Abstract
Purpose
Management and outcomes of pregnant women with coronavirus disease 2019 (COVID-19) admitted to intensive care unit (ICU) remain to be investigated.
Methods
A retrospective multicenter study conducted in 32 ICUs in France, Belgium and Switzerland. Maternal management as well as maternal and neonatal outcomes were reported.
Results
Among the 187 pregnant women with COVID-19 (33 ± 6 years old and 28 ± 7 weeks’ gestation), 76 (41%) were obese, 12 (6%) had diabetes mellitus and 66 (35%) had pregnancy-related complications. Standard oxygenation, high-flow nasal oxygen therapy (HFNO) and non-invasive ventilation (NIV) were used as the only oxygenation technique in 41 (22%), 55 (29%) and 18 (10%) patients, respectively, and 73 (39%) were intubated. Overall, 72 (39%) patients required several oxygenation techniques and 15 (8%) required venovenous extracorporeal membrane oxygenation. Corticosteroids and tocilizumab were administered in 157 (84%) and 25 (13%) patients, respectively. Awake prone positioning or prone positioning was performed in 49 (26%) patients. In multivariate analysis, risk factors for intubation were obesity (cause-specific hazard ratio (CSH) 2.00, 95% CI (1.05–3.80), p = 0.03), term of pregnancy (CSH 1.07, 95% CI (1.02–1.10), per + 1 week gestation, p = 0.01), extent of computed tomography (CT) scan abnormalities > 50% (CSH 2.69, 95% CI (1.30–5.60), p < 0.01) and NIV use (CSH 2.06, 95% CI (1.09–3.90), p = 0.03). Delivery was required during ICU stay in 70 (37%) patients, mainly due to maternal respiratory worsening, and improved the driving pressure and oxygenation. Maternal and fetal/neonatal mortality rates were 1% and 4%, respectively. The rate of maternal and/or neonatal complications increased with the invasiveness of maternal respiratory support.
Conclusion
In ICU, corticosteroids, tocilizumab and prone positioning were used in few pregnant women with COVID-19. Over a third of patients were intubated and delivery improved the driving pressure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06833-8.
Keywords: Acute respiratory distress syndrome, COVID-19, Mechanical ventilation, Neonates, Oxygenation, Pregnancy, Prone positioning, Prognosis
Take-home message
| In this retrospective multicenter and international study, corticosteroids, tocilizumab and prone positioning were used in few pregnant women with coronavirus disease 2019 (COVID-19) admitted to the intensive care unit (ICU). Over a third of patients were intubated and had to be delivered during ICU stay mainly due to maternal respiratory worsening, which improved the driving pressure. Despite low maternal and fetal/neonatal mortality rates, the rate of maternal and/or neonatal complications increased with the invasiveness of maternal ventilatory support. |
Introduction
Since December 2019, SARS-CoV-2 is an emerging coronavirus responsible of a worldwide pandemic of SARS-CoV-2-related pneumonia, known as coronavirus disease (COVID-19) [1].
Pregnancy is a risk factor for severe form of SARS-CoV-2 infection [2] due to pregnancy-related changes in physiology, immunity and respiratory mechanics [3, 4]. Pregnant women > 35 years old, obese, with chronic and/or gestational hypertension, diabetes and pre-eclampsia are at risk of developing a severe form of SARS-CoV-2 pneumonia [5–8]. Furthermore, SARS-CoV-2 infection may also increase the rate of obstetric complications with more frequent premature deliveries, more cesarean sections and more frequent postpartum hemorrhage [2, 5, 7–10].
Pregnant women with COVID-19 are more likely to be admitted to intensive care unit (ICU) [11] and to require invasive mechanical ventilation or venovenous extracorporeal membrane oxygenation (VV-ECMO) [5, 6, 11–14]. Nevertheless, only few case series have specifically focused on ventilatory management and outcomes of pregnant women with COVID-19 admitted to ICU [15–18] and although French recommendations address the criteria for ICU admission, there are currently no specific recommendations for ICU management of pregnant women with COVID-19 [19, 20].
The first aim of this study was to assess the ventilatory management of pregnant women with COVID-19 admitted to ICU. The second aims were to assess obstetric management and to report on maternal and neonatal outcomes.
Methods
This retrospective multicenter and international study was conducted in 29 French ICUs, 2 Belgian ICUs and 1 Swiss ICU and was approved by the Ethics committee of the Société de Réanimation de Langue Française (CE SRLF 21–78) and by the Ethic committee of Erasme Hospital (P2020/253). Informed consent was waived but all patients or next of kin were informed about the study. The study complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines (Supplemental methods).
We included all consecutive pregnant women over 18 years old admitted to the different ICUs for SARS-CoV-2 pneumonia from March 2020 to December 2021 with a positive real-time reverse transcriptase-polymerase chain reaction assay for SARS-CoV-2 in nasal swabs or pulmonary samples. There were no exclusion criteria.
Ventilatory management and measurements
Ventilatory support in the different ICUs included standard oxygenation, high-flow nasal oxygen therapy (HFNO), non-invasive ventilation (NIV) or invasive mechanical ventilation, according to the severity of respiratory failure and local protocols. Indications for intubation were left at the discretion of the attending physician but was based on usual clinical and/or oxygenation parameters in critically ill patients.
Mechanically ventilated patients were sedated by propofol (1–3 mg/kg/h) or midazolam (0.1–0.2 mg/kg/h) associated with sufentanil (0.1–0.5 μg/kg/h) or remifentanil (0.05–0.25 μg/kg/h), according to local protocols. Neuromuscular blocker agents and prone positioning sessions were used according to current recommendations in non COVID-19 patients [21]. Awake prone positioning sessions were performed in patients under HFNO or NIV [22].
In patients under standard oxygenation, inspired oxygen fraction (FiO2) was calculated as follows: FiO2 = (oxygen flow × 3) + 21 [23]. The compliance of the respiratory system was calculated as tidal volume/(plateau pressure − total positive end-expiratory pressure). The driving pressure was calculated as plateau pressure − total positive end-expiratory pressure.
Fetal monitoring
All but one of the maternity units associated with each participating ICU (level 2A) were level-3 maternity units. In all ICUs, fetal monitoring included fetal heart rate monitoring and/or ultrasound according to the term of pregnancy. The frequency of these exams depended on local protocols and results of fetal heart rate monitoring.
Data collection and outcomes
Demographic characteristics, comorbidities of patients, clinical, biological and radiological data, therapeutics as well as clinical outcomes were collected and analyzed. Biological and radiological data were collected at ICU admission. The closest ventilatory and oxygenation parameters before and after delivery (i.e., within hours) were also collected in patients who were intubated. The severity of computed tomography (CT) scan abnormalities was assessed by the radiologist and divided into five categories according to the extent of ground-glass opacities and consolidations as a percentage of the total lung parenchyma: < 10%, 10–25%, 25–50%, 50–75 and > 75% [24].
Respiratory outcomes were the intubation rate in pregnant women with COVID-19, the proportion of patients only treated with HFNO and NIV, the proportion of patients in whom prone positioning was performed, the risk factors of intubation and the duration of invasive mechanical ventilation. Obstetric outcomes were the proportion of patients requiring delivery, the maternal ICU mortality rate and the maternal and neonatal complications rate during ICU and/or hospital stay.
Maternal complications included obstetric complications (postpartum hemorrhage and gynecologic infection) and all complications related to ICU stay. Neonatal complications included fetal or neonatal death, preterm birth (at < 32 and < 37 weeks’ gestation), small for gestational age [25], organ failure or need for ICU admission. Preterm birth at < 32 weeks’ gestation included both live and stillbirths at > 20 weeks’ gestation but < 32 weeks’ gestation [25]. Preterm birth at < 37 weeks’ gestation included both live and stillbirths at > 20 weeks’ gestation but < 37 weeks’ gestation [25].
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) according to normal distribution and categorical variables as numbers (percentages). Between-group comparisons were performed by Student or Mann–Whitney tests for continuous variables and by Pearson’s Chi-square or Fisher exact tests for categorical variables. Within-group comparisons were performed by paired Student or Wilcoxon tests for continuous variables and by Mc Nemar or Fisher exact tests for categorical variables.
Risk factors for intubation were identified with a Cox cause-specific model, with ICU discharge alive or death in ICU without intubation as competing events and results were given as cause-specific hazard ratio (CSH) with their 95% confidence interval (CI). For this analysis, patients who were intubated before ICU admission were excluded (n = 145). All covariates related to ICU management (ventilatory management and treatments received during ICU stay) were assessed as time-dependent covariate. Covariates included in the Cox cause-specific model were selected a priori based on clinical relevance: term of pregnancy, obesity, extent of CT scan abnormalities, HFNO and NIV use and corticosteroids’ administration.
The effects of delivery on respiratory mechanics and oxygenation in intubated patients were investigated only in the subgroup of patients with a complete dataset for all respiratory mechanics and oxygenation variables before and after delivery (n = 27). Analyses concerning the rate of maternal and/or neonatal complications were stratified on the invasiveness of the oxygenation technique. For this purpose, patients who received more than one of the four techniques were classified according to the most invasive technique used, assuming intubation to be more invasive than NIV, NIV to be more invasive than HFNO and HFNO to be more invasive than standard oxygenation [26].
Analyses were performed with R 3.1.1 (R foundation for Statistical Computing Vienna, Austria). Descriptive statistics were only carried out on the available data. The percentage of missing data for each variable is detailed in Table S1. Missing values for covariates included in the multivariable model were handled by multiple imputations with chained equations [27]. All tests were two-sided, and a p value < 0.05 was considered statistically significant.
Results
Study population
Among the 2480 pregnant women with COVID-19 hospitalized in the different participating centers during the study period, 187 (8%) were admitted to ICU for severe SARS-CoV-2 pneumonia. Among them, 82 (44%) were followed in the maternity unit associated with the participating ICU since the beginning of pregnancy and 105 (56%) were transferred due to the severity of COVID-19. Patients were 33 ± 6 years old and had a gestation length of 28 ± 7 weeks: 14 (7%) patients were in the first trimester, 71 (38%) were in the second trimester, 102 (55%) were in the third trimester of pregnancy and 9 (5%) patients had twin pregnancy. Overall, 76 (41%) patients were obese, 12 (6%) had diabetes mellitus and 66 (35%) had pregnancy-related complications (Table 1). Among patients eligible for vaccination according to recommendations in France, Switzerland and Belgium during the study period, 95% were not vaccinated against SARS-CoV-2. The delay from the onset of symptoms to ICU admission was 9 ± 5 days and CT scan was performed in 142 (76%) patients. Overall, 157 (84%) patients received corticosteroids for SARS-CoV-2 infection at ICU admission, 41 (22%) patients received betamethasone for fetal lung maturation and 6 (3%) patients received methylprednisolone for unresolved ARDS. Tocilizumab was administered in 25 (13%) patients within 48 h of ICU admission, if there was increased blood inflammatory biomarkers and no respiratory improvement (Table 1).
Table 1.
Characteristics and ICU management in pregnant women with COVID-19
| No intubation (n = 114) | Intubation (n = 73) | p value | |
|---|---|---|---|
| Characteristics | |||
| Age (years) | 33 ± 6 | 34 ± 5 | 0.14 |
| Body mass index (kg/m2) | 29.1 (26.4–33.5) | 31.5 (27.1–34.5) | 0.02 |
| SAPS-2 score | 18 (14–26) | 27 (20–39) | < 0.001 |
| SOFA score at ICU admission | 2 (1–3) | 4 (3–7) | < 0.001 |
| Obesity, n (%) | 35 (31) | 41 (56) | < 0.001 |
| Diabetes mellitus, n (%) | 6 (5) | 6 (8) | 0.54 |
| Smokers, n (%) | 8 (7) | 3 (4) | 0.53 |
| Asthma, n (%) | 10 (9) | 4 (5) | 0.40 |
| Immunosuppression, n (%) | 2 (2) | 0 (0) | 0.52 |
| CT scan, n (%) | 84 (74) | 58 (79) | 0.37 |
| Extent of CT Scan abnormalities, n (%) | < 0.001 | ||
| < 10% | 3 (4) | 3 (5) | |
| 10–25% | 26 (31) | 3 (5) | |
| 25–50% | 32 (38) | 12 (21) | |
| 50–75% | 20 (23) | 29 (50) | |
| > 75% | 3 (4) | 11 (19) | |
| Obstetric history | |||
| Term of pregnancy at ICU admission (weeks’ gestation) | 27 ± 8 | 29 ± 6 | 0.04 |
| Previous pregnancy, n (%) | 53 (46) | 33 (45) | 0.86 |
| Twin pregnancy, n (%) | 5 (4) | 4 (5) | 0.74 |
| Gestational hypertension, n (%) | 3 (3) | 7 (10) | 0.05 |
| Gestational diabetes, n (%) | 23 (20) | 25 (34) | 0.03 |
| Preeclampsia, n (%) | 2 (2) | 6 (8) | 0.06 |
| Oxygenation variables at ICU admission | |||
| FiO2 (%) | 40 (30–51) | 70 (50–97) | < 0.001 |
| PaO2 (mmHg) | 81 (68–100) | 85 (70–120) | 0.06 |
| PaO2/FiO2 ratio | 198 (148–302) | 147 (96–206) | < 0.001 |
| SaO2 (%) | 96 (94–98) | 97 (94–98) | 0.79 |
| PaCO2 (mmHg) | 30 (27–33) | 31 (26–37) | 0.05 |
| pH | 7.44 (7.40–7.46) | 7.40 (7.31–7.44) | < 0.001 |
| Lactate (mmol/L) | 0.9 (0.7–1.4) | 1.0 (0.7–1.3) | 0.47 |
| Ventilatory management | |||
| Standard oxygenation, n (%) | 41 (36) | 0 (0) | < 0.001 |
| HFNO, n (%) | 70 (61) | 50 (68) | 0.32 |
| NIV, n (%) | 18 (16) | 23 (32) | 0.01 |
| Intubation, n (%) | 0 (0) | 73 (100) | < 0.001 |
| Awake prone positioning, n (%) | 5 (4) | 3 (4) | 1.00 |
| Awake prone positioning under HFNO, n (%) | 5 (7) | 3 (6) | 1.00 |
| Awake prone positioning under NIV, n (%) | 1 (5) | 0 (0) | 0.47 |
| Prone positioning, n (%) | 0 (0) | 41 (56) | < 0.001 |
| Number of prone positioning sessions | 0 (0) | 3 (1–6) | NA |
| Neuromuscular blocker agents, n (%) | 0 (0) | 64 (88) | < 0.001 |
| Venovenous ECMO, n (%) | 0 (0) | 15 (21) | < 0.001 |
| Veno-arterial ECMO, n (%) | 0 (0) | 3 (4) | 0.06 |
| Tracheostomy, n (%) | 0 (0) | 6 (8) | < 0.01 |
| Treatments during ICU stay for SARS-CoV-2 infection | |||
| Corticosteroids, n (%) | 100 (88) | 57 (78) | 0.08 |
| Dexamethasone, n (%) | 91 (91) | 55 (96) | |
| Prednisolone, n (%) | 3 (3) | 1 (2) | |
| Prednisone, n (%) | 3 (3) | 0 (0) | |
| Hydrocortisone, n (%) | 3 (3) | 1 (2) | |
| Tocilizumab, n (%) | 14 (12) | 11 (15) | 0.58 |
| Delays and duration of ventilatory support | |||
| Delay from onset of symptoms to hospital admission (days) | 7 (4–9) | 6 (3–7) | 0.26 |
| Delay from onset of symptoms to ICU admission (days) | 9 (7–10) | 8 (5–10) | 0.68 |
| Duration of HFNO (days) | 3 (2–4) | 2 (1–3) | 0.01 |
| Duration of NIV (days) | 4 (2–5) | 1 (1–3) | 0.01 |
| Duration of invasive mechanical ventilation (days) | 0 (0–0) | 9 (5–18) | < 0.001 |
Variables are summarized as mean ± standard deviation, median (interquartile range) or number (percentages)
Patients who were intubated < 24 h for fetal extraction only were considered non-intubated
CT computed tomography, ECMO Extracorporeal membrane oxygenation, FiO2 inspired oxygen fraction, HFNO high-flow nasal oxygen therapy, ICU intensive care unit, NA non-available, NIV non-invasive ventilation, PaO2 partial arterial pressure of oxygen, PaCO2 partial arterial pressure of carbon dioxide, SaO2 arterial oxygen saturation, SAPS simplified acute physiology score, SOFA sepsis-related organ failure assessment
Ventilatory management in COVID-19 pregnant women
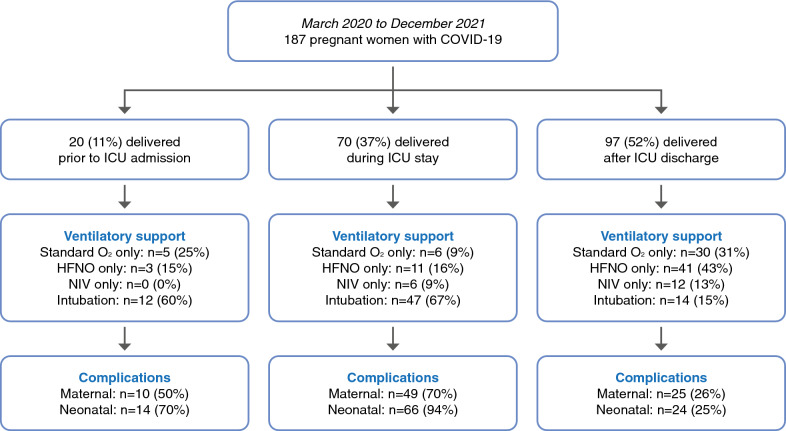
Standard oxygenation, HFNO and NIV were used as the only oxygenation technique in 41 (22%), 55 (29%) and 18 (10%) patients, respectively, and 73 (39%) patients were intubated. Overall, 72 (29%) patients required several oxygenation techniques, 64 (34%) received neuromuscular blocker agents and 15 (8%) patients required VV-ECMO (Table 1, Fig. 1). The VV-ECMO was implanted before delivery in 4 (27%) patients and after delivery in 11 (73%) patients. Awake prone positioning or prone positioning was performed in 49 (26%) patients and before delivery in 37% of them (Table 1).
Fig. 1.
Flowchart of the study summarizing ventilatory and obstetric management as well as maternal and neonatal complications in pregnant women with COVID-19 (n = 187). Maternal complications included postpartum hemorrhage, gynecologic infection and ICU-related complications. Neonatal complications included fetal or neonatal death, preterm birth, small for gestational age, organ failure or need for ICU admission. ICU intensive care unit, Standard O2 standard oxygenation, HFNO high-flow nasal oxygen, NIV non-invasive ventilation
The delay from the onset of symptoms and ICU admission to intubation was 9 (6–11) days and 1 (0–2) days, respectively: 3 (4%) patients were intubated in the first trimester, 26 (36%) in the second trimester and 44 (60%) in the third trimester. The duration of mechanical ventilation was 9 (5–18) days (Table 1). Among intubated patients, 50 (68%) were treated with HNFO and 23 (32%) with NIV as first-line ventilatory support, 64 (88%) received neuromuscular blocker agents and prone positioning was performed in 41 (56%) of them with a median of 3 (1–6) sessions (Table 1).
Risk factors of intubation in COVID-19 pregnant women during ICU stay
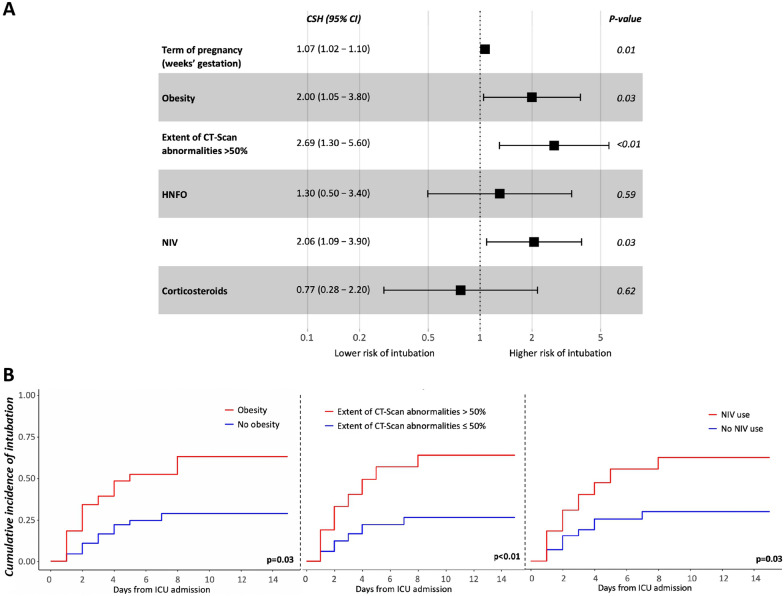
Obesity, pregnancy-related complications, a more advanced term of pregnancy and NIV use were more frequent in patients who required to be intubated (Table 1). In multivariate analysis, obesity (CSH 2.00, 95% CI (1.05–3.80), p = 0.03), term of pregnancy (CSH 1.07, 95% CI (1.02–1.10), per + 1 week gestation, p = 0.01), extent of CT scan abnormalities > 50% (CSH 2.69, 95% CI (1.30–5.60), p < 0.01) and NIV use (CSH 2.06, 95% CI (1.09–3.90), p = 0.03) were associated with a higher risk of intubation (Fig. 2).
Fig. 2.
A Risk factors for intubation during the intensive care unit stay (n = 145). CI Confidence interval, CSH cause-specific hazard ratio, HFNO high-flow nasal oxygen therapy, NIV non-invasive ventilation. B Cumulative incidence of intubation after admission in intensive care unit (ICU) according to obesity, extent of CT scan abnormalities > 50% and non-invasive ventilation (NIV) use
Obstetric management and maternal and neonatal outcomes in COVID-19 pregnant women
Delivery occurred before ICU admission in 20 (11%) patients, during ICU stay in 70 (37%) patients and after ICU discharge in 97 (52%) patients, including one patient who decided to voluntarily terminate her pregnancy after ICU discharge (Table 2, Fig. 1). Among the 70 patients who delivered during ICU stay, 47 (67%) were intubated, 6 (9%) were treated by NIV, 11 (15%) by HFNO and 6 (9%) patients received standard oxygenation. Indications for delivery during ICU stay were maternal respiratory worsening in 56 (80%) patients, fetal distress in 7 (10%) patients and spontaneous delivery in 7 (10%) patients. Except for the patients who delivered spontaneously, all deliveries required cesarean section (Table 2).
Table 2.
Obstetric management and maternal/neonatal outcomes in pregnant women with COVID-19
| No intubation (n = 114) | Intubation (n = 73) | p value | |
|---|---|---|---|
| Obstetric management | |||
| Timing of delivery | |||
| Term of delivery (weeks’ gestation) | 37 (34–39) | 31 (28–36) | < 0.001 |
| Delivery before ICU admission, n (%) | 8 (7) | 12 (16) | 0.04 |
| Delivery during ICU stay, n (%) | 23 (20) | 47 (64) | < 0.001 |
| Delivery after ICU discharge, n (%) | 83 (73) | 14 (19) | < 0.001 |
| Indications and modalities of delivery during ICU staya | |||
| Maternal respiratory worsening, n (%) | 16 (70) | 40 (85) | 0.20 |
| Fetal distress, n (%) | 2 (9) | 5 (11) | 1.00 |
| Spontaneous delivery, n (%) | 5 (22) | 2 (4) | 0.03 |
| Cesarean birth, n (%) | 19 (83) | 44 (94) | 0.21 |
| Maternal outcomes and complications | |||
| Mortality, n (%) | 0 (0) | 2 (3) | 0.15 |
| ICU length of stay (days) | 3 (2–5) | 13 (8–27) | < 0.001 |
| Hospital length of stay (days) | 10 (8–16) | 24 (15–40) | < 0.001 |
| Obstetric complications | |||
| Postpartum hemorrhage, n (%) | 5 (4) | 6 (8) | 0.34 |
| Gynecologic infection, n (%) | 2 (2) | 13 (18) | < 0.001 |
| ICU complications | |||
| Pulmonary embolism, n (%) | 3 (3) | 16 (22) | < 0.001 |
| Pneumoniae, n (%) | 20 (18) | 49 (67) | < 0.001 |
| Urinary tract infection, n (%) | 3 (3) | 9 (12) | 0.01 |
| Catheter infection, n (%) | 2 (2) | 5 (7) | 0.11 |
| Severe hemorraghe, n (%) | 1 (1) | 9 (12) | < 0.01 |
| Neonatal outcomes and complications | |||
| Fetal or neonatal mortality, n (%) | 3 (3) | 5 (7) | 0.27 |
| Term of delivery | |||
| Miscarriage < 20 weeks’ gestation, n (%) | 2 (2) | 2 (3) | 0.65 |
| Stillbirth > 20 week’s gestation, n (%) | 1 (1) | 2 (3) | 0.56 |
| Preterm birth (< 32 weeks’ gestation)b, n (%) | 9 (8) | 38 (52) | < 0.001 |
| Preterm birth (< 37 weeks’ gestation)c, n (%) | 25 (22) | 54 (74) | < 0.001 |
| Full-term birth, n (%) | 88 (77) | 19 (26) | < 0.001 |
| Birth’s weight (g) | 3060 (2375–3495) | 1902 (1275–2968) | < 0.001 |
| Small for gestational age, n (%) | 1 (1) | 8 (11) | < 0.01 |
| Organ failure, n (%) | 16 (14) | 28 (38) | < 0.001 |
| ICU admission, n (%) | 21 (19) | 38 (54) | < 0.001 |
| ICU length of stay (days) | 8 (3–17) | 22 (6–44) | 0.09 |
| Hospital length of stay (days) | 6 (3–14) | 41 (8–65) | < 0.001 |
Variables are summarized as median (interquartile range) or number (percentages)
Patients who were intubated < 24 h for fetal extraction only were considered non-intubated
ICU intensive care unit
aAmong patients who were delivered during ICU stay: n = 23 for “no intubation" group and n = 47 for “intubation” group
bPreterm birth at < 32 weeks’ gestation included both live and stillbirths at > 20 weeks’ gestation but < 32 weeks’ gestation
cPreterm birth at < 37 weeks’ gestation included both live and stillbirths at > 20 weeks’ gestation but < 37 weeks’ gestation
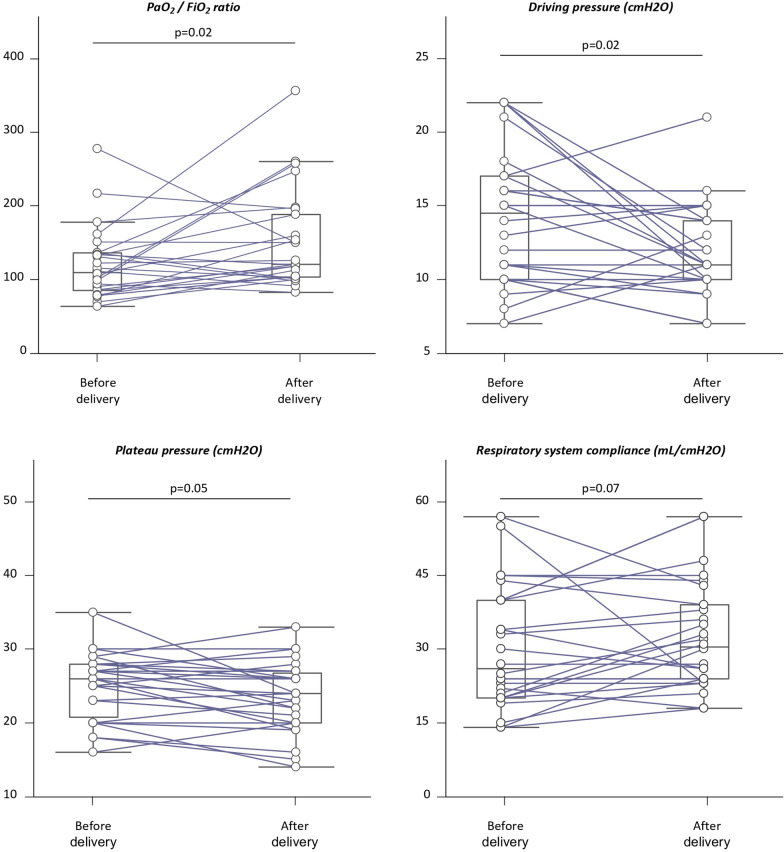
Delivery during ICU stay (64% in intubated vs. 20% in non-intubated patients, p < 0.001), cesarean section and preterm birth were more frequent in case of maternal intubation (Table 2). Overall, delivery significantly increased PaO2/FiO2 ratio by 9% (p = 0.02) and significantly decreased the driving pressure by 27% (p = 0.02). There was also a trend towards an 8% decrease in plateau pressure (p = 0.05) and a 26% increase in respiratory system compliance (p = 0.07), whereas the other ventilatory and oxygenation parameters remained unchanged (Table S2, Fig. 3). The 10 patients with decreased driving pressure tended to be more frequently obese (80 vs. 56%, p = 0.19), while other patient characteristics did not differ.
Fig. 3.
Effects of delivery on respiratory mechanics and oxygenation in intubated pregnant women with COVID-19 (n = 27). The box shows the 25th and 75th percentiles, the line in the box the median and the whiskers the minimum and maximum values. Lines represent the individual changes. FiO2 inspired oxygen fraction, PaO2 partial arterial pressure of oxygen
The maternal ICU mortality rate was 1%, 26 (14%) patients had obstetric complications and 117 (62%) had ICU-related complications. The main ICU-related complications were infection and pulmonary embolism (47% and 10% of patients, respectively) (Table 2). The fetal and neonatal mortality rate was 4% with 4 (2%) miscarriage at < 20 weeks’ gestation, 3 (2%) stillbirth at > 20 weeks’ gestation, 47 (25%) preterm birth at < 32 weeks, 79 (42%) preterm birth at < 37 weeks and 107 (57%) full-term birth. Overall, 59 (32%) neonates required ICU admission with an ICU length of stay of 15(4–42) days, 44 (24%) presented at least one organ failure and 9 (5%) were small for gestational age (Table 2). The rate of maternal and/or neonatal complications increased with the invasiveness of maternal ventilatory support (Table 2, Fig. S1).
Discussion
To our knowledge, we report the first international and largest cohort of pregnant women admitted in ICU for a severe form of SARS-CoV-2 infection. We found that 8% of pregnant women with COVID-19 required to be admitted to ICU. Corticosteroids, tocilizumab and prone positioning were used in few pregnant women with COVID-19, over a third of patients required intubation and delivery improved the driving pressure and oxygenation. Term of pregnancy, obesity, extent of CT scan abnormalities > 50% and NIV use were associated with a higher risk of intubation. Despite low maternal and fetal/neonatal mortality rates, the rate of maternal and/or neonatal complications increased with the invasiveness of maternal ventilatory support.
Non-invasive ventilatory support was mainly preferred as first-line ventilatory support but 39% of patients required to be intubated although all of them received corticosteroids. This rate of intubation is similar to the 30% rate shown in pregnant women with pneumonia non-related to SARS-CoV-2 infection [17] but much lower than the 63% rate found in a recent small cohort of 26 critically ill pregnant women with COVID-19 [18]. This discrepancy should be considered with caution as the intubation rate depends on ICU admission criteria and the ventilatory and/or obstetric management of patients, which may differ from country to country due to potentially different local organization of care in the absence of strong international recommendations. Risk factors for intubation were term of pregnancy, obesity, extent of CT scan abnormalities > 50% and NIV use. Previous studies showed that obesity was a risk factor of intubation in pregnant women with COVID-19 [5, 8] and that NIV but not HFNO use was not associated with a reduction in oxygenation failure in a large cohort of COVID-19 [26], probably because NIV was mainly used as a rescue therapy before intubation, as this was the case in our cohort. Awake prone positioning or prone positioning was performed in 26% of patients and before delivery in only 37% of them, although both of these postural maneuvers improve oxygenation in COVID-19 and non-COVID-19 patients [21, 22, 28], suggesting that physicians feared potential difficulties with fetal monitoring [29].
We found that 84% and 15% of pregnant women with COVID-19 received corticosteroids and tocilizumab, respectively, for SARS-CoV-2 infection, whereas the proportion of patients receiving corticosteroids and Tocilizumab varies from 12 to 100% and 0 to 100% in the existing literature [10, 18, 25]. These differences between the different studies and the low proportion we found in our cohort may be explained by the lack of strong recommendation, which may result in significant variability in practice between countries and centers within a country regarding indications and timing of administration.
Regarding obstetric management, 37% of patients had to be delivered during their ICU stay, mainly due to maternal respiratory worsening, and cesarean section was used in 90% of cases. Delivery improved both maternal respiratory mechanics (decreased driving pressure) and oxygenation (increased PaO2/FiO2 ratio). To our knowledge, this is the first study to report such effects on driving pressure. However, these results should be considered with caution due to the large interindividual variability and limited sample size for these analyses, and larger studies are needed to confirm these potential beneficial effects of delivery on respiratory mechanics. We also found a 42% rate of preterm births at < 37 weeks’ gestation. Preterm births resulted from spontaneous delivery in 10% of cases and from induced deliveries due to maternal respiratory worsening and/or fetal distress in 90% of cases. Our results are consistent with the existing literature, reporting a rate of delivery during ICU stay for maternal respiratory worsening ranging from 38 to 69% with a rate of cesarean section from 54 to 86% [8, 17, 18] and a rate of prematurity ranging from 27 to 48% [8, 17]. Compared to non- or less severe pregnant women with COVID-19 [5, 9, 25, 30–32], we found a higher rate of maternal and neonatal complications, which increased with the invasiveness of maternal ventilatory support. Maternal complications appeared to be due to ICU-related complications rather than obstetric complications, the most common being infections and pulmonary embolism. Rather than a direct fetal impact of SARS-CoV-2 infection, neonatal complications were most probably related to the severity of the maternal respiratory failure, leading to an increased risk of prematurity [2, 5, 7–10]. Thus, delivery during ICU stay and preterm birth were more frequent in case of maternal intubation.
Our results may suggest that all pregnant women with COVID-19 requiring ICU admission should be systematically admitted to a hospital with units capable of managing preterm neonates, given the 42% incidence of preterm births at < 37 weeks. Moreover, the respiratory management in pregnant women with a severe form of SARS-CoV-2 infection might be similar to that of other patients with COVID-19 and that non- or minimally invasive oxygenation strategies might be preferred. In the most severe patients with persistent or refractory hypoxemia delivery might be considered to improve maternal respiratory mechanics and oxygenation. However, the exact timing of delivery should always be discussed between intensivists, obstetricians and pediatricians.
We acknowledge some limitations to our study. First, standards of care have changed over the different pandemic waves and ventilatory and obstetric management of pregnant women may differ between the different participating centers. However, we used a competing risk model that took into account some of these changes to strengthen our results. Second, data on SARS-CoV-2 variants were not available at all centers and it was, therefore, not possible to assess the impact of each variant on maternal and neonatal outcomes. Third, our results are not generalizable to newly emerging SARS-CoV-2 variants and to pregnant women who have received immunomodulatory treatments and/or who have been vaccinated. It has been suggested very recently that vaccination may be associated with lower maternal respiratory severity without adverse peripartum outcomes [33–35]. Finally, we did not study the management of analgesia during labor in patients who were not intubated for delivery and further studies are needed to specifically address this issue.
Conclusions
Corticosteroids, tocilizumab and prone positioning were used in few pregnant women with COVID-19 admitted to ICU. Over a third of patients were intubated and had to be delivered during ICU stay, mainly due to maternal respiratory worsening, which improved the driving pressure. Despite low maternal and fetal/neonatal mortality rates, delivery during ICU stay and preterm birth were more frequent in case of maternal intubation and the rate of maternal and/or neonatal complications increased with the invasiveness of maternal ventilatory support.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the members of the COVIDPREG study group: Laurent Argaud, MD PhD (Hospices Civils de Lyon, Hôpital Edouard Herriot, Service de Médecine Intensive-Réanimation, Lyon, France); Cécile Aubron, MD PhD (Service de Médecine Intensive et Réanimation, CHU de Brest, 29200 Brest, France; Université de Brest, Inserm, EFS, UMR 1078, GGB, 29200 Brest, France); Nicolas Bèle, MD (Service de réanimation, Hôpital de Fréjus, 83608 Fréjus, France); François Beloncle, MD PhD (Département de Médecine Intensive—Réanimation et Médecine Hyperbare, Vent’Lab, CHU d’Angers, Université d’Angers, Angers, France); Pierre-Marie Bertrand, MD (Service de Médecine Intensive et Réanimation, Centre Hospitalier de Cannes, 15 avenue des Broussailles, 06400 Cannes); Laetitia Bodenes, MD (Service de Médecine Intensive et Réanimation, CHU de Brest, 29200 Brest, France); Filippo Boroli, MD (Division of Intensive Care, Geneva University Hospitals, 4 Rue Gabrielle Perret-Gentil, 1211 Geneva 14, Switzerland); Belaid Bouhemad, MD PhD (Service de réanimation chirurgicale, Centre Hospitalier Universitaire de Dijon, Dijon, France); Lucie Braconnier, MD (Pôle de Médecine Intensive et Réanimation, Hôpital Roger Salengro, CHU Lille, Lille, France); William Buffieres, MD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Universitaire Purpan, 31300 Toulouse, France); Cédric Darreau, MD (Service de réanimation médico-chirurgicale et USC, centre hospitalier le Mans, 194 avenue Rubillard 72037 le Mans cedex 9, France); Jean Dellamonica, MD PhD (Service de Médecine Intensive Réanimation, Centre Hospitalier Universitaire de Nice, Hôpital l’Archet 1, 151 rue saint Antoine de Ginestière, 06200 Nice, France; Équipe 2 CARRES, UR2CA—Unité de Recherche Clinique Côte d'Azur, Université Côte d’Azur, Nice, France); Stephan Ehrmann, MD PhD (Service de Médecine Intensive et Réanimation, CHRU de Tours, France; INSERM CIC 1415, CRICS-TriGGERSep F-CRIN research network, CHRU de Tours, Tours, France; Centre d'Étude des Pathologies Respiratoires (CEPR) INSERM U1100, Université de Tours, Tours, France); Mélanie Faure, MD (Service de Médecine Intensive et Réanimation, CHRU de Tours, France); Sébastien Gibot, MD PhD (Service de Médecine Intensive et Réanimation, Hôpital central, Nancy, France); Claudine Gniadek, RN (Service de Médecine Intensive et Réanimation, CHU de Lapeyronie, Montpellier, France); Marine Goudelin, MD (Service de réanimation médico-chirurgicale, CHU Dupuytren, 87000 Limoges, France); Pierre-Alban Guenier, MD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Universitaire de Saint Etienne, France); Christophe Guitton, MD PhD (Service de réanimation médico-chirurgicale et USC, centre hospitalier le Mans, 194 avenue Rubillard 72037 le Mans cedex 9, France); Etienne Haussaire, MD (Hospices Civils de Lyon, Hôpital Edouard Herriot, Service de Médecine Intensive-Réanimation, Lyon, France); Julie Helms, MD PhD (Service de Médecine Intensive et Réanimation, Nouvel Hôpital Civil, Strasbourg, France); Matthieu Jamme, MD (Réanimation polyvalente, hôpital privé de l’ouest parisien, Ramsay Générale de Santé, Trappes, France; CESP, Équipe Épidémiologie clinique, INSERM U1018, Villejuif, France); Michel Kaidomar, MD (Service de réanimation, Hôpital de Fréjus, 83608 Fréjus, France); Jean-Baptiste Lascarrou, MD PhD (Service de Médecine Intensive et Réanimation, CHU Nantes, Nantes, France); Christophe Le Terrier, MD (Division of Intensive Care, Geneva University Hospitals, 4 Rue Gabrielle Perret-Gentil,1211 Geneva 14, Switzerland); Julien Maizel, MD PhD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Universitaire d’Amiens, France); Ferhat Meziani, MD PhD (Service de Médecine Intensive et Réanimation, Nouvel Hôpital Civil, Strasbourg, France); Jean-Paul Mira, MD PhD (Service de Médecine Intensive et Réanimation, Hôpitaux Universitaires Paris Centre, Hôpital Cochin, Assistance Publique—Hôpitaux de Paris, 27 rue du faubourg Saint Jacques, 75014 Paris, France; Université de Paris, Paris, France); Lucas Morand, MD (Service de Médecine Intensive Réanimation, Centre Hospitalier Universitaire de Nice, Hôpital l’Archet 1, 151 rue saint Antoine de Ginestière, 06200 Nice, France; Équipe 2 CARRES, UR2CA—Unité de Recherche Clinique Côte d’Azur, Université Côte d’Azur, Nice, France); Grégoire Muller, MD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Régional d'Orléans, Orléans, France); Benjamin Pequignot, MD (Service de Médecine Intensive et Réanimation, Hôpital Brabois, CHRU Nancy, Vandœuvre-lès-Nancy, France); Gaël Piton, MD PhD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Universitaire de Besançon, Besançon, France); Jérôme Pugin, MD PhD (Division of Intensive Care, Geneva University Hospitals, 4 Rue Gabrielle Perret-Gentil, 1211 Geneva 14, Switzerland; University of Geneva Faculty of Medicine, 4 Rue Michel Servet, 1204 Geneva, Switzerland); Alexandre Robert, MD (Service de Médecine Intensive et Réanimation, Centre Hospitalier de Cannes, 15 avenue des Broussailles, 06400 Cannes, France; Université Côte d'Azur, INSERM U1065, Laboratoire C3M, Nice, France); Michael Siino, MD (Service de réanimation, Hôpital de Fréjus, 83608 Fréjus, France); Bertrand Souweine, MD PhD (Service de Médecine Intensive et Réanimation, CHU Gabriel-Montpied, Clermont-Ferrand, France); Mattieu Stanowski, MD (Service de Médecine Intensive et Réanimation, Hôpital central, Nancy, France); Fabienne Tamion, MD PhD (Service de Médecine Intensive et Réanimation, CHU Rouen, 76000 Rouen, France; Normandie Université, UNIROUEN, Inserm U1096, FHU-REMOD-VHF, 76000 Rouen, France); Nicolas Terzi, MD PhD (Service de Médecine Intensive et Réanimation, CHU Grenoble-Alpes, Grenoble, France; INSERM, U1042, Université Grenoble-Alpes, HP2, Grenoble, France); Guillaume Thiery, MD PhD (Service de Médecine Intensive et Réanimation, Centre Hospitalier Universitaire de Saint Etienne, France; Research on Healthcare Performance RESHAPE, INSERM U1290, Université Claude Bernard Lyon 1).
Author contributions
EP, FB and MJ conceived and designed the study. All the authors and members of the COVIDPREG study group collected data in each participating center. EP, FB and MJ analyzed the data. EP, FB and MJ drafted the first version of the manuscript. All the authors contributed drafting the manuscript and approved the final version of the manuscript.
Funding
None.
Availability of data and materials
All data are available after request to EP and MJ.
Declarations
Conflicts of interest
The authors have no conflict of interest to declare.
Footnotes
The members of the COVIDPREG Study Group are listed in Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mathieu Jozwiak, Email: jozwiak.m@chu-nice.fr.
the COVIDPREG Study Group:
Laurent Argaud, Cécile Aubron, Nicolas Bèle, François Beloncle, Pierre-Marie Bertrand, Laetitia Bodenes, Filippo Boroli, Belaid Bouhemad, Lucie Braconnier, William Buffieres, Cédric Darreau, Jean Dellamonica, Stephan Ehrmann, Mélanie Faure, Sébastien Gibot, Claudine Gniadek, Marine Goudelin, Pierre-Alban Guenier, Christophe Guitton, Etienne Haussaire, Julie Helms, Matthieu Jamme, Michel Kaidomar, Jean-Baptiste Lascarrou, Christophe Le Terrier, Julien Maizel, Ferhat Meziani, Jean-Paul Mira, Lucas Morand, Grégoire Muller, Benjamin Pequignot, Gaël Piton, Jérôme Pugin, Alexandre Robert, Michael Siino, Bertrand Souweine, Mattieu Stanowski, Fabienne Tamion, Nicolas Terzi, and Guillaume Thiery
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornish EF, Filipovic I, Åsenius F, Williams DJ, McDonnell T. Innate immune responses to acute viral infection during pregnancy. Front Immunol. 2020;11:572567. doi: 10.3389/fimmu.2020.572567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger SE, Weiss ST, Cohen WR, Weiss JW, Johnson TS. Pregnancy and the lung. Am Rev Respir Dis. 1980;121:559–581. doi: 10.1164/arrd.1980.121.3.559. [DOI] [PubMed] [Google Scholar]
- 5.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBolt CA, Bianco A, Limaye MA, Silverstein J, Penfield CA, Roman AS, et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224:510.e1–510.e12. doi: 10.1016/j.ajog.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223:914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayem G, Lecarpentier E, Deruelle P, Bretelle F, Azria E, Blanc J, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49:101826. doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crovetto F, Crispi F, Llurba E, Pascal R, Larroya M, Trilla C, et al. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021;73:1768–1775. doi: 10.1093/cid/ciab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225:77.e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellington S. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huntley BJF, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136:303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 13.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K (2010) Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 303:1517–1525. https://www.google.com/search?client=firefox-b-d&q=Siston+A.M.%2C+Rasmussen+S.A.%2C+Honein+M.A.%2C+Fry+A.M.%2C+Seib+K.+Pandemic+2009+influenza+A%28H1N1%29+virus+illness+among+pregnant+women+in+the+United+States.+JAMA.+2010%3B303%3A1517%E2%80%931525. Accessed 29 Aug 2021 [DOI] [PMC free article] [PubMed]
- 14.Elshafeey F, Magdi R, Hindi N, Elshebiny M, Farrag N, Mahdy S, et al. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int J Gynaecol Obstet. 2020;150:47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 16.Lapinsky SE, Rojas-Suarez JA, Crozier TM, Vasquez DN, Barrett N, Austin K, et al. Mechanical ventilation in critically-ill pregnant women: a case series. Int J Obstet Anesth. 2015;24:323–328. doi: 10.1016/j.ijoa.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. 2020;2:100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Genderen ME, van Uitert E, Duvekot JJ, Gommers D, COVPREG Study Group Management and outcome of critically ill pregnant women with COVID-19. Intensive Care Med. 2022 doi: 10.1007/s00134-022-06653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prise en charge aux urgences maternité d’une patiente enceinte suspectée ou infectée par le coronavirus (covid-19) [internet]. Collège National des Gynécologues et Obstétriciens Français; [cited 2020 March]. https://syngof.fr/wp-content/uploads/2020/03/COVID-19-CNGOF.pdf
- 20.Recommandations régionales Covid-19. Femmes enceintes et prise en charge en soins critiques [internet]. Agence Régionale de Santé Ile de France; [cited 2021 April]. https://www.iledefrance.ars.sante.fr/system/files/2021-04/011_2021-04-23_Doctrine_Femmes-enceintes_Soins-critiques-v3def.pdf
- 21.Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coudroy R, Frat J-P, Girault C, Thille AW. Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask. Thorax. 2020;75:805–807. doi: 10.1136/thoraxjnl-2020-214863. [DOI] [PubMed] [Google Scholar]
- 24.Shang Y, Xu C, Jiang F, Huang R, Li Y, Zhou Y, et al. Clinical characteristics and changes of chest CT features in 307 patients with common COVID-19 pneumonia infected SARS-CoV-2: a multicenter study in Jiangsu, China. Int J Infect Dis. 2020;96:157–162. doi: 10.1016/j.ijid.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators (2021) Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 47:60–73. 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed]
- 27.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Guérin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong MJ, Bharadwaj S, Lankford AS, Galey JL, Kodali BS. Mechanical ventilation and prone positioning in pregnant patients with severe COVID-19 pneumonia: experience at a quaternary referral center. Int J Obstet Anesth. 2021 doi: 10.1016/j.ijoa.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256–e2029256. doi: 10.1001/jamanetworkopen.2020.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman M, Navér L, Söderling J, Ahlberg M, Hervius Askling H, Aronsson B, et al. Association of maternal SARS-CoV-2 infection in pregnancy with neonatal outcomes. JAMA. 2021;325:2076–2086. doi: 10.1001/jama.2021.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara A, Hedderson MM, Zhu Y, Avalos LA, Kuzniewicz MW, Myers LC, et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022 doi: 10.1001/jamainternmed.2022.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022 doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022 doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available after request to EP and MJ.