenditures in clinics and nonfederal hospitals.3
For the COVID-19 analysis, expenditures for COVID-19 drugs (defined below), in aggregate for the total market, by week, were evaluated in clinics and nonfederal hospitals to compare spending in 2019 through 2021. We then separately assessed expenditure trends in clinics and nonfederal hospitals for individual treatments commonly used in patients with COVID-19. We included inhaled and systemic (nontopical) drugs with suspected and demonstrated effectiveness for the treatment of COVID-19 regardless of evidence demonstrating lack of effectiveness (eg, ivermectin). COVID-19 drugs included albuterol (given alone and in combination with ipratropium), azithromycin, baricitinib, casirivimab/imdevimab, dexamethasone, hydroxychloroquine, ivermectin, nirmatrelvir/ritonavir, remdesivir, sotrovimab, tocilizumab, and zinc sulfate. No data were available for bamlanivimab/etesevimab and molnupiravir. Newly available medications authorized under an emergency use authorization (EUA) were not available in the data until full FDA approval was received or a product was distributed through traditional commercial channels. For example, remdesivir data were available only after FDA approval on October 22, 2020. COVID-19 vaccine expenditures data is still not available because those costs continue to be covered by the federal government. Some of the COVID-19 monoclonal antibody therapies transitioned back and forth between standard commercial distribution and the federal government distributing and covering the costs. Expenditures for COVID-19 drugs are presented by week, starting the week of January 24, 2019, through the week of December 31, 2021. Weekly new COVID-19 case counts in the US are reported to help readers visualize timing of expenditures in relation to COVID-19 dissemination. Total case counts were available starting January 24, 2020, and extending through December 31, 2021. These case counts were sourced from the Johns Hopkins University COVID-19 Data Repository and compiled by the Our World in Data group at the University of Oxford.4
Specialty drugs were defined by IQVIA as those used to treat specific rare and/or complex chronic diseases and which meet 4 or more of the following criteria: (1) initiated and maintained by a specialist; (2) generally injectable and/or not self-administered; (3) products that require an additional level of care in their chain of custody; (4) annual cost of therapy of $6,000 or more; (5) unique distribution; (6) requires extensive or in-depth monitoring/patient counseling; and/or (7) requires reimbursement assistance.5 We identified the top specialty drugs by expenditures and examined specialty drug expenditures by setting, with a focus on clinics and nonfederal hospitals.
Results
Historical trends in prescription expenditures.
Prescription drug spending in the US in 2021 totaled $576.9 billion, as shown in Table 1. Retail pharmacies were the largest sector based on expenditures—accounting for 42.2% ($243.2 billion) of all spending. The sector with the next highest spending was mail-order pharmacies (27.7% of the total, or $159.6 billion), followed by clinics (18.2%, or $105.0 billion), and nonfederal hospitals (6.9%, or $39.6 billion). Other sectors combined accounted for less than 10% of expenditures in 2021.
Table 1.
Prescription Drug Expenditures and Growth by Sector in 2021
| Sectora | 2021 expenditures ($ millions) |
Percent of total expenditures | Percent change from 2020 |
|---|---|---|---|
| Retail pharmacies | 243,194 | 42.2 | 6.5 |
| Mail-order pharmacies | 159,561 | 27.7 | 10.4 |
| Clinics | 104,989 | 18.2 | 7.7 |
| Nonfederal hospitals | 39,584 | 6.9 | 8.4 |
| Long-term care | 15,219 | 2.6 | 4.1 |
| Home health care | 8,461 | 1.5 | 10.1 |
| Federal facilities | 2,648 | 0.5 | –2.0 |
| Staff-model HMOs | 2,026 | 0.4 | –13.3 |
| Other | 1,185 | 0.2 | –5.5 |
| Total | 576,866 | 100.0 | 7.7 |
Abbreviation: HMO, health maintenance organization.
aRetail pharmacies include standalone chain and independent stores, as well as mass merchandisers and food and convenience stores with a licensed pharmacy. Mail-order pharmacies include licensed mail-service pharmacies, including both private-sector and federal facilities. Clinics include physician offices and outpatient clinics, including general, family medicine, and specialty clinics covering oncology, nephrology, dialysis, family planning, orthopedics, and urgent care centers. Nonfederal hospitals include all non–federally owned facilities licensed as hospitals, including inpatient treatment and rehabilitation facilities, in addition to general and specialty acute care institutions. Long-term care includes nursing homes and residential care facilities. Staff-model HMOs include closed-panel HMO pharmacies and hospitals, union clinics and pharmacies, and workers’ compensation clinics. Home healthcare includes licensed home health organizations and visiting nurse entities. Federal facilities include Public Health Service and other federal hospitals, and US ships at sea (Veterans Health Administration facilities were previously included in the federal facility sector, but data on these expenditures were not available after December 31, 2013). “Other” covers a variety of otherwise unclassified government accounts, as well as entities such as jails, prisons, and veterinary hospitals and clinics.
The increase in prescription drug spending in 2021 compared to 2020 was 7.7%, also shown in Table 1. This increase was somewhat larger than those in the previous 2 years (5.5% and 4.9%, respectively, for 2019 and 2020), and slightly higher than anticipated.6,7 In fact, all the major sectors experienced growth in drug spending in 2021, with mail-order pharmacies and home healthcare both exceeding a 10% increase compared to 2020, as shown in Table 1.
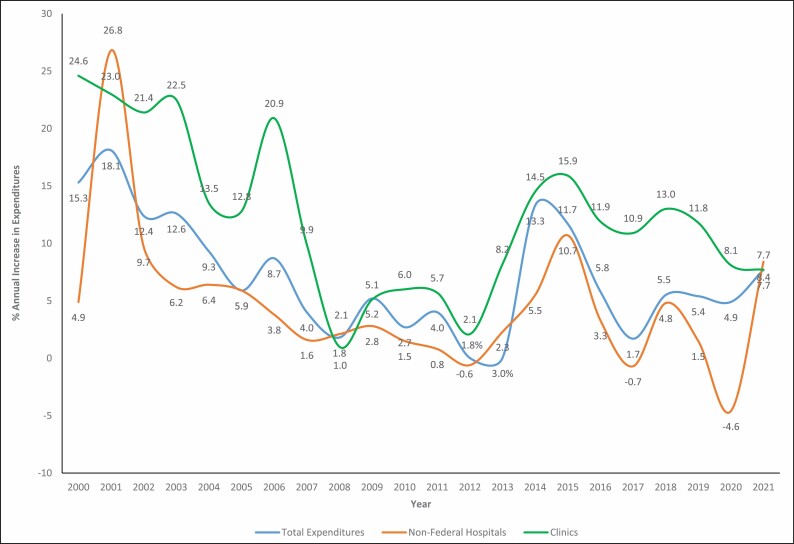
Pharmaceutical expenditures in nonfederal hospitals grew 8.4% in 2021, which was more than the 7.7% growth observed for clinics. While this growth rate was predicted for clinics, the expenditure growth rate for hospitals in 2021 exceeded expectations.7 This was the first time since 2008 that hospital drug expenditure growth outpaced that of clinics, which can be seen in Figure 1. This figure, which displays the percentage increase or decrease in pharmaceutical spending compared to the previous year, also shows the remarkable rebound in hospital drug spending in 2021. Hospital medication spending shrank 4.6% in 2020 compared to 2019, then grew 8.4% in 2021 compared to 2020.
Figure 1.
Annual growth in drug expenditures compared to previous year, 2000-2021.
Factors driving growth.
The increase in overall drug spending in 2021 in clinics, nonfederal hospitals, and overall was driven primarily by increases in volume of utilization. Specifically, the 7.7% overall increase in drug expenditures in 2021 compared to 2020 was a product of a 4.8% increase due to volume/mix, a 1.9% increase due to price, and a 1.1% increase due to new products. For definitions of these drivers of growth, see Table 2.
Table 2.
Factors Driving Growth of Pharmaceutical Expenditures in Clinics and Nonfederal Hospitals in 2021, by Product Categorya
| Clinics | Nonfederal hospitals | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent growth due to factor | Percent growth due to factor | |||||||
| Product category | Total percent growth | New products | Price | Volume and mix | Total percent growth | New products | Price | Volume and mix |
| All products | 7.7 | 0.8 | –1.3 | 8.2 | 8.4 | 0.9 | –1.4 | 8.8 |
| Injectables | 6.7 | 0.6 | –1.6 | 7.7 | 9.0 | 0.9 | –1.5 | 9.6 |
| Branded | 6.7 | 0.5 | –1.3 | 7.5 | 12.6 | 0.6 | 0.7 | 11.3 |
| Generics | –5.4 | 2.6 | –10.2 | 2.2 | 0.5 | 2.7 | –8.2 | 6.0 |
| Branded generics | 17.2 | 0.4 | 1.0 | 15.8 | –1.8 | 0.3 | –5.9 | 3.8 |
| Noninjectables | 11.4 | 1.5 | –0.1 | 10.0 | 6.3 | 0.9 | –1.1 | 6.5 |
| Branded | 13.7 | 1.3 | 1.1 | 11.3 | 6.6 | 0.5 | 1.1 | 5.0 |
| Generics | –4.5 | 3.7 | –9.8 | 1.6 | 3.0 | 2.2 | –8.3 | 9.1 |
| Branded generics | 11.5 | 0.2 | 2.0 | 9.3 | 9.7 | 0.4 | 0.8 | 8.5 |
aTotal growth comprised growth attributable to 3 factors: (1) new products (products that were not on the market in the previous year), primarily newly approved and marketed agents; (2) price (changes in the unit cost of drugs that were on the market in the previous year); and (3) volume and mix (changes in volume of utilization of existing products or changes in utilization patterns [eg, a shift from one product to another, as when prescribing moves from branded to generic products]).
For clinics, the 7.7% increase in spending in 2021 compared to 2020 was the product of an 8.2% increase in volume, a 0.8% increase from new products, and negative growth (–1.3%) from price changes, as shown in Table 2. The impact on spending growth due to increased utilization was consistent across injectable and noninjectable products and for both branded and generic drugs. There was a pattern of negative pressure due to price reductions, which was more significant for generic products. Most of the spending in clinics in 2021 was on injectables (78.3%, not shown in table), of which most was for branded products. While less was spent on noninjectables, that segment of the market had the largest increase in expenditures, 11.4% in 2021 compared to 2020—again driven by volume increases.
In nonfederal hospitals, the 8.4% growth in pharmaceutical spending in 2021 compared to 2010 was driven by volume changes (8.8%) and new products (0.9%), balanced by a 1.4% downward effect of price reductions. Again, the impact of volume of utilization on spending growth was seen for injectables and noninjectables and for branded and generic products, while generic products likely had a greater negative impact on spending growth as a result of price reductions. In nonfederal hospitals, injectables accounted for 77.3% of spending in nonfederal hospitals.
Trends in overall drug spending.
Figure 1 displays long-term trends in annual growth of prescription expenditures in the US. The impact of major disruptions to the economy, as occurred in 2008, are obvious. The higher rate of growth and volatility in the clinic sector is also apparent. The complete impact of the COVID-19 pandemic on these trends is still unfolding.
Top drugs overall.
The top 25 drugs by expenditures for the overall US market in 2021 are shown in Table 3. Adalimumab ($28.5 billion) and apixaban ($15.8 billion) repeated as the number 1 and number 2 drugs, with dulaglutide ($12.2 billion) rounding out the top 3 in 2021. Robust growth was observed for semaglutide (90.1%), dupilumab (55.2%), empagliflozin (40.8%), dulaglutide (40.2%), ustekinumab (28.3%), and apixaban (23.2%). Liraglutide was the only drug in the top 25 with decreased expenditures (–3.7%) relative to 2020 expenditures.
Table 3.
Top 25 Drugs by Expenditures Overall in 2021
| Druga | 2021 expenditures ($ thousands) |
Percent change from 2020 |
|---|---|---|
| Adalimumab | 28,498,153 | 14.6 |
| Apixaban | 15,795,250 | 23.2 |
| Dulaglutide | 12,198,284 | 40.2 |
| Semaglutide | 10,718,460 | 90.1 |
| Ustekinumab | 10,681,921 | 28.3 |
| Insulin glargine | 10,027,314 | 3.2 |
| Pembrolizumab | 9,860,807 | 18.7 |
| Bictegravir/emtricitabine/ tenofovir alafenamide |
9,570,249 | 19.0 |
| Etanercept | 8,094,614 | 4.2 |
| Empagliflozin | 8,022,438 | 40.8 |
| Rivaroxaban | 7,135,012 | 7.5 |
| Sitagliptin | 6,406,544 | 2.4 |
| Insulin aspart | 6,043,212 | 2.1 |
| Insulin lispro | 5,700,806 | 2.7 |
| Dupilumab | 5,604,661 | 55.2 |
| Immune globulin | 5,371,428 | 6.7 |
| Secukinumab | 4,613,406 | 3.9 |
| Liraglutide | 4,546,309 | –3.7 |
| Epinephrine | 4,382,123 | 13.9 |
| Nivolumab | 4,222,753 | 6.8 |
| Ibrutinib | 4,163,655 | 8.1 |
| Budesonide/formoterol | 4,155,298 | 0.1 |
| Ocrelizumab | 4,132,431 | 16.7 |
| Lisdexamfetamine | 4,100,293 | 8.9 |
| Lurasidone | 4,025,640 | 7.4 |
aFor each drug listed, the expenditures shown are the total for branded and generic products (including biosimilars) and of various dosage forms unless otherwise stated.
Top drugs in clinics.
The top 25 drugs by expenditures in the clinic setting in 2021 are shown in Table 4. Pembrolizumab ($8.6 billion) and nivolumab ($3.6 billion) repeated as the top 2 drugs, with ocrelizumab ($2.8 billion) rounding out the top 3. Robust growth was observed for daratumumab/hyaluronidase (351.1%), adalimumab (29.7%), the combination human immunodeficiency virus (HIV) product bictegravir/emtricitabine/tenofovir alafenamide (21.3%), and pembrolizumab (20.7%). Ten drugs in the clinic sector experienced decreased expenditures, led by 5 drugs facing biosimilar competition: trastuzumab (–19.8%), pegfilgrastim (–16.8%), infliximab (–13.6%), bevacizumab (–9.0%) and rituximab (–8.8%).
Table 4.
Top 25 Drugs by Expenditures in Clinics in 2021
| Druga | 2021 expenditures ($ thousands) |
Percent change from 2020 |
|---|---|---|
| Pembrolizumab | 8,611,231 | 20.7 |
| Nivolumab | 3,617,512 | 8.5 |
| Ocrelizumab | 2,848,788 | 18.9 |
| Denosumab | 2,783,345 | 9.7 |
| Pegfilgrastim | 2,584,766 | -16.8 |
| Rituximab | 2,465,607 | -8.8 |
| Infliximab | 2,419,144 | -13.6 |
| Bevacizumab | 2,227,567 | -9.0 |
| Vedolizumab | 2,211,013 | 18.8 |
| Daratumumab/hyaluronidase | 1,896,835 | 351.1 |
| Immune globulin | 1,892,955 | 13.8 |
| Ranibizumab | 1,689,376 | -1.5 |
| Trastuzumab | 1,568,833 | -19.8 |
| Atezolizumab | 1,558,146 | 13.8 |
| Erythropoietin alfa | 1,526,132 | -4.8 |
| Pertuzumab | 1,318,593 | -0.9 |
| Abatacept | 1,267,777 | 10.4 |
| Ibrutinib | 1,264,826 | 2.1 |
| Inactivated influenza virus | 1,224,234 | -6.7 |
| Bictegravir/emtricitabine/ tenofovir alafenamide | 1,113,918 | 21.3 |
| Durvalumab | 1,093,285 | 4.9 |
| Ipilimumab | 1,071,407 | 13.0 |
| Pemetrexed | 1,024,605 | -2.2 |
| Adalimumab | 968,133 | 29.7 |
| Vaccine varicella | 961,935 | 15.2 |
aFor each drug listed, the expenditures shown are the total for branded and generic products (including biosimilars) and of various dosage forms unless otherwise stated.
Top drugs in nonfederal hospitals.
The top 25 drugs by expenditures in nonfederal hospitals in 2021 are listed in Table 5. The antiviral remdesivir ($3.1 billion) repeated as the top drug, with more expenditures than the next 3 drugs combined: pembrolizumab ($1.1 billion), immune globulin ($980 million) and alteplase ($888 million). Daratumumab/hyaluronidase (314.8%), remdesivir (163.6%), tocilizumab (107.9%), sugammadex (29.4%), bupivacaine (25.6%), adalimumab (24.2%) and denosumab (22.4%) each had robust growth in expenditures in 2021. Drug expenditures decreased for 9 drugs on the top-25 list, with rituximab (–23.1%), bevacizumab (–18.3%), infliximab (–9.5%) and their biosimilar competitors leading the decline.
Table 5.
Top 25 Drugs by Expenditures in Nonfederal Hospitals in 2021
| Druga | 2021 expenditures ($ thousands) |
Percent change from 2020 |
|---|---|---|
| Remdesivir | 3,147,107 | 163.6 |
| Pembrolizumab | 1,104,794 | 7.4 |
| Immune globulin | 980,178 | 7.9 |
| Alteplase | 887,526 | 7.5 |
| Vasopressin | 711,970 | 19.2 |
| Rituximab | 645,962 | –23.1 |
| Tocilizumab | 641,673 | 107.9 |
| Inactivated influenza virus | 620,434 | –2.5 |
| Natalizumab | 572,070 | –9.2 |
| Nivolumab | 543,309 | –0.5 |
| Ocrelizumab | 479,529 | –3.0 |
| Bictegravir/emtricitabine/tenofovir alafenamide | 444,296 | 16.3 |
| Pegfilgrastim | 444,218 | –1.6 |
| Infliximab | 435,378 | –9.5 |
| Sugammadex | 433,072 | 29.4 |
| Denosumab | 407,403 | 22.4 |
| Immunoglobulin, antithymocyte | 393,931 | 11.4 |
| Iohexol | 354,556 | 14.2 |
| Bupivacaine | 353,996 | 25.6 |
| Albumin | 352,884 | 6.3 |
| Adalimumab | 328,839 | 24.2 |
| Bevacizumab | 328,018 | –18.3 |
| Vedolizumab | 295,073 | 0.3 |
| Piperacillin/tazobactam | 294,382 | –8.0 |
| Daratumumab/hyaluronidase | 279,943 | 314.8 |
aFor each drug listed, the expenditures shown are the total for branded and generic products (including biosimilars) and of various dosage forms unless otherwise stated.
Table 6 displays the top 25 therapeutic drug categories based on drug expenditures in nonfederal hospitals; these categories accounted for 96.7% of drug spending in nonfederal hospitals in 2021. Antineoplastics ($6.6 billion) continued as the top therapeutic category despite decreasing expenditures (–1.4%) for the second consecutive year. Antiviral drugs ($4.4 billion) leapt into the second-highest expenditure spot with the strongest therapeutic category growth (74.4% growth in expenditures compared to 2020). Antiarthritics were the only other therapeutic category to show robust growth (37.2% growth compared to 2020). Significant decreases in expenditures were observed for neurological disorder drugs (–29.7%) and analgesics (–28.0%).
Table 6.
Top 25 Therapeutic Drug Category by Expenditures in Nonfederal Hospitals in 2021
| Drug category | 2021 expenditures ($ thousands) |
Percent of total 2021 expenditures | Percent change from 2020 |
|---|---|---|---|
| Antineoplastic agents | 6,635,822 | 16.8 | –1.4 |
| Antiviral drugs | 4,420,592 | 11.2 | 74.4 |
| Immunologic agents | 3,056,736 | 7.7 | 14.7 |
| Hemostatic modifiers | 3,046,581 | 7.7 | 8.6 |
| Hospital solutions | 2,157,151 | 5.4 | 2.5 |
| Biologicals | 2,145,347 | 5.4 | 0.2 |
| Miscellaneous | 1,741,376 | 4.4 | 18.3 |
| Antiinfectives, systemica | 1,577,317 | 4.0 | 0.6 |
| Blood factors | 1,467,055 | 3.7 | 5.5 |
| Hormones | 1,424,203 | 3.6 | 13.5 |
| Anesthetics | 1,230,038 | 3.1 | –0.2 |
| Diagnostic aids | 1,208,349 | 3.1 | 0.5 |
| Antiarthritics | 1,124,360 | 2.8 | 37.2 |
| Respiratory therapy agents | 1,078,487 | 2.7 | 0.5 |
| Gastrointestinal agents | 982,669 | 2.5 | –5.6 |
| Musculoskeletal agents | 819,623 | 2.1 | 13.2 |
| Psychotherapeutics | 710,425 | 1.8 | 2.7 |
| Diabetes therapies | 593,970 | 1.5 | 11.5 |
| Neurological disorder drugs | 528,689 | 1.3 | –29.7 |
| Analgesics | 447,852 | 1.1 | –28.0 |
| Cardiac agents | 425,726 | 1.1 | 0.8 |
| Vascular agents | 420,833 | 1.1 | 1.2 |
| Antifungal agents | 380,325 | 1.0 | 9.5 |
| Enzymes | 355,259 | 0.9 | 3.2 |
| Ophthalmic agents | 315,066 | 0.8 | 1.0 |
aIncludes mostly antibacterials along with some antiparasitic agents, with the latter being minimal in terms of expenditures.
Trends in specialty drugs.
Specialty drug expenditures by sector in the US in 2021 are shown in eTable 1. Spending on specialty drugs in 2021 was $284.9 billion for all sectors, which was 49.4% of total prescription expenditures. Specialty drug expenditures increased 8.7% in 2021 compared to 2020. The 3 biggest sectors accounted for 88.8% of spending on specialty drugs: mail-order pharmacies ($130.3 billion, or 45.7% of all specialty drug spending); clinics, including physician offices and outpatient clinics ($84.2 billion, or 29.5% of all specialty drug spending); and retail pharmacies ($38.5 billion, or 13.5% of all specialty drug spending). The top 5 specialty drugs in mail-order pharmacies were self-injectable drugs (adalimumab, ustekinumab, etanercept, dupilumab, and secukinumab), whereas the top 5 specialty drugs in clinics were pembrolizumab, nivolumab, ocrelizumab, denosumab, and pegfilgrastim, reflecting wide use of infusion-based and provider-administered medications in clinics.
The top 25 drugs in clinics, shown in Table 4, were mostly specialty drugs except for influenza vaccines. The fastest-growing sector for specialty drugs was home healthcare, with spending increasing by 12.3%, from $6.6 billion in 2020 to $7.4 billion in 2021. The top 5 specialty drugs in home healthcare were immune globulin, vedolizumab, ocrelizumab, infliximab, and emicizumab. Nonfederal hospitals spent $17.9 billion on specialty drugs in 2021, a 3.5% increase from $17.3 billion in 2020. The top 5 specialty drugs in nonfederal hospitals were pembrolizumab, immune globulin, rituximab, tocilizumab, and natalizumab. Nonfederal hospital spending on specialty drugs grew relatively slower than spending in other sectors and overall specialty drug market growth in previous years.
Trends in biosimilars.
The US biosimilar portfolio in 2021 continued to grow with FDA approval of 4 biosimilars and the launch of 3 biosimilars. As of February 2022, there were 34 FDA-approved biosimilars, 21 of which are available on the US market.8 Nine of the 34 products will have delayed launches, primarily due to patent litigation between the makers of the reference biologic and biosimilar manufacturers. In 2019 and 2020, a total of 13 biosimilars were launched, primarily in oncology. Market shifts were observed in 2020; however, in 2021 the pivot to biosimilar preference was significant and rapid. The newest additions to the biosimilar landscape are listed in eTable 2; they include insulin glargine-yfgn, ranibizumab-nuna, insulin glargine-aglr, and adalimumab-aqvh.
Beyond the increased numbers of new biosimilar approvals and launches, important new biosimilars were approved and use of biosimilars increased in 2021, beginning with the landmark FDA decisions to give interchangeability designations to 2 biosimilars: insulin glargine-yfgn and adaliumumab-adbm. Another key milestone was the approval of the first biosimilar for ophthalmology indications, ranibizumab-nuna. The reference product, ranibizumab, is indicated in the management of neovascular age-related macular degeneration, which is the leading cause of irreversible visual impairment in the elderly. Clinic expenditures on ranibizumab in 2021 totaled $1.7 billion.9 In terms of expenditures, the biosimilar market continued to see increased utilization in both the clinic and nonfederal hospital settings. In clinics, as shown in eTable 3, biosimilar expenditures increased to $5.3 billion in 2021 from $4.3 billion in 2020. Biosimilars for bevacizumab, rituximab, trastuzumab, and infliximab attracted significantly increased expenditures in the clinic setting as they gained market share. Bevacizumab biosimilar market uptake continued to gain momentum, with 2021 market share at 62.9% compared to 35.6% in 2020, while rituximab biosimilar market share in 2021 increased to 44.9% from 18.3% in 2020. Trastuzumab biosimilars experienced a significant increase in market share, from 35.7% in 2020 to 63.2% in 2021.
In nonfederal hospitals, biosimilar adoption continued to gain significant traction. Biosimilar expenditures in 2021 increased to $1.1 billion, compared to $0.7 billion in 2020. Key biosimilars driving expenditures in the nonfederal hospital setting include biosimilars for bevacizumab, rituximab, and trastuzumab. Market share increases were observed for bevacizumab biosimilars (56.1%), rituximab biosimilars (47.8%), and trastuzumab biosimilars (52.5%). Other biosimilars with significantly increased utilization in 2021 versus 2020 included infliximab (27.9% market share) and erythropoietin alfa (26.4% market share).
Recent and anticipated drug approvals.
Adoption of new drugs often substantially contributes to increased pharmaceutical expenditures. Below we review important new drugs approved in 2021. We also identify drugs that are anticipated to be approved in 2022 that may increase drug expenditures.
Drugs approved in 2021.
In 2021, FDA approved 50 new drugs and biological products (not including vaccines and cellular and gene therapy products). Twenty-seven of the 50 novel drugs approved in 2021 were first-in-class drugs. More than half (26) of FDA’s novel drug approvals were to treat rare or orphan diseases. The annual FDA novel drug approval program has averaged 43 novel drug approvals per year for the past decade. Of the 50 drugs newly approved in 2021, 22 were small-molecule drugs and 17 were monoclonal antibodies.
The 2021 drug approvals included drugs for use in therapeutic areas such as oncology, neurology, infectious disease, and cardiovascular diseases. With about 30% of new approvals, oncology was the therapeutic area with the most new agents. Two antibody-drug conjugates (ADCs) were among the approved drugs. Loncastuximab tesirine-ipyl is a CD19-targeted ADC for B-cell lymphoma, and tisotumab vendotin-tftv is a tissue factor–targeted ADC for recurrent or metastatic cervical cancer in patients with disease progression on or after chemotherapy. FDA also approved the third bispecific antibody, amivantamab-vmjw, for the management of non–small cell lung cancer associated with EGFR exon 20 mutation.10 Perhaps the most controversial FDA drug approved in 2021 was aducanumab for Alzheimer’s disease. Based on the cost and size of the potential target population, this agent could quickly become a top-expense drug for clinics and hospitals; however, concerns about the quality of data supporting its use, high cost, lack of clarity on reimbursement, and complex monitoring requirements limited expenses to less than $2 million for clinics and hospitals combined in 2021.11
Anticipated and approved new drugs in 2022.
Selected novel agents that may receive or have received FDA approval for marketing by the end of this year are shown in Table 7. The drugs shown in the table are anticipated to have significant clinical and/or financial impact in clinics and hospitals and were selected from 57 novel drugs expected to be considered for approval in 2022, as reported in the IPD Analytics pipeline database (see Methods section in eSupplement for details).12 Prescription Drug User Fee Act (PDUFA) dates are listed as an indicator of the expected approval dates.
Table 7.
Selected Drugs and Biologicals That Have Already or May Receive FDA-Approved Labeling in 2022a
| Drug or biological | Manufacturer | Indication | Route | Type of application | PDUFA date (month or quarter)b |
|---|---|---|---|---|---|
| Tebentafusp-tebn | Immunocore | Uveal melanoma | IV | BLA | Approved (Jan) |
| Sutimlimab-jome | Sanofi | Cold agglutinin disease | IV | BLA | Approved (Feb) |
| Mitapivat sulfate | Agios | Pyruvate kinase deficiency | Oral | NDA | Approved (Feb) |
| Ciltacabtagene autoleucel | Janssen | Relapsed or refractory multiple myeloma | IV | BLA | Approved (Feb) |
| Penpulimab | Akeso Biopharma | Nasopharyngeal carcinoma | IV | BLA | Q1 |
| Nivolumab/relatlimab | Bristol-Myers Squibb | Unresectable or metastatic melanoma | IV | BLA | Q1 |
| Ganaxolone | Marinus | Seizures associated with cyclin-dependent kinase-like 5 deficiency disorder | Oral, IV | NDA | Q1 |
| Vadadustat | Akebia Therapeutics | Anemia due to chronic kidney disease | Oral | NDA | Q1 |
| Tirzepatide | Eli Lilly | Type 2 diabetes | SC | NDA | Q2 |
| Toripalimab | Coherus biosciences | Advanced recurrent or metastatic nasopharyngeal carcinoma | IV | BLA | Q2 |
| Vutrisiran | Alnylam Pharmaceuticals | Polyneuropathy of hereditary transthyretin-mediated amyloidosis | SC | NDA | Q2 |
| Mavacamten | Bristol-Myers Squibb | Hypertrophic cardiomyopathy | Oral | NDA | Q2 |
| Spesolimab | Boehringer Ingelheim | Pustular psoriasis | IV | BLA | Q2 |
| Tebipenem pivoxil hydrobromide | Spero therapeutics | Complicated UTI | Oral | NDA | Q2 |
| Tauroursodeoxycholic acid and sodium phenylbutyrate | Amylyx Pharmaceuticals | Amyotrophic lateral sclerosis | Oral | NDA | Q2 |
| Teclistamab | Janssen | Relapsed or refractory multiple myeloma | SC | BLA | Q3 |
| Bulevirtide | Gilead | Hepatitis delta virus infection | SC | BLA | Q3 |
| Poziotinib | Spectrum Pharmaceutical | Non–small cell lung cancer | Oral | NDA | Q3 |
| Tislelizumab | Novartis | Unresectable recurrent locally advanced or metastatic esophageal squamous cell carcinoma | IV | BLA | Q3 |
| Betibeglogene autotemcel | Bluebird bio | Beta thalassemia | IV | BLA | Q3 |
| Deucravacitinib | Bristol-Myers Squibb | Moderate to severe plaque psoriasis | Oral | NDA | Q3 |
| Elivaldogene autotemcel | Bluebird bio | Cerebral adrenoleukodystrophy | IV | BLA | Q3 |
Abbreviations: FDA, Food and Drug Administration; PDUFA, Prescription Drug User Fee Act; IV, intravenous; BLA, biologic license application; Q, quarter; NDA, new drug application; SC, subcutaneous.
aInformation for this table was extracted from the IPD Analytics Brand and Biosimilar Pipeline database (see extended Methods description in eSupplement).
bExtrapolated on the basis of new drug application submission date and review status (ie, 10 months for standard review and 6 months for priority review). Some agents listed may have been approved by the time of publication.
As of February 1, 2022, 3 COVID-19 vaccines were available for use in the US (the Pfizer-BioNTech, Spikevax/Moderna, and Johnson & Johnson/Janssen COVID-19 vaccines) through FDA authorization or approval. Besides remdesivir, which is the first antiviral approved by FDA for adults and certain pediatric patients with COVID-19, 14 COVID-19 treatments are listed as available under EUA.13 Substantial resources have been invested to develop COVID-19 vaccines and therapeutics. Due to the uncertainty of the COVID-19 pandemic, it is unclear how COVID-19 vaccines and treatments with an EUA may influence drug expenditures after FDA approval in 2022. Outside of COVID-19, new medications expected to be approved in 2022 include innovative therapies to treat a variety of cancers. For patients with solid tumors, approvals are expected in the areas of melanoma, gastric cancer, esophageal squamous cell carcinoma, and nasopharyngeal carcinoma treatment. Four immune checkpoint inhibitors are predicted to be approved in 2022: penpulimab, toripalimab, tislelizumab, and relatlimab/nivolumab. Penpulimab and toripalimab, anti–programmed cell death protein 1 (PD-1) monoclonal antibodies, are being developed for nasopharyngeal carcinoma.14 Tislelizumab, a humanized anti–PD-1 antibody inhibitor, is being developed for use in the management of unresectable recurrent locally advanced or metastatic esophageal squamous cell carcinoma in people who have received prior systemic therapy.15 Relatlimab/nivolumab is a novel fixed-dose combination dual immune checkpoint inhibitor that targets lymphocyte-activation gene 3 (LAG 3) and PD-1. The combination yielded positive data in untreated or unresectable melanoma compared to nivolumab monotherapy. Approval of these 4 agents would bring the total of FDA approved immune checkpoint inhibitors available in the US to 12.16 Collectively, the immune checkpoint inhibitors are indicated for use in 16 distinct cancers as monotherapy, in combination with chemotherapy, or in combination with targeted therapy. Although price competition is currently uncommon for immuno-oncology therapies, it may occur as more agents are approved.
As in other recent years, new therapeutic classes of drugs for treatment of rare diseases may be approved in 2022. Sutimlimab-jome is the first approved treatment for patients with cold agglutinin disease, a rare autoimmune disorder causing hemolysis. A one-time gene therapy infusion, betibeglogene autotemcel, may be available for beta thalassemia, an inherited blood disorder requiring regular red blood cell transfusions. In addition, mitapivat surfate may become the first therapy for pyruvate kinase deficiency affecting red blood cells. Ganaxolone is a novel γ-aminobutyric acid (GABA) receptor modulator that can be administered by both the injection and oral routes and is being studied for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. Another gene therapy, elivaldogene autotemcel, is under development as a treatment for the rare genetic disorder cerebral adrenoleukodystrophy.
Several other first-in-class drugs may become available. If approved, vadadustat would be the first hypoxia-inducible factor prolyl hydroxylase inhibitor for patients with anemia due to chronic kidney disease. A novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) receptor agonist, tirzepatide, may be available this year, joining currently approved GLP-1 agonists for treating type 2 diabetes. Lastly, deucravacitinib, a first-in-class oral selective tyrosine kinase 2 inhibitor, may be approved for patients with moderate to severe plaque psoriasis in 2022.
Generic drug trends and patent expiration.
FDA continues to increase the availability of generic medications, with full or tentative approval of 776 agents in 2021 (a 14.6 % decrease from 2020); there were fewer approvals of abbreviated new drug applications but 93 first-time generic approvals in 2021 (a 22.5% increase from 2020).17 In 2021, expenditures on generic products (including branded generics) in the US totaled $99.4 billion, a 0.02% increase from 2020. Generics accounted for 17.3% of total pharmaceutical expenditures in 2021. This was a slight change in the trend since 2015 of a decreasing percentage of the total drug spend being attributable to generics. From 2020 to 2021, generics’ share of total expenditures increased 0.1%, after a consistent decrease from 2015 to 2020.
Trends in clinics and nonfederal hospitals.
Table 2 provides information on the expenditures for branded and generic medications in clinics and nonfederal hospitals in 2021, including factors that influenced increases and decreases in those expenditures. In clinics, there was an overall increase of 6.1% in spending on generics, including branded generics, in 2021 compared to 2020. This increase was driven by increasing volume and utilization of branded generic products. Expenditures on injectable generics, excluding branded generics, in clinics decreased by 5.4% in 2021 compared to 2020; this was driven by price decreases. Meanwhile, the 2% increase in clinic expenditures on noninjectable generics (including branded generics) in 2021 was driven by price decreases and volume and case mix, while new products and price changes left overall expenditures for these agents nearly the same as in 2020. Clinic expenditures on noninjectable generic products decreased by 4.5% in 2021, largely due to price decreases.
In nonfederal hospitals, generic drug spending (including spending on branded generics) increased by 1.4% in 2021 compared to 2020. This rise was driven by a mix of increasing utilization and decreasing prices.
Anticipated patent expirations.
Table 8 lists selected branded agents expected to lose patent protection in 2022. Generic availability dates are always fluid, as they depend on results of patent litigation or market exclusivity agreements and delays in drug manufacturing. Among the branded agents that may lose patent protection in 2022, the most important agents in terms of recent overall expenditures for clinics and hospitals are pemetrexed ($1.2 billion); bortezomib ($1.1 billion); vasopressin ($712 million); regadenoson ($652 million); lenalidomide ($495 million); sitagliptin, sitagliptin/metformin, and sitagliptin/metformin ER ($181 million in total); lacosamide ($161 million); octreotide long-acting ($145 million); and dabigatran ($13 million).
Table 8.
Selected Potential Patent Expirations for 2022
| Drug | Brand name(s) | Indication |
|---|---|---|
| Bortezomib | Velcade | Cancer treatment |
| Dabigatran | Pradaxa | Anticoagulant |
| Lacosamide | Vimpat | Epilepsy |
| Lenalidomide | Revlimid | Cancer treatment |
| Octreotide acetate long-acting | Sandostatin LAR | Carcinoid tumors |
| Pemetrexed | Alimta | Cancer treatment |
| Regadenoson | Lexiscan | Pharmacologic stress test |
| Sitagliptin, sitagliptin/metformin, and sitagliptin/metformin ER | Januvia, Janumet, Janumet XR | Diabetes |
| Vasopressin | Vasostrict | Vasoactive agent |
Influence of COVID-19.
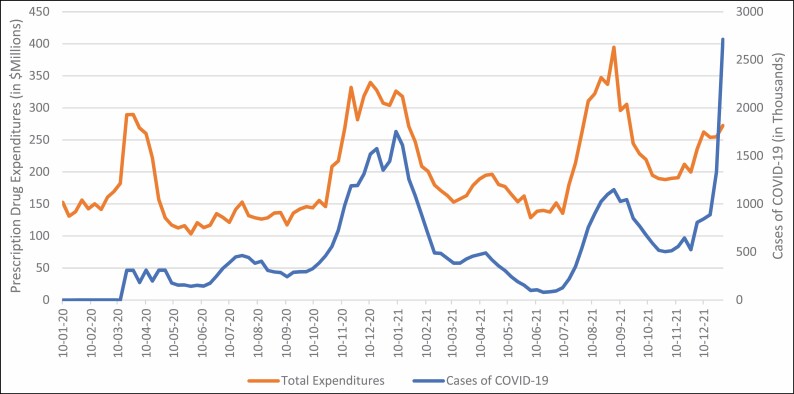
Across all healthcare sectors, 2021 expenditures for COVID-19 drugs were $11.3 billion, a 21.5% increase from 2020. COVID-19 drug expenditures followed trends similar to trends in cases of COVID-19 in the US (Figure 2).
Figure 2.
Trends in expenditures for COVID-19 treatments and COVID-19 cases.
Clinic expenditures for medications to treat COVID-19 grew last year by 25.4% (from $1.3 billion in 2020 to $1.7 billion in 2021). In clinics, expenditures for azithromycin (25.6% decreased growth), albuterol (6.5% decreased growth), zinc (3.6% growth), and dexamethasone (6.7% growth) moderated in 2021 after atypical increases in 2020. Continuing the trend in mid-2020, decreases in growth were observed in 2021 for hydroxychloroquine (58.6% decreased growth). However, ivermectin expenditures experienced a staggering 228.0% increase in growth. While tocilizumab expenditures only modestly increased (13.8% increased growth) in 2021 compared to 2020, increases in growth were observed for sotrovimab (unavailable in 2020). After FDA approval in the fourth quarter of 2020, remdesivir had a 167.7% expenditure increase in clinics in 2021. Baricitinib, a Janus kinase inhibitor that is FDA approved for rheumatoid arthritis, received an EUA for the treatment of COVID-19 when administered in combination with remdesivir. Baricitinib expenditures grew by a staggering 827.4% in clinics, from $5.0 million in 2020 to $46.6 million in 2021.
Nonfederal hospital expenditures for medications to treat COVID-19 grew by 121.1% (from $1.9 billion in 2020 to $4.2 billion in 2021). As the top drug by expenditures in nonfederal hospitals, remdesivir continued to be the dominant COVID-19 drugs in this sector. Baricitinib experienced similarly large growth as clinics, growing by nearly 25-fold (from $8 million in 2021 to $198.9 million in 2021). The monoclonal antibody tocilizumab experienced greater than 100% increased growth, and sotrovimab expenditures exceeded $10.2 million. Similar to trends in clinics, expenditures for azithromycin (25.1% decreased growth), albuterol (13.5% decreased growth), dexamethasone (2.3% growth), and zinc (8.1% growth) started to return to pre–COVID-19 levels in nonfederal hospitals. With regard to treatments for which definitive evidence demonstrated ineffectiveness for COVID-19, expenditures for hydroxychloroquine decreased (–92.5%), but expenditures for ivermectin increased significantly (by 147.0%). There were no expenditures for casirivimab/imdevimab or nirmatrelvir in clinics or nonfederal hospitals, since these costs were covered by the federal government and the drugs only became available at the end of 2021.
Discussion
Biosimilar and generic drugs.
Biosimilars and traditional generic drugs are the 2 main forces restraining drug expenditures in the US. The US healthcare system is poised to see further growth in biosimilars in 2022 as a result of increased market share for existing products, lowering of prices by innovators in response to biosimilar competition, and new biosimilar competition for therapeutic agents for which biosimilars do not currently exist. In the coming years, biosimilars will continue to be a force in moderating medication expenditures. The overall market for biosimilars will develop as biosimilars for more top-expenditure drugs enter the market. The recent advent of interchangeable biosimilars will quicken the pace of biosimilar adoption for products with that designation. Moreover, legislative efforts such as the Advancing Education on Biosimilars Act, signed into law in April 2021, may advance education and awareness of biosimilars among healthcare providers, further enhancing biosimilar uptake in the marketplace.18
Despite challenges, clinics and hospital systems are becoming more adept at implementing biosimilars into practice.19,20 The increasing adoption of biosimilars contributes to the overall decreasing drug costs for clinics and hospitals (Table 2.)19,20 Expenditures for bevacizumab, rituximab, and trastuzumab illustrate the influence of biosimilars on drug expenditures. In 2018, expenditures for those 3 key oncology therapeutics in the clinic setting totaled approximately $7.7 billion; in 2019, after 4 biosimilars for bevacizumab, trastuzumab, and rituximab were launched, total expenditures for the 3 reference products decreased to $5.4 billion. In 2021, a total of 10 biosimilars for these key biologics had reached the market, further decreasing expenditures for the reference products to $2.86 billion. Similar trends were observed in the hospital setting for these 3 key biologics, which historically were responsible for driving expenditures. In 2018, hospital expenditures for these agents totaled approximately $2.4 billion; in 2021, with the adoption of biosimilars, hospital expenditures decreased to $590 million.
New generic drug launches are poised to have a substantial impact in restraining clinic and hospital expenditures in 2022, with drugs accounting for over $4 billion in expenditures facing new competition. Specifically, vasopressin, the 5th-highest medication expense for hospitals, will face competition after marketing exclusivity resulting from the Unapproved Drug Initiative (UDI) ends in early 2021. Generics for bortezomib, pemetrexed, regadenoson, and lacosamide will also contribute to restraining expenses with their use in both the clinic and hospital sectors.
Cancer drugs.
In 2021, overall oncology drug clinic expenditures increased to $44.1 billion, compared to $39.9 billion in 2020. Key drivers of oncology expenditures were innovation and continuous adoption of newer therapies, which will continue to be the case in 2022. The oncology pipeline for 2022 appears to be robust, with new molecular entities as well as expanded indications for several novel biologic agents anticipated. Another key driver of clinic expenditures on oncology agents in 2021 was the continued incorporation of immuno-oncology agents into regimens for multiple cancers despite FDA’s withdrawal of 5 previously approved indications under accelerated circumstances.21 Clinic expenditures on immune checkpoint inhibitors in 2021 totaled approximately $16.2 billion, which accounted for 36.7% of oncology drug expenditures in clinics. Oncology clinic drug expenditures on immuno-oncology agents increased approximately 16% in 2021. 2022 will likely see continued uptake and an expanded number of immuno-oncology therapies, leading to increased expenditures. Oncology biosimilars will likely continue to erode reference products’ market share; however, that will likely not be enough to offset expenditures for other novel biologic agents and immuno-therapies.
COVID-19 pandemic.
The ongoing pandemic continues to increase clinic and nonfederal hospital prescription drug expenditures. In 2021, COVID-19 drug expenditure trends followed COVID-19 case trends in the US. Drugs used to treat COVID-19, especially monoclonal antibodies and antivirals, continue to significantly impact prescription drug expenditures. Expenditures for tocilizumab in 2021, although high, were likely moderated due to drug shortages and inability of the manufacturer to respond to demand.22 Use of these treatments will obviously increase if cases of COVID-19 increase, and booster doses could become a routine aspect of healthcare. However, the potential impact on clinic and hospital expenditures remains uncertain, since many of these therapies are currently paid for by the federal government, and it is difficult to anticipate when the cost of these therapies may shift to clinics and hospitals. Given that remdesivir accounted for 8% of hospital drug expenditures in 2021, we anticipate that COVID-19 vaccines will become top-expenditure drugs in all sectors once they move into traditional distribution models and are no longer paid for directly by the federal government.
Public policy.
In September 2021, Secretary of Health and Human Services Xavier Becerra released the report Comprehensive Plan for Addressing High Drug Prices. This document outlines the Biden administration’s playbook of administrative levers and legislative action aimed towards reforming drug pricing in the US.23 The plan focuses on supporting drug price negotiation with manufacturers, reducing the rate of price increases, enabling market changes that strengthen supply chains, promoting biosimilars and generics, and increasing transparency.
The specific legislative actions currently under pursuit by the Biden administration are contained within the Build Back Better (BBB) Act.24 This bill was introduced in the US Congress and is currently under consideration. If the bill is passed, beginning in 2023 the Centers for Medicare & Medicaid Services (CMS) could begin negotiating with pharmaceutical manufacturers on certain medications covered under Medicare Parts B and D, with negotiated prices becoming effective in 2025. Other provisions of the BBB bill relevant to pharmaceutical expenditures include mechanisms to slow price increases over time for existing drugs; efforts to speed market entry of biosimilar and generic drugs, including shortening the period of exclusivity; and policies in Medicare Part B to increase the prescribing of biosimilars by clinicians and prohibition of “pay-for-delay” agreements and other anticompetitive practices by manufacturers.
At the time of writing, it appeared unlikely the BBB would be approved by the US Senate. However, it is possible that specific provisions will be separately passed individually by Congress. While none of these actions are likely to have a material impact on prescription drug expenditures in 2022, they represent the leading policy approach to manage drug expenditures and would influence expenditures in the latter part of this decade if enacted.
Inflation is another public policy variable that may impact drug expenditures in 2022. During the 3 decades that this annual report has been published, we have not observed a direct relationship between general economic inflation and prescription drug expenses, as new drugs and volume/mix (rather than price) tend to be the largest drivers. Notably, general inflation has been restrained during most of that time, and overall drug expenditures typically grew more than inflation. In 2021, the US Consumer Price Index increased by the highest rate in 4 decades, while drug prices for clinics and hospitals decreased by 1.3% and 1.4% respectively.25 Inflation tends to slowly work its way through different sectors of the economy, and we expect some prescription drug price increases in 2022 as manufacturers attempt to pass along their increased input costs.
In June of 2021, FDA reversed the November 2020 Trump administration decision to suspend the UDI.26 The UDI, which was launched in 2006, allows pharmaceutical manufacturers to obtain FDA approval and exclusivity for drugs that were never previously approved because they were on the market before contemporary FDA regulatory requirements. Drug approvals through the UDI process have led to substantial expenditure increases during the years of marketing exclusivity, and healthcare leaders will need to be aware that drugs following this pathway can contribute to increased expenses.27 Notably, vasopressin previously gained exclusivity through the UDI and was the number 5 drug in terms of hospital expenditures in 2021. It is difficult to anticipate which drugs may undergo approval through this pathway and result in increased expenditures.
Specialty drugs.
The rate of growth for specialty drug expenditures slowed during the pandemic (an increase of 11.3% in 2019 vs 8.7% in 2021). However, specialty drugs still drove overall growth in the mail-order, clinic, and home healthcare sectors in 2021. Self-injectable biologics for treatment of inflammatory conditions led the recent growth in mail-order pharmacies. For example, adalimumab has been the overall number 1 drug by expenditures the past several years among all FDA-approved drugs. We anticipate the recent trend of orphan drug approvals will continue in 2022, as there are several novel drugs targeting rare diseases in this year’s pipeline. Orphan drug therapies are intended for a small patient population; however, due to their extremely high cost, drugs for rare diseases will increasingly influence specialty drug expenditures in the future. Another trend influencing drug expenditures is FDA’s approvals of additional indications for already approved specialty drugs. Dupilumab was approved in 2017 for atopic dermatitis and received approval for several additional indications, including moderate to severe asthma and chronic rhinosinusitis. Adalimumab has obtained 9 additional indications since its initial approval in 2002 for rheumatoid arthritis. Supplemental indications may continue to increase utilization and expand the specialty pharmacy market. As specialty drug expenditures continue to grow and expand as a share of total drug expenditures, pharmacy leaders must understand the factors influencing increased specialty drug expenditures, as these products will be of increasing importance to health-system medication expenditure trends.
Drug expenditure forecast for 2022.
Taking into account the historical trends and anticipated new drug and biosimilar/generic availability developments reviewed above, together with other policy, public health, and economic factors and our analytic modeling, we predict that in 2022, for the overall market, pharmaceutical expenditures will grow 4.0% to 6.0% compared to 2021. Further, we estimate that drug spending in clinics and nonfederal hospitals will increase by 7.0% to 9.0% and 3.0% to 5.0%, respectively, in 2021 compared to 2020.
Summary.
In this paper, we provide information to help health-system leaders understand drug expenditure patterns and anticipate future growth in spending. Projecting future pharmaceutical spending at either the national level, as we have done here, or at a local level (through an institution’s drug budget) is complex. Actual future spending is determined by many different factors, some of which are unknown at the time of budget projection formation. Key factors include changes in patient volume, disease patterns, and/or acuity; changes in local or national policies or economic conditions; availability and adoption of new technology or medications; price changes; changes in prescribing practices and utilization of medications; and many other developments. Remaining mindful of changes in the local and national landscape is critical for leaders to be able to explain variances that occur when comparing budgeted to actual spending. Close monitoring of spending will also help identify measures to proactively manage actual spending so that it does not exceed budgeted amounts. Leaders also need to understand the value of the sectors of business that they manage, since exceeding expenses in areas that produce net operating income is likely to be unfavorable to the overall financial performance of the enterprise.
Limitations.
This analysis of historical spending and the projections for future growth in national drug expenditures should be interpreted with understanding of the limitations. Our observations of spending in 2021 do not account for COVID-19 vaccinations and treatments that were directly paid for by the federal government and, therefore, underrepresent true costs. Because projections for 2022 are based on experience in prior years, these are also impacted by the pandemic. More generally, there are many unknown factors—whether related to COVID-19 or not—that can impact future spending on drugs in any given year in each of the settings on which we focused. Supplemental material that delves deeper into the list of these limitations can be found in the eSupplement.
Conclusion
We predict continued moderate growth of 4.0% to 6.0% in overall drug expenditures (across the entire US market) in 2022. We expected the clinic sector to continue to experience high growth in drug spending (7.0%-9.0%) in 2022. Finally, for nonfederal hospitals we anticipate moderating growth, in the range of 3.0% to 5.0%. These estimates are at the national level. Health-system pharmacy leaders should carefully examine their own local drug utilization patterns to determine their own organization’s anticipated spending in 2022.
Supplementary Material
Acknowledgments
The authors thank all of the individuals who served as reviewers for this paper and the ASHP Section of Pharmacy Practice Managers for supporting this effort. We also recognize IQVIA and IPD Analytics for their contributions to this work.
 An audio interview that supplements the information in this article is available on AJHP’s website at www.ashp.org/ajhp-voices.
An audio interview that supplements the information in this article is available on AJHP’s website at www.ashp.org/ajhp-voices.
Contributor Information
Eric M Tichy, Mayo Clinic, Rochester, MN, USA.
James M Hoffman, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Katie J Suda, Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, PA, and Department of Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Matthew H Rim, College of Pharmacy, University of Illinois at Chicago, Chicago, IL, USA.
Mina Tadrous, Ontario Drug Policy Research Network (ODPRN), St. Michael’s Hospital, Toronto, Canada, and Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Canada.
Sandra Cuellar, College of Pharmacy, University of Illinois at Chicago, Chicago, IL, USA.
John S Clark, Michigan Medicine, University of Michigan, Ann Arbor, MI, and University of Michigan College of Pharmacy, Ann Arbor, MI, USA.
Jennifer Ward, IQVIA, Plymouth Meeting, PA, USA.
Glen T Schumock, College of Pharmacy, University of Illinois at Chicago, Chicago, IL, USA.
Disclosures
Dr. Tichy, Dr. Hoffman, and Dr. Rim are AJHP contributing editors. Dr. Suda is a member of the AJHP Editorial Advisory Board. The other authors have declared no potential conflicts of interest.
Additional information
The statements, findings, conclusions, and views contained and expressed herein are those of the authors and do not necessarily represent the views of ASHP, the US government, the Department of Veterans Affairs, or IQVIA or any of its affiliated or subsidiary entities.
References
- 1. A timeline of COVID-19 developments in 2020. AJMC. Published January 1, 2021. Accessed February 10, 2022. https://www.ajmc.com/view/a-timeline-of-covid19-developments-in-2020 [Google Scholar]
- 2. Martin AB, Hartman M, Benson J, Catlin A; National Health Expenditure Accounts Team. National health spending in 2020: growth driven by federal spending in response to the COVID-19 pandemic. Health Aff (Millwood). 2022;41:13-25. [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration. Biosimilar interchangeable and interchangeable products. Published October 2018. Accessed February 9, 2022. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- 4. Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus pandemic (COVID-19). Our World in Data. Accessed February 13, 2022. https://ourworldindata.org/coronavirus
- 5. IQVIA Specialty Drug Definition [proprietary data]. IQVIA; 2021. Accessed February 28, 2022. [Google Scholar]
- 6. Tichy EM, Schumock GT, Hoffman JM, et al. National trends in prescription drug expenditures and projections for 2020. Am J Health-Syst Pharm. 2020;77:1213-1230. [DOI] [PubMed] [Google Scholar]
- 7. Tichy EM, Schumock GT, Hoffman JM, et al. National trends in prescription drug expenditures and projections for 2021. Am J Health-Syst Pharm. 2021;78:1293-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. Biosimilar product information. Accessed February 25, 2022. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
- 9. Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25: S172-S181. [PubMed] [Google Scholar]
- 10. Mullard, A. Cancer drug approvals and setbacks in 2021. Nat Cancer 2021;2:1246-1247. [DOI] [PubMed] [Google Scholar]
- 11. Walker J. Medicare proposal on Alzheimer’s drug draws criticism from drugmakers. Wall Street Journal. Published February 23, 2022. Accessed February 25, 2022. https://www.wsj.com/articles/medicare-proposal-on-alzhermers-drug-draws-criticism-from-drugmakers-11645639032 [Google Scholar]
- 12. Brand and Biosimilar Pipeline Database [proprietary data]. IPD Analytics; 2022. Accessed February 1, 2022. [Google Scholar]
- 13. US Food and Drug Administration. Coronavirus Treatment Acceleration Program (CTAP). Published February 14, 2022. Accessed February 23, 2022. https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap#dashboard
- 14. Xu R-H, Mai H-Q, Chen Q-Y, et al. JUPITER-02: the randomized, double-blind phase 3 study of toripalimab or placebo plus cisplatin and gemcitabine as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (NPC). J Clin Oncol. 2021;39(suppl 15):LBA2. [Google Scholar]
- 15. Shen L, Kato K, Kim S-B, et al. RATIONALE 302: randomized, phase 3 study of tislelizumab vs chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma. Poster presentation at: American Society of Clinical Oncology Annual Meeting; June 2021.
- 16. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration. Generic Drugs Program activities report — monthly performance. Accessed February 21, 2022. https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/generic-drugs-program-activities-report-monthly-performance
- 18. Advancing Education on Biosimilars Act of 2021, S164, 117th Cong (2021-2022). [Google Scholar]
- 19. Lam SW, Amoline K, Marcum C, Leonard M. Healthcare system conversion to a biosimilar: trials and tribulations, Am J Health-Syst Pharm, 2022;78:2159-2163. [DOI] [PubMed] [Google Scholar]
- 20. Kar I, Kronz M, Kolychev E, et al. Biosimilar strategic implementation at a large health system, Am J Health-Syst Pharm, 2022;79: 268-275. [DOI] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration. Withdrawn — cancer accelerated approvals. Accessed February 25, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-cancer-accelerated-approvals
- 22. University of Utah Drug Information Service. Current drug shortages. Accessed February 25, 2022. https://www.ashp.org/drug-shortages/current-shortages/drug-shortage-detail.aspx
- 23. Office of the Assistant Secretary for Planning and Evaluation, US Department of Health & Human Services. Comprehensive Plan for Addressing High Drug Prices: A Report in Response to the Executive Order on Competition in the American Economy. Published September 2021. Accessed February 22, 2022. https://aspe.hhs.gov/reports/comprehensive-plan-addressing-high-drug-prices [Google Scholar]
- 24. Build Back Better Act, HR5376, 117th Cong (2021-2022). Congress.gov. Accessed February 14, 2022. https://www.congress.gov/bill/117th-congress/house-bill/5376 [Google Scholar]
- 25. Gryta T. Inflation is everywhere, including places you might not expect. Wall Street Journal. Published February 13, 2022. Accessed February 25, 2022. https://www.wsj.com/articles/inflation-is-everywhere-including-places-you-might-not-expect-11644748202 [Google Scholar]
- 26. US Food and Drug Administration. Unapproved drugs. Accessed March 15, 2022. https://www.fda.gov/drugs/enforcement-activities-fda/unapproved-drugs
- 27. Gupta R, Dhruva SS, Fox ER, et al. The FDA Unapproved Drugs Initiative: an observational study of the consequences for drug prices and shortages in the United States. J Manag Care Spec Pharm. 2017;23:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.