Abstract
Background
Resection of initially oligometastatic pancreatic ductal adenocarcinoma (PDAC) following response to first-line chemotherapy is controversial. We herein updated a previous case series to investigate the oncologic outcomes and preoperative factors that could drive the decision-making process.
Methods
This retrospective analysis was limited to patients with liver-only synchronous metastases who experienced complete regression of the metastatic component and underwent pancreatectomy between October 2008 and July 2020 at two high-volume institutions. Clinical-pathologic variables were captured, and inflammation-based prognostic scores were calculated. Recurrence and survival analyses were performed using standard statistical methods.
Results
Overall, 52 patients were included. FOLFIRINOX was the most employed chemotherapy regimen (63.5%). Post-treatment tumor size, serum carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA) were significantly decreased relative to baseline evaluation. The median time from diagnosis to pancreatectomy was 10.2 months, while the median time from chemotherapy completion to pancreatectomy was 2 months. Major postoperative complications occurred in 26.9% of patients, while postoperative mortality was nil. The median disease-free survival (DFS) and overall survival (OS) from pancreatectomy were 16.5 and 23.0 months, respectively, and the median OS from diagnosis was 37.2 months. At multivariable analysis, vascular resection, operative time, prognostic nutrition index (PNI) and neutrophil-to-lymphocyte ratio (NLR) were associated with OS. Operative time, platelet × neutrophil/lymphocyte count (SII), and PNI were associated with DFS.
Conclusions
We confirm promising outcomes of selected patients who underwent pancreatectomy following downstaging of liver metastases. The absence of vascular involvement of the primary tumor, good nutritional status, and low inflammatory index scores could be useful to select candidates for resection.
According to the current cancer statistics,1 nearly 50% of patients with pancreatic ductal adenocarcinoma (PDAC) present with metastatic disease, mainly to the liver. Although the diagnosis of metastatic disease has always been considered as an absolute contraindication for resection,2 there have been various reports of pancreatectomies with synchronous liver metastasectomy in patients with low metastatic burden (up to three lesions, defined as oligometastatic PDAC hereafter). This practice, resulting in median overall survival (OS) duration in the range from 7.6 to 14.5 months,3–10 did not prompt the uptake of a selective resection policy because primary chemotherapy with FOLFIRINOX or gemcitabine + nanoalbumin-bound(nab)-paclitaxel was associated with intention-to-treat median OS ranging from 8 to 12 months.11,12 Owing to the high response rate of these multiagent regimens, several patients with initially oligometastatic PDAC experienced substantial reduction of the metastatic burden, up to complete downstaging. Whether resection in this selected group of responders could be associated with improved prognosis has been a matter of further debate.13–18 In a previous paper including 24 patients who underwent resection following chemotherapy at the authors’ institutions, we showed a margin-free resection rate of 88%, a 17% rate of complete pathologic responses, a median disease-free survival (DFS) of 27 months, and a median OS of 56 months.19 Subsequent systematic reviews with analyses limited to patients receiving first-line chemotherapy showed median survival outcomes ranging from 23 to 56 months.20,21
On these premises, we updated our previous series and investigated the outcomes of patients with PDAC initially metastatic to the liver who underwent resection following first-line chemotherapy and complete regression of the metastatic component. We also sought to identify preoperative factors that could drive the decision-making process in this challenging clinical scenario.
Patients and Methods
All patients with PDAC initially metastatic to the liver who received systemic chemotherapy and subsequent resection between October 2008 and July 2020 at the Unit of Pancreatic Surgery, Pederzoli Hospital, Peschiera del Garda, Verona, and the Unit of Pancreatic Surgery, University of Verona Hospital Trust, Italy, were retrospectively analyzed from a prospectively collected database. Following baseline diagnosis, patients received first-line systemic chemotherapy either at the two hub centers or at spoke, local institutions, according to the patient’s area of residence. The hub centers assisted with chemotherapy regimen recommendation and patient follow-up, which was planned on a 3-month basis. Following restaging, patients were re-evaluated by the hub centers’ multidisciplinary boards. Criteria for surgical eligibility were disappearance of liver metastases at cross-sectional imaging, consisting of triple phase, thin-slice computed tomography (MDCT) and gadoxetic acid-enhanced magnetic resonance imaging (MRI) with diffusion-weighted imaging. Fluorodeoxyglucose–positron emission tomography (18FDG–PET) was performed to functionally characterize a persistent liver nodule. Only patients with negative 18FDG-PET were considered surgical candidates. Furthermore, in secretors, a serum carbohydrate antigen (CA19-9) decrease threshold >50% relative to baseline was employed to define biochemical response.
Perioperative Management
The intraoperative strategy has been previously described.19 Demographic and perioperative data included chemotherapy regimen, duration of treatment, time between diagnosis and surgery, time between last chemotherapy and surgery, postoperative pancreatic fistula (POPF), postoperative bleeding (PPH), delayed gastric emptying (DGE), operative time, blood transfusion, postoperative length of stay (LOS), and 30-day mortality. Complications were defined according to the International Study Group of Pancreatic Surgery (ISGPS)22–24 and their severity was classified per the Clavien– Dindo system.25
After discharge, all patients were referred for adjuvant chemotherapy if indicated. Follow-up was planned on a 3-month basis with triple-phase MDCT scan or MRI, serum CA19-9, and outpatient or telehealth evaluation due to distance or coronavirus disease 2019 (COVID-19)-related travel restrictions. Inflammation-based prognostic scores known to have a role in cancer progression, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), platelet × neutrophil/lymphocyte count (SII), and prognostic nutrition index (PNI; 10 × s-albumin g/dL + 0.005 × total lymphocyte in peripheral blood/mm3), were calculated at baseline and before surgery.26 Pathologic data included residual tumor evaluation, nodal status, tumor stage per American Joint Committee on Cancer (AJCC) criteria,27 margin status, and vascular and perineural infiltration. Follow-up details included date and first site of recurrence, recurrence treatment, and date of death. OS was calculated from the time of diagnosis and from the time of pancreatectomy, while DFS was calculated from the time of pancreatectomy.
Statistical Analysis
Continuous variables were reported as means and standard deviation or median and interquartile range (IQR), as appropriate. Student’s t-test or Mann–Whitney U test were used to compare continuous variables. Categorical variables were reported as frequencies with percentages and compared using Fisher’s exact test. Survival and follow-up were calculated from the time of diagnosis to the date of death or last follow-up. Cumulative survival was analyzed using the Kaplan–Meier method. Univariable and multivariable Cox regression models were employed to investigate variables associated with survival. The predictive values of the inflammation-based prognostic scores at the time of recurrence were evaluated by receiver operating characteristic (ROC) analysis. Prognostic accuracy was assessed by calculating the area under the curve (AUC). Statistical analysis was performed using SPSS v.25 (IBM Corporation, Armonk, NY, USA).
Results
Overall, 52 patients were included in the present analysis. Baseline demographics and clinicopathologic characteristics are summarized in Table 1. At the time of diagnosis, 73.1% of patients had more than 2 liver metastases, and in 54.2% of patients the primary tumor was anatomically resectable per National Comprehensive Cancer Network (NCCN) guidelines. FOLFIRINOX was the most employed chemotherapy regimen (63.5% of cases), while 26.9% of patients received gemcitabine + nab-paclitaxel and only 9.6% received gemcitabine alone. Data on adjuvant chemotherapy were available for 32/53 patients (61.5%). Baseline nutritional data and inflammation scores are reported in Table 1. Post-treatment CA19-9 and carcinoembryonic antigen (CEA) were markedly decreased relative to baseline values (from 11,167.2 U/mL to 50.6 U/mL, p < 0.001; and from 6.7 ng/mL to 4.4 ng/mL, p = 0.042). Notably, 67.3% of patients had normalized CA19-9 post-treatment. Primary tumor size was also markedly reduced, from 32.6 to 17.6 mm (p < 0.002). The median interval between diagnosis and surgery was 10.2 months (range 3–32), while the median interval between chemotherapy completion and pancreatectomy was 2 months. Time-trend analysis showed a reduction in the period off systemic therapy (from 3.8 months in 2008–2014 to 1.8 months in 2015–2020, respectively). Resection procedures included pancreatoduodenectomy (PD, 69%), distal pancreatectomy (DP, 27%), and total pancreatectomy (TP, 4%) (Table 2). Segmental vascular resections were performed in 7 patients (13.5%). The overall morbidity and 30-day mortality rates were 57.7% and 0%, respectively. Major complications (Clavien–Dindo III–IV) occurred in 26.9% of cases, the POPF rate was 13.4%, and no patients required reoperation. The mean LOS was 14.6 days (IQR 5–60). Histopathological examination showed complete response in 17.3% of patients, and the R0 resection rate was 86.5%. The N0 rate was 53.9%, with a median lymph node ratio (LNR) of 0.04.
Table 1.
Baseline characteristics
| Age, years [median (range)] | 58 (34–77) |
| Sex [n (%)] | |
|
Male Female |
30 (57.7) 22 (42.3) |
| BMI [median (range)] | 24.4 (17.8–35.4) |
| Liver metastasis [n (%)] | |
|
One Two Multiple |
9 (17.3) 5 (9.6) 38 (73.1) |
| Tumor location [n (%)] | |
|
Head Body and tail |
36 (69.2) 16 (30.7) |
| Primary tumor resectability at diagnosis [n (%)] | |
| Resectable | 28 (54.2) |
| BLR/LAPC | 24 (45.8) |
| Primary chemotherapy regimen [n (%)] | |
| FOLFIRINOX | 33 (63.5) |
| Gemcitabine + nab-paclitaxel | 14 (26.9) |
| Gemcitabine | 5 (9.6) |
| Chemotherapy cycles [median (range)] | 9.4 (1–20) |
|
CA19-9, U/mL [median (range)] Baseline Restaging Normal value before surgery [n (%)] |
p < 0.001 1167.2 (0.6–9824) 50.6 (0.6–277) 35 (67.3) |
|
CEA, ng/mL [median (range)] Baseline Re-staging |
p < 0.042 32.7 (16-45) 17.6 (0-37) |
Significant results (p < 0.05) are highlighted in bold
BMI body mass index, BLR borderline resectable, LAPC locally advanced pancreatic cancer, CA carbohydrate antigen, CEA carcinoembryonic antigen
Table 2.
Perioperative data and pathologic details
| Surgical resection | ||
|
PD DP TP |
36 14 2 |
(69.2) (26.9) (3.8) |
| Vascular resection | ||
|
No Yes |
45 7 |
(86.5) (13.5) |
| Median operative time, min (range) | 370.9 | (130–620) |
| Intraoperative blood transfusion | ||
|
No Yes |
47 5 |
(90.4) (9.6) |
| Postoperative complications | ||
|
No Yes |
22 30 |
(42.3) (57.7) |
| Abdominal complications | ||
|
No Yes POPF PPH DGE Other* |
26 26 7 3 9 7 |
(50.0) (50.0) (13.4) (5.7) (1.3) (1.4) |
| Clavien–Dindo >II | ||
|
No Yes |
38 14 |
(73.1) (26.9) |
| Median postoperative stay, days (range) | 14.6 | (5–60) |
| Complete pathological response | ||
|
No Yes |
43 9 |
(82.7) (17.3) |
| R status | ||
|
R0 R1 |
45 7 |
(86.5) (13.5) |
| Microvascular embolization | ||
|
No Yes |
23 29 |
(44.2) (55.8) |
| Perineural infiltration | ||
|
No Yes |
20 32 |
(38.5) (61.5) |
| Nodal status | ||
|
N0 N1 N2 |
28 17 7 |
(53.9) (32.7) (13.4) |
| LNR [median (range)] | 0.04 | (0.00–0.6) |
Data are expressed as n (%) unless otherwise specified
PD pancreaticoduodenectomy, DP distal pancreatectomy, TP total pancreatectomy, POPF postoperative pancreatic fistula, PPH postoperative hemorrhage, DGE Delay Gastric Emptying, LNR lymph node ratio (positive nodes/harvested nodes)
*Wound infection, anemia with no signs of bleeding, fever
Survival Analysis and Prognostic Factors
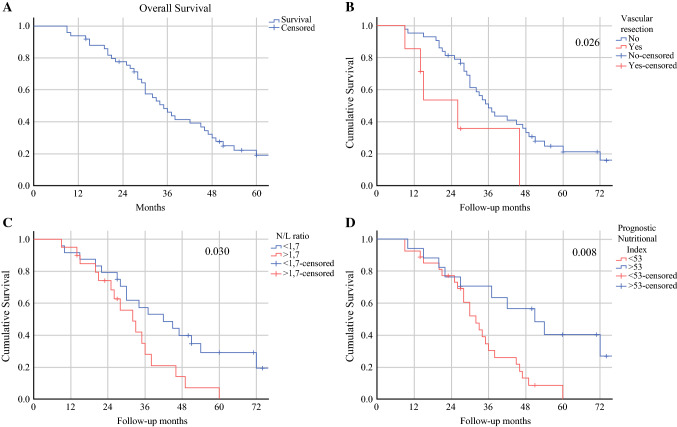
The median OS from the time of diagnosis was 37.2 months, while the median DFS and median OS post-pancreatectomy were 16.5 months and 23.0 months, respectively (Table 3, Fig. 1). Overall, 39/52 patients experienced recurrence, mainly in the liver (48.7%), followed by resection bed (18%), peritoneum (10.2%), other distant sites (7.7%), and multiple sites (15.4%). Complete pathological response did not provide a benefit in survival relative to patients with residual tumor (36 vs. 28 months; p = 0.972), as was for the subset of patients receiving chemotherapy for longer than 10 months (p = 0.291). On univariable analysis (Table 4), poor nutritional status, inflammation parameters (PNI, NLR, and SII), vascular resection, and omission of adjuvant chemotherapy were associated with disease recurrence. Vascular resection, length of operation, and microscopic vascular embolization were associated with shorter OS. On multivariable analysis (Table 5), vascular resection, operative time, PNI >53 and NLR <1.7, were independently associated with OS. Operative time, SII, and PNI were independently associated with DFS.
Table 3.
Survival and recurrence information following pancreatectomy
| OS, months (range) | 37.2 | (26–54) |
| DFS, months (range) | 16.5 | (6–25) |
| PFS, months (range) | 23.9 | (9–46) |
| Survival post-pancreatectomy, months (range) | 23.0 | (16–40) |
| Recurrence [n (%)] | ||
|
No Yes |
13 39 |
(25.0) (75.0) |
| Recurrence pattern [n (%)] | ||
|
Liver only Local only Peritoneal dissemination Other distant site Multisite |
19 7 4 3 6 |
(48.7) (18.0) (10.2) (7.7) (15.4) |
| Interval from initial diagnosis to pancreatectomy, months (range) | 10.2 | (3–32) |
| Interval from chemotherapy completion to pancreatectomy, months (range) | 2.0 | (1.2–2.8) |
| Adjuvant chemotherapy [n (%)] | ||
|
No Yes |
12 20 |
(37.5) (62.5) |
OS overall survival, DFS disease-free survival, PFS progression-free survival
Fig. 1.
a Overall survival stratified according to b vascular resection (p = 0.026), c NLR <1.7 (p = 0.030), and d Prognostic Nutritional Index >53 (p = 0.008). NLR neutrophil-to-lymphocyte ratio
Table 4.
Univariable analysis of factors associated with recurrence and overall survival
| Variable | Recurrence | Overall survival |
|---|---|---|
| CA19-9 pre-chemotherapy | 0.046 | 0.830 |
| CEA pre-chemotherapy | 0.059 | 0.292 |
| CA19-9 post-chemotherapy | 0.306 | 0.437 |
| CEA post-chemotherapy | 0.489 | 0.930 |
| Vascular resection | 0.014 | 0.029 |
| IO transfusion | 0.911 | 0.633 |
| Operation time 360 min | 0.787 | 0.010 |
| Postoperative complications | 0.572 | 0.910 |
| Microvascular embolization | 0.968 | 0.040 |
| Perineural infiltration | 0.339 | 0.190 |
| N+ | 0.213 | 0.216 |
| LNR | 0.218 | 0.171 |
| Chemotherapy type | 0.504 | 0.498 |
| Adjuvant chemotherapy N | 0.033 | 0.806 |
| NLR | 0.001 | 0.085 |
| NLR <1.7 | 0.001 | 0.314 |
| P/L ratio | 0.066 | 0.679 |
| SII | 0.009 | 0.389 |
| PNI | 0.125 | 0.093 |
| PNI >53 | 0.001 | 0.434 |
Significant results (p < 0.05) are highlighted in bold
NLR neutrophil-to-lymphocyte ratio, LNR lymph node ratio (positive nodes/harvested nodes), P/L platelets/lymphocyte, SII Systemic Inflammatory Index, PNI Prognostic Nutritional Index, CA carbohydrate antigen, CEA carcinoembryonic antigen
Table 5.
Multivariable analysis for the association of relevant variables with overall survival and disease-free survival
| OS >12 months | RR | OS >24 months | RR | DFS >6 months | RR | |
|---|---|---|---|---|---|---|
| Sex (male) | 0.128 | – | 0.026 | 2.2 (1.11–4.45) | 0.057 | – |
| No vascular resection | 0.005 | 3.6 (1.46–8.81) | 0.076 | – | 0.286 | – |
| Operation time <330 min | 0.039 | 1.8 (1.10–3.63) | 0.032 | 2.5 (1.10–5.64) | 0.143 | – |
| NLR <1.7 | 0.007 | 2.5 (1.30–4.93) | 0.019 | 2.5 (1.16–5.39) | 0.050 | 2.0 (1.01–3.95) |
| PNI >53 | 0.012 | 2.6 (1.23–5.30) | 0.008 | 3.1 (1.35–7.25) | 0.113 | – |
Significant results (p < 0.05) are highlighted in bold
OS overall survival, RR risk ratio, DFS disease-free survival, NLR neutrophil-to-lymphocyte ratio, PNI Prognostic Nutritional Index
Discussion
The present study updates a previous analysis of pancreatectomies for initially metastatic PDAC with complete response of liver metastases following first-line chemotherapy.19 Data from 52 patients showed acceptable perioperative outcomes, with a major complications rate of 26.9% and zero mortality. Complete pathologic responses were 17.3%, nearly 90% of patients received a margin-free resection. The median OS from the time of diagnosis was 37.2 months, while the median DFS and OS post-pancreatectomy were 16.5 months and 23 months, respectively. Most recurrences were in the liver (48.9%), although it was not possible to ascertain whether the disease relapsed at the initial metastatic site or as new lesion(s). Although direct comparison with our previous experience is not necessarily appropriate, the median survival duration herein reported is relatively shorter (37.2 vs. 56 months), yet similar to, other series of patients who underwent resection following primary chemotherapy.13–18 This reflects a strict selection process based on a combination of radiologic, biochemical and clinical parameters. At the time of diagnosis, high signal intensity on diffusion-weighted MRI was deemed enough to define metastatic liver lesions, with confirmation biopsy being performed in 21 patients (40.4%). In our recent practice, upfront pancreatectomy with synchronous metastasectomy was never an option. According to a systematic review of retrospective data, upfront resection is mostly carried out in patients unexpectedly found with low-burden metastatic disease at surgical exploration and was associated with median OS duration ranging from 7.6 to 14.5 months.20 This is comparable with intention-to-treat survival outcomes in randomized trials of first-line multiagent chemotherapy.11,12 Hence, it can be argued that the principle of upfront ‘cancer-directed surgery’ in oligometastatic PDAC does not portend better survival rates relative to chemotherapy alone, with the adjunct burden of a highly morbid surgical procedure.28
Following first-line chemotherapy for a median of nine cycles, only patients who experienced complete radiologic response of liver metastases were considered for surgical exploration by our multidisciplinary boards. In the absence of high-level evidence, the minimal acceptable degree of residual liver disease for attempting resection has not been clearly established. Notably, for some authors, stable disease post-treatment was not a contraindication to surgery, with a rate of synchronous single hepatic segment or atypical resections as high as 39%.2
Another lingering question in patients with oligometastatic PDAC is whether a specific chemotherapy regimen is associated with a greater response rate. Despite the fact that this should be better addressed in a prospective fashion, in the present series there was no survival difference between patients receiving platinum- or gemcitabine-based chemotherapy, such that the value of a treatment regimen as a surrogate endpoint for survival could not be ascertained, with the initial choice remaining at the discretion of the treating oncologist. Interestingly, the median interval from chemotherapy completion to pancreatectomy became shorter over time (<2 months in the last 5 years). While the impact of the chemotherapy holiday has never been investigated, it might be speculated that a certain period off-treatment helps excluding patients with unexpected disease progression on preoperative restaging.
Regarding biochemical response, there is no evidence as to whether the magnitude of post-treatment CA19-9 decline could aid in the patient selection process. In localized disease, some studies defined the optimal CA19-9 response as the presence of normal values post-treatment,29 while others showed that a decline in CA19-9 levels >50% was an independent predictor of post-resection survival.30,31 In the present series, the median difference between pre- and post-treatment values was >90%, and more than 60% of patients had normalized CA19-9 levels. Nonetheless, neither CA19-9 decrease nor post-treatment normalization were independently associated with survival. Even other well-documented prognostic factors, including N status, margin status, and complete pathologic response, did not impact survival. Whether this depends on a peculiar biologic behavior of initially metastatic PDAC or on the small sample size can only be speculated. Notably, patients undergoing synchronous vascular resection displayed significantly worse outcomes. The presence of macroscopic vascular involvement at the time of pancreatectomy could be a surrogate of a more advanced disease and has been associated with an increased rate of postoperative morbidity, thereby reducing the opportunity to receive adjuvant chemotherapy.32,33 Furthermore, patients with a systemic inflammatory state and impaired nutritional conditions showed worse DFS and OS, as already reported in patients with earlier-stage PDAC.26,34 In particular, the combination of lymphocytopenia and hypoalbuminemia indicates immunosuppression and compromised immune-nutritional status, which may lead to reduced adjuvant chemotherapy tolerance and earlier recurrence.35 While poor nutritional status following first-line chemotherapy can be interpreted as a marker of a biologically aggressive disease, prehabilitation programs in responders could be implemented to address nutritional issues and improve the functional capability of a patient.36
The present study has several major limitations. First, it is retrospective and lacks a control group. Second, the results apply to a super-selected group of initially oligometastatic PDAC patients who underwent first-line multiagent chemotherapy with complete radiologic response of liver metastases, significant drop of serum CA19-9 levels, and good conditional status. This is an exiguous proportion of the whole collective of patients with metastatic PDAC evaluated at baseline. Third, there was no standard practice with respect to chemotherapy regimen, duration, and time interval between chemotherapy completion and pancreatectomy. With these limitations in mind, we suggest that local resectability (without vascular involvement), good nutritional status, and low inflammatory index scores could be useful indicators to select patients with oligometastatic PDAC who respond to chemotherapy and could benefit from surgical resection.
Funding
No financial or material support was received for this work.
Disclosure
Isabella Frigerio, Giuseppe Malleo, Matteo de Pastena, Giacomo Deiro, Niccolò Surci, Filippo Scopelliti, Alessandro Esposito, Paolo Regi, Alessandro Giardino, Valentina Allegrini, Claudio Bassi, Roberto Girelli, Roberto Salvia, and Giovanni Butturini have no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roberto Salvia and Giovanni Butturini shared senior authorship on this work.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 202. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 3.Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2006;14:118–127. doi: 10.1245/s10434-006-9131-8. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou E, Margonis GA, Sasaki K, et al. Is resection of pancreatic adenocarcinoma with synchronous hepatic metastasis justified? A review of current literature. ANZ J Surg. 2016;86:973–977. doi: 10.1111/ans.13738. [DOI] [PubMed] [Google Scholar]
- 5.Buc E, Orry D, Antomarchi O, Gagnière J, Da Ines D, Pezet D. Resection of pancreatic ductal adenocarcinoma with synchronous distant metastasis: is it worthwhile? World J Surg Oncol. 2014;12:347. doi: 10.1186/1477-7819-12-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160:136–144. doi: 10.1016/j.surg.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43:358–363. doi: 10.1016/j.ejso.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Tao L, Yuan C, Ma Z, Jiang B, Xiu D. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: a population-based study. Cancer Manag Res. 2017;9:471–479. doi: 10.2147/CMAR.S145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol. 2017;23:1872–1880. doi: 10.3748/wjg.v23.i10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Wang D, Lou W. Current role of surgery in pancreatic cancer with synchronous liver metastasis. Cancer Control. 2020;27:1073274820976593. doi: 10.1177/1073274820976593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandel P, Wallace MB, Stauffer J, et al. Survival of patients with oligometastatic pancreatic ductal adenocarcinoma treated with combined modality treatment including surgical resection: a pilot study. J Pancreat Cancer. 2018;4:88–94. doi: 10.1089/pancan.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42:1533–1539. doi: 10.1016/j.ejso.2016.06.398. [DOI] [PubMed] [Google Scholar]
- 15.Byun Y, Han Y, Kang JS, et al. Role of surgical resection in the era of FOLFIRINOX for advanced pancreatic cancer. J Hepato-Biliary-Pancreat Sci. 2019;26:416–425. doi: 10.1002/jhbp.648. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Heckler M, Mihaljevic AL, et al. CT response of primary tumor and CA19-9 predict resectability of metastasized pancreatic cancer after FOLFIRINOX. Eur J Surg Oncol. 2019;45:1453–1459. doi: 10.1016/j.ejso.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Wright GP, Poruk KE, Zenati MS, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage IV pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg. 2016;20:1830–1835. doi: 10.1007/s11605-016-3256-2. [DOI] [PubMed] [Google Scholar]
- 18.Nie D, Lai G, An G, et al. Individualized prediction of survival benefits of pancreatectomy plus chemotherapy in patients with simultaneous metastatic pancreatic cancer. Front Oncol. 2021;11:719253. doi: 10.3389/fonc.2021.719253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frigerio I, Regi P, Giardino A, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24:2397–2403. doi: 10.1245/s10434-017-5885-4. [DOI] [PubMed] [Google Scholar]
- 20.Crippa S, Cirocchi R, Weiss MJ, et al. A systematic review of surgical resection of liver-only synchronous metastases from pancreatic cancer in the era of multiagent chemotherapy. Updates Surg. 2020;72:39–45. doi: 10.1007/s13304-020-00710-z. [DOI] [PubMed] [Google Scholar]
- 21.De Simoni O, Scarpa M, Tonello M, et al. Oligometastatic pancreatic cancer to the liver in the era of neoadjuvant chemotherapy: Which role for conversion surgery? A systematic review and meta-analysis. Cancers. 2020;12:3402. doi: 10.3390/cancers12113402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)–an international study group of pancreatic surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabłońska B, Pawlicki K, Mrowiec S. Associations between nutritional and immune status and clinicopathologic factors in patients with pancreatic cancer: a comprehensive analysis. Cancers. 2021;13:5041. doi: 10.3390/cancers13205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 28.Pausch TM, Liu X, Cui J, et al. Survival benefit of resection surgery for pancreatic ductal adenocarcinoma with liver metastases: a propensity score-MAtched SEER database analysis. Cancers. 2021;14:57. doi: 10.3390/cancers14010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai S, George B, Wittmann D, et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. 2020;271:740–747. doi: 10.1097/SLA.0000000000003049. [DOI] [PubMed] [Google Scholar]
- 30.Reni M, Zanon S, Balzano G, et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann Oncol. 2017;28:2786–2792. doi: 10.1093/annonc/mdx495. [DOI] [PubMed] [Google Scholar]
- 31.Maggino L, Malleo G, Marchegiani G, et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 2019;154:932–942. doi: 10.1001/jamasurg.2019.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleive D, Sahakyan MA, Berstad AE, et al. Trends in indications, complications and outcomes for venous resection during pancreatoduodenectomy. Br J Surg. 2017;104:1558–1567. doi: 10.1002/bjs.10603. [DOI] [PubMed] [Google Scholar]
- 33.Belfiori G, Fiorentini G, Tamburrino D, et al. Vascular resection during pancreatectomy for pancreatic head cancer: a technical issue or a prognostic sign? Surgery. 2021;169:403–410. doi: 10.1016/j.surg.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa K, Sho M, Akahori T, et al. Significance of the inflammation-based prognostic score in recurrent pancreatic cancer. Pancreatology. 2019;19:722–728. doi: 10.1016/j.pan.2019.05.461. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2014;13:474–481. doi: 10.1016/S1499-3872(14)60284-8. [DOI] [PubMed] [Google Scholar]
- 36.Perlmutter BC, Ali J, Turgut BC, et al. Correlation between physical status measures and frailty score in patients undergoing pancreatic resection. Surgery. 2022;171:711–717. doi: 10.1016/j.surg.2021.10.030. [DOI] [PubMed] [Google Scholar]