Abstract
Atherosclerosis is accompanied by a CD4 T cell response to apolipoprotein B (APOB). Major Histocompatibility Complex (MHC)-II tetramers can be used to isolate antigen-specific CD4 T cells by flow sorting. Here, we produce, validate and use an MHC-II tetramer, DRB1*07:01 APOB-p18, to sort APOB-p18-specific cells from peripheral blood mononuclear cell samples from 8 DRB1*07:01+ women with and without subclinical cardiovascular disease (sCVD). Single cell RNA sequencing showed that transcriptomes of tetramer+ cells were between regulatory and memory T cells in healthy women and moved closer to memory T cells in women with sCVD. TCR sequencing of tetramer+ cells showed clonal expansion and V and J segment usage similar to those found in regulatory T cells. These findings suggest that APOB-specific regulatory T cells may switch to a more memory-like phenotype in women with atherosclerosis. Mouse studies showed that such switched cells promote atherosclerosis.
Editor summary:
Atherosclerosis is accompanied by an autoimmune response that includes CD4 T cellsrecognizing epitopes in apolipoprotein B (APOB). Saigusa et al. analyzed thetranscriptomes and T cell receptors (TCRs) of APOB-specific CD4 T cells by single cell RNA sequencing using MHC-II tetramers in women with atherosclerosis, and showed that APOB-specific regulatory T cells switch to a more memory-like phenotype in atherosclerosis.
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease of large and medium-sized arteries. Its autoimmune component is manifest in autoantibodies to apolipoprotein B (APOB), the main lipoprotein in low density lipoprotein (LDL), and to oxidized LDL (oxLDL),1 as well as robust CD4 T cell responses.2–5 Individuals without cardiovascular disease (CVD) have a detectable number of FoxP3+ CD4 T cells that specifically recognize epitopes in APOB,2 suggesting that these APOB-specific CD4 T cells may be regulatory T cells (Treg). Although FoxP3 is the canonical, lineage-defining transcription factor for Treg, it is not known whether these APOB-specific T cells indeed have regulatory function. This cannot be addressed directly, because the number of APOB-specific CD4 T cells recovered by tetramer is far too low for in vitro suppression assays.2
Several previous studies have shown that adoptive transfer of Treg is atheroprotective. Ait-Oufella et al (2006)6 showed that co-transfer of polyclonal (not ApoB-specific) CD4+CD25+ Tregs reduced atherosclerosis induced by Cd28−/− splenocytes. Mor et al (2007)7 showed that transfer of Tregs (CD4+CD25+) from age-matched Apoe−/− mice resulted in significant attenuation of atherosclerosis compared with adoptive transfer of CD4+CD25− T cells or PBS. In a loss-of-function experiment, Klingenberg et al (2013)8 showed that irradiated Ldlr−/− mice that received FoxP3+ Treg-depleted (by FoxP3-DTR treated with diphteria toxin, DT) bone marrow had a 2.1-fold increase in atherosclerotic lesions measured by image analysis software on cryosections. Wolf et al (2020)3 showed that the number of small, developing lesions in the descending and abdominal aorta was reduced by transfer of ApoB-specific Tregs (by MHC-II multimer) into Apoe−/− mice.
Mouse studies have shown that APOB-specific Tregs lose expression of many Treg genes with both age and a high fat diet and become exTregs.3 In vitro expanded ApoB-specific T cells from mice vaccinated with a single mouse ApoB peptide in Complete Freund’s Adjuvant (CFA) are pro-atherogenic.9 In these experiments, lymph node cells were harvested after 10 days of immunization, cultured with ApoB peptide for 4-5 days and transferred into Apoe−/− recipient mice on western diet (WD). Mice that had received the ApoB-p6-specific cells showed exacerbated aortic lesions. Zhou et al10 immunized donor mice with oxLDL and transferred 12 million purified splenic CD4 T cells into each Apoe−/−scid/scid mouse. Mice receiving CD4T cells from oxLDL-immunized mice had substantially larger lesions compared with those receiving irrelevant T cells. Elevated circulating levels of IFNγ suggested that a Th1 response was responsible for the acceleration of atherosclerosis. Here, we directly tested the effect of exTregs on atherosclerosis by transferring exTregs from Treg lineage tracker mice immunized with an ApoB peptide into Apoe−/− recipient mice on WD.
Humans with subclinical cardiovascular disease (sCVD) harbor APOB-specific CD4 T cells that express FoxP3 along with T-helper transcription factors like ROR-γt and T-bet,2 suggesting that, like in mice, these cells are no longer regulatory but instead assume a changed, pro-inflammatory phenotype. Flow cytometry data2 showed expression of the 5 main lineage-defining transcription factors (FoxP3, RORγt, T-bet, GATA3 and Bcl6) but no other cellular details. Here, we use single cell RNA-sequencing (scRNA-Seq) to obtain single cell transcriptomes and T cell receptor (TCR) sequences of human APOB-specific CD4 T cells and compare them with transcriptomes of bona fide Tregs to address transcriptomic similarities and differences. Studying the TCR repertoire addresses the clonality of the APOB-specific CD4 T cells.
CD4 T cells express TCRs that are heterodimers of one α and one β chain and the subunits of CD3 needed for surface expression and signaling.11 The CD3 subunit sequences are invariant, but the α and β TCR chains undergo somatic recombination, using different alleles of the V, (D) and J subunits and a template-free section that together defines the most variable CDR3 region. With 5’ scRNA-Seq, TCR CDR3 sequences can be assembled,12 thus reflecting the full repertoire of TCRs found in the sample. Cells expressing the same TCRα and β are called clonotypes.13 Our sorting scheme was informed by the fact that human Tregs express high levels of the high affinity IL-2 receptor CD25, but not the IL-7 receptor CD127 (CD25hiCD127−).4 Other CD4 T cells serve as helpers. Antigen-experienced memory CD4 T cells do not express CD45RA (CD45RA−). In human blood, many Th1 cells express the chemokine receptor CXCR3 (CD45RA-CXCR3+).14
APOB-specific CD4 T cells can be detected by MHC-II peptide tetramers in humans2 and mice.2,3 Tetramers are recombinant MHC-II molecules loaded with an epitope-defining peptide. In the case of human APOB, the best-defined epitope is APOB-p18, sequence SLFFSAQPFEITAST. We previously tested multiple tetramers, and successfully constructed and validated a human tetramer constructed from the MHC-II allele DRB1*07:01 loaded with p18 (APOB-p18 DRB1*07:01 tetramer).2 The transcriptomes of Tet+ cells in sCVD have not been studied before. No TCR sequences of APOB-specific human CD4 T cells have been reported. The only transcriptomic data available is for mouse APOB-specific CD4 T cells.3 Mouse APOB-specific CD4 T cells were found to be oligoclonal, with half of them expressing TCRBV02-01. scRNA-Seq showed that in 8 week-old Apoe−/− mice, some of these cells expressed Treg genes like FoxP3, Ctla4 and Tgfb1, but other expressed Th17 genes like Il17f and Rorc and yet other expressed Th1 genes like Cxcr3, Cxcr6 and Tbx21 (T-bet).3 This suggested that ApoB-specific CD4 T cells show a mixed phenotype in mice. No transcriptomes or TCR sequences of human APOB-specific CD4 T cells have been reported.
The Women’s Interagency HIV Study (WIHS) is a multi-center, prospective, observational cohort study of over 4,000 women with or at risk of HIV infection that was initiated in 1994.15 WIHS is now part of the MACS/WIHS Combined Cohort Study.16 Cardiovascular risk in people living with HIV is elevated 2-fold compared to uninfected controls.17 Atherosclerosis was assessed by carotid artery ultrasound.18 In the present study, we show the autoimmune response to APOB-p18 in a re-stimulation assay. We sorted all of the small population of Tet+ cells from frozen peripheral blood mononuclear cells (PBMCs) from 8 WIHS participants. In addition, we sorted larger populations of Tregs (CD25hi CD127−), Th1 cells (CD45RA−CXCR3+) and other (CD45RA−CXCR3−) memory T cells (Tmem). To minimize batch effects, all four cell types were hash-tagged, as were donors, such that four cell types (Tet+, Treg, Th1, Tmem) from 8 donors were run on each 10x Genomics Chromium microfluidic device.
RESULTS
APOB-p18 peptide-specific immune response in human PBMCs
To measure the endogenous T cell response to APOB-p18 peptide (SLFFSAQPFEITAST), we isolated PBMCs from an HLA-typed DRB1*0701 donor and expanded the cells with a pool of 20 human MHC-II-restricted APOB peptides,3 including p18. On Day 14, the response to the APOB-p18 was monitored by human IFNγ ELISpot assays (Figure 1A), showing that APOB-p18 peptide evokes a measurable T cell response in human PBMCs.
Figure 1. Re-stimulation assay and gating strategy.

A, PBMCs from an HLA-typed DRB1*0701 donor were expanded with a pool of 20 MHC-II restricted APOB-derived peptides, including p18. On Day 14, the response to p18 was monitored in human IFNγ ELISpot assays (p18 Stim). Re-stimulation with the pool (APOB20) and untreated cells (Unstim) served as positive and negative controls, respectively. Representative data from IFNγ ELISpot. B, All PBMCs were gated for dump (CD8, CD14, CD16, CD19, CD56) negative live (live/dead aqua negative). C, CD3+ TCRαβ+. D, CD4+ T cells. E, APC and PE APOB-p18 DRB1*07:01 tetramer positive cells were sorted as Tet+ cells. Treg, Th1 and Tmem cells were sorted from tetramer negative cells (panel D) for CD4+CD45RA− (not naïve, F). Tregs were identified as CD25hi CD127− (G). From the remaining cells, CXCR3+ cells were considered Th1 and CXCR3− cell other memory T cell (Tmem) (H). I, Sorted cells were multiplexed by hashtags, processed and loaded to 10x Genomics Chromium Controller.
APOB-p18 DRB1*07:01 tetramer specificity validation
For the single cell RNA sequencing experiments, we used an APOB-p18 specific tetramer (APOB-p18 DRB1*07:01), validated by mismatched control, back-gating and confocal microscopy. PBMCs from subjects not expressing DRB1*701 showed no tetramer binding (Extended Data Fig. 1A). To minimize non-specific staining, the tetramer was conjugated with both PE and APC. APC and PE double positive cells were Dump-CD3+CD4+ cells (Extended Data Fig. 1B), confirming specificity. Confocal microscopy showed that APC− and PE− labeled APOB-p18 DRB1*07:01 tetramers colocalized with the T-cell receptor in CD4 T cells (Extended Data Fig. 1C).
Rare Tet+ cells were successfully sorted and sequenced
scRNA-seq and TCR-seq was conducted on frozen PBMCs from 8 participants (Supplementary table 1) from the WIHS cardiovascular cohort. Subclinical cardiovascular disease (sCVD) was assessed by standardized carotid ultrasound.19 After thawing, average PBMC viability was 88.5 ± 5.1% (Supplementary table 2). All PBMCs were gated for dump negative. From CD3+TCRαβ+CD4+ T cells, APC and PE APOB-p18 DRB1*07:01 tetramer positive cells were sorted as Tet+ cells. Treg, Th1 and Tmem cells were sorted from tetramer negative cells using standard markers (Figure 1B–H). The four cell types were hashtagged, multiplexed and loaded into the 10x Genomics Chromium controller (Figure 1I). The number of cells sequenced ranged between 1933 and 3097 per participant (Supplementary table 2). TCRαβ pairs were successfully assembled in 12,598 cells. In all cases, the most limiting number were the p18-DRB1*07:01 tetramer positive (Tet+) cells, of which 10 to 41 cells were sequenced per participant. Tet+ cells were found in each donor. The washing and loading procedure was optimized to preserve as many of these cells as possible (Extended Data Fig. 2).
TCRα and β sequences
First, we analyzed the TCRα and β sequences in all sequenced cells. In total, we found 12,598 clonotypes, of which 11,996 were unique (Supplementary Data 1A). Alpha and β chains were successfully assembled in approximately the same number of cells (15,774 and 16,647, respectively). 262 clonotypes were found in multiple cells, with the most common one (ct3326) found in 61 cells (Figure 2A).
Figure 2. TCR clonotypes of the combination of TCRα and β sequences and VDJ usage.
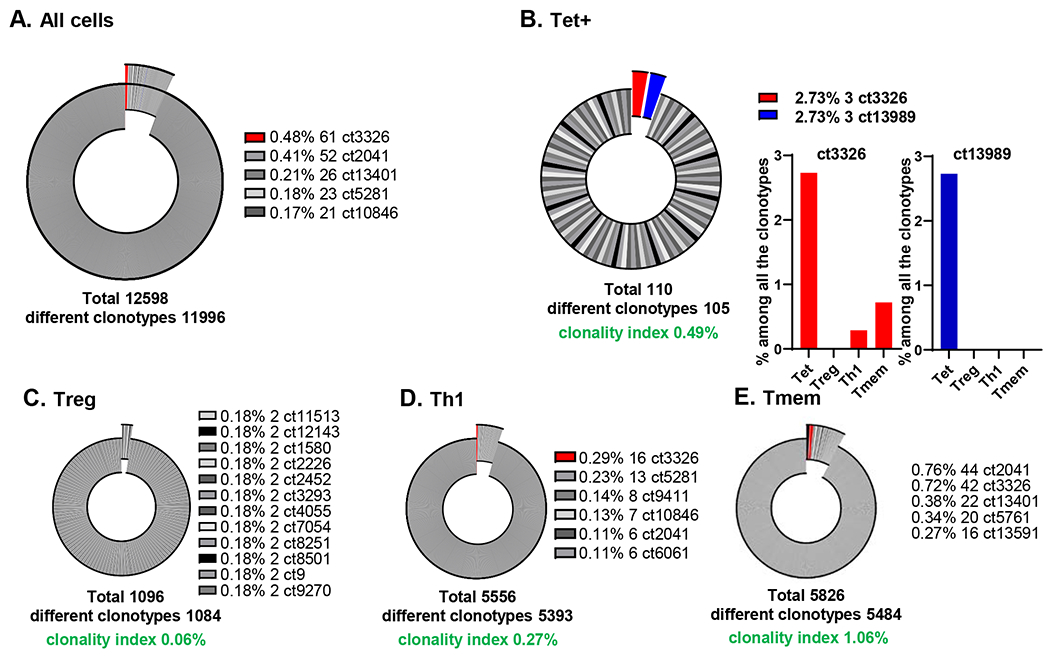
A, Pie chart for all the TCR clonotypes from all the cells. B-E, Pie charts for each of the 4 cell types (B, Tet+; C, Treg; D, Th1; E, Tmem), with clonality index. Clonotypes with more than 1 clone exploded in the graph. Top 5 clonotypes with more than 1 clone are as shown. ct, clonotype. Clonotype3326, which was expanded in Tet+ cells, was shared with other cell types, and highlighted in red.
Next, we analyzed the clonality of Tet+, Treg, Th1, and Tmem cells from all donors (Figure 2B–E). Among the Tet+ TCR clonotypes, two were represented by 3 cells each and the remaining 108 were uniquely expressed in one cell each. The most common clonotype in Th1 cells was found in 16 cells, in Tmems in 44 cells, and in Tregs in 2 cells (Figure 2B–E). Clonotype 3326 (ct3326) was clonally expanded (61 T cells in total), and accounted for 2.73% of all the clonotypes of Tet+ cells. This percentage is the much higher than other cell types (0.48% in all the cells, 0% in Tregs, 0.29% in Th1, and 0.72% in Tmem cells). Since the number of clonotypes found multiple times is dependent on the total number of TCRs analyzed, we calculated the clonality index. The highest clonality index (1.06%) was found in Tmems, followed by Tet+ cells (0.49%) and Th1 (0.27%). Tregs had the lowest clonality index (0.06%), meaning that they had the most diverse TCR repertoire of all CD4 T cells studied. Among the non-expanded TCRα/β clonotypes of Tet+ cell, ct10760, ct13262, ct6674, ct7704, and ct9694 were shared with a Th1 cell, ct11806 and ct1834 with Tmem, ct13401 with 3 Th1 and 22 Tmem cells, ct2041 with 6 Th1 and 44 Tmem cells (Supplementary Data 1C). All TCRα and β sequences are listed in Supplementary Data 1. False positive rates (cells that were non-specifically stained by tetramer) were extremely low, less than 0.01% (Extended Data Fig. 1B). We also used TCRαβ sequences to estimate the false negative rates, i.e. cells that the tetramer missed. To find false negatives, we looked for tetramer-negative cells with the same clonotype (TCRα and TCRβ) found in the tetramer+ population (both expanded and non-expanded). We calculated false negative rate as 18% (the sum of % of a clonotype shared with tetramer in each of the 3 other cell types (Treg, Th1 and Tmem), divided by % of the same clonotype in tet+). When comparing TCRβ only or TCRα only, the Tmem and Th1 cells are most oligoclonal, followed by Tregs, and Tet+ are most polyclonal (Extended Data Fig. 3).
TCRβ clonotypes between Tet+ cells and Tregs are similar
To compare our data to previous studies based on TCRβ sequences obtained by DNA sequencing,20 we re-analyzed the data for Vβ and Jβ combinations. Looking at V segment usage in HIV−sCVD− participants, Vβ6-1, 18 and 20-1 were the most common in Tet+ cells. Vβ20-1 was also in the top 3 Vβ segments in Th1, Tmem and Treg (Supplementary Data 2A). Vβ6-1 and 20-1 were also repeatedly found in Tet+ cells from HIV−sCVD+ participants (Supplementary Data 2B). In addition, Vβ9 and Vβ29-1 were found repeatedly, suggesting that these Vβ chains are preferentially used in Tet+ cells in the presence of sCVD. All these Vβ chains were also used in some Th1, Tmem and Treg cells.
J segment usage in Tet+ cells was also different between participants with and without sCVD. Jβ1-1, 1-3 and 1-5 were repeatedly found in HIV−sCVD− participants (Supplementary Data 2E). In HIV− sCVD+ participants, Jβ2-1 was the most common (Supplementary Data 2F).
Figure 3A shows that most Tet+ cells (51) shared Vβ and Jβ with all other cell types. No Tet+ cells shared Vβ and Jβ with Tregs only. 17 Vβ Jβ sequences were private to Tet+ cells. Constructing heatmaps for Th1 and Tmem (Figure 3B, C) showed Vβ20-1 and Jβ1-1 as the most common combination. In Tregs, Vβ20-1 Jβ1-2 (Figure 3D) and in Tet+ cells, Vβ20-1 Jβ2-1 (Figure 3E) were most common, respectively. Based on Vβ and Jβ usage, we found 14 expanded clones among the Tet+ cells (Figure 3F, Supplementary Data 3). We ranked these from largest to smallest and interrogated the Tregs, Th1 and Tmem Vβ and Jβ combinations. Vβ and Jβ combinations were similar between Tet+ cells and Tregs, but significantly different from Th1 (p<0.001, Kolmogorov-Smirnov test) and Tmem (p<0.001) (Figure 3F). This finding supports the notion that Tet+ cells may have originated from Tregs.
Figure 3. TCRβ sequences and VDJ usage.

A, Venn diagram of TCRβ clonotypes (Vβ and Jβ combination) in each cell type. B-F, Heat maps of TCRβ combinations in Th1 cells (B), Tmem (C), Treg cells (D), and Tet+ cells (E). Scale bars show the percentages of specific TCRβ clonotypes in all the clonotypes. F, Cumulative histogram of the frequency of TCRβ which was observed more than once in Tet+ cells.
Transcriptomes of APOB-p18 DRB1*07:01 tetramer+ CD4 T cells
Next, we clustered all cells based on their transcriptomes. UMAP with Louvain clustering identified 8 clusters (Extended Data Fig. 4A). Treg transcriptomes clustered together (Figure 4A). Th114 and Tmem were intermingled, suggesting that their transcriptomes were not very different (Extended Data Fig. 4B and C). Indeed, transcriptomic analysis showed that CXCR3− Tmem still contained cells expressing TBX21 or IFNG (Extended Data Fig. 4D). We checked FOXP3 expression in CD3+CD4+CD127−CD25+ cells by flow cytometry and found that 91±4% of CD127−CD25+ cells were FoxP3 positive (Extended Data Fig. 4E), confirming that CD127−CD25+ are mostly FOXP3+ Treg, as described previously.21 Projecting the transcriptomes of Tet+ cells onto the Th1, Tmem and Treg UMAP showed that very few Tet+ cells from HIV− women without sCVD fell within the Treg cluster (Figure 4B). Instead, their transcriptomes placed them in the Th1 and Tmem clusters (Figure 4C).
Figure 4. UMAP with Louvain clustering.

A, Treg cells from all 8 donors are highlighted in blue in UMAP, other cell types light grey. B, C, APOB-p18 DRB1*07:01 tetramer positive cells (Tet+ cells, solid circles) are plotted on the combined UMAP of cells from all 8 donors. Treg distribution (B), and Th1 and Tmem distribution (C) are shown as contour plots of density.
Distance of transcriptomes of Tet+ cells from Th1 and Tmem
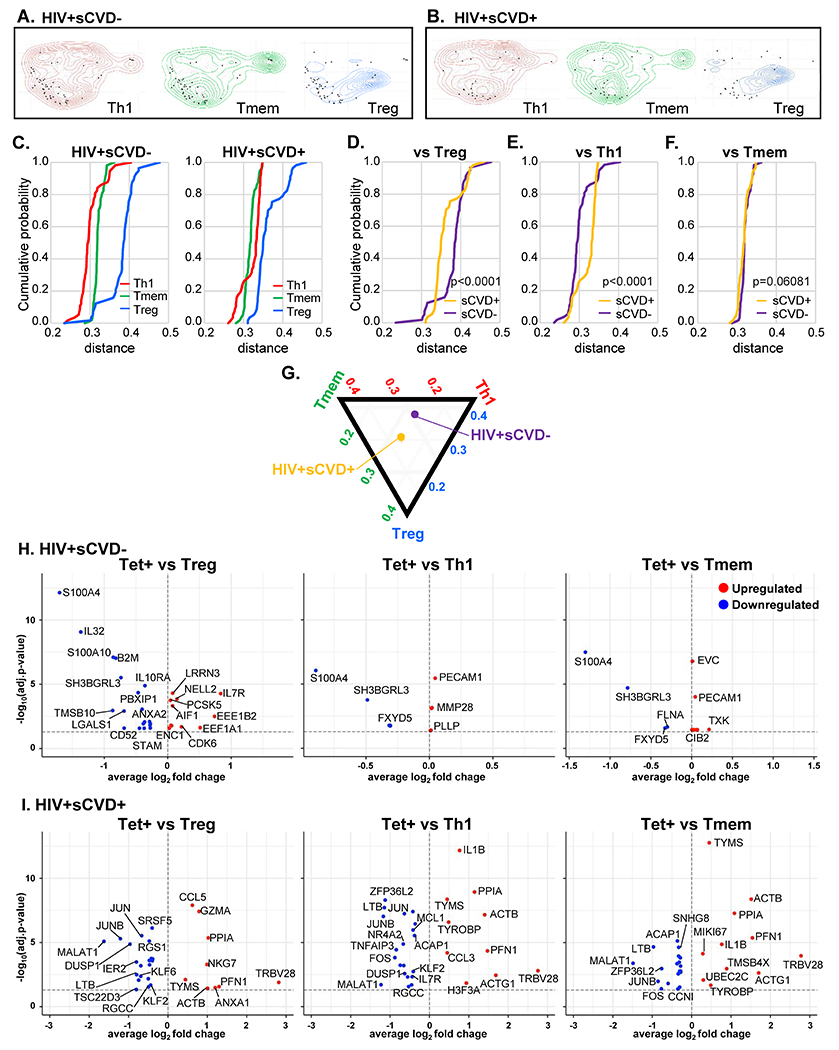
To study the influence of sCVD on the transcriptomes of Tet+ cells, we reclustered all HIV− Th1, Tmem and Treg cells separately for sCVD− and sCVD+ for each participant and projected the corresponding Tet+ cells on the contour plots (Extended Data Fig. 5A, B). To quantitatively analyze the distance between Tet+ and other cell types, we calculated the distance of each Tet+ cell transcriptome from the calculated bulk transcriptomes of Treg, Th1 and Tmem, separately for women with and without sCVD (all HIV−). Tet+ cells were equidistant to Th1 and Tmem transcriptomes in both CVD− and CVD+ participants (Figure 5A). They were significantly (p< 0.001, Kolmogorov-Smirnov test) more different from Treg transcriptomes in both CVD− and CVD+ participants. Next, we plotted the cumulative histograms of the distances of Tet+ cells from Treg (Figure 5B), Th1, and Tmem (Figure 5C). Tet+ cells from women with sCVD were significantly closer to Tmem transcriptomes (Figure 5C) and significantly further removed from Tregs than those from women without sCVD (Figure 5B). There was no difference in the distance from Th1. These findings are summarized in Figure 5D in a modified ternary plot. Thus, we conclude that Tet+ cells from women with sCVD are closer to memory T cell transcriptomes and less like Treg transcriptomes than in women without sCVD.
Figure 5. Comparison of Tet+ transcriptome to other cell types in HIV−.

A, Cumulative histogram of the distances of each Tet+ cells against Tmem (green), Treg (blue) and Th1 (red) in HIV−. B, C, Cumulative histograms of the distances of each of the Tet+ cells from Treg and Tmem cells, separately for sCVD+ (yellow) and sCVD− (purple) and HIV− in the first 6 PCA components. Significance by Kolmogorov-Smirnov test (two-sided). D, A ternary plot of relative median positions of p18-DRB1*07:01 tetramer positive cells relative to pseudobulk mean of Tregs, Th1 and Tmem in the first 6 PCA components in HIV− women. E, F, Volcano plots comparing gene expression in single cells of Tet+ cells compared to Treg, Th1, and Tmem in HIV−sCVD− (E), and HIV−sCVD+ (F). We performed differential expression analysis using Seurat’s non-parametric Wilcoxon rank-sum test to extract marker genes. Significant markers were selected based on Bonferroni-adjusted P-Values <0.05. Colored dots (upregulated genes in red, and downregulated genes in blue) indicate significantly differentially expressed genes (adjusted p-value <0.05). Dashed line indicates adjusted p-value of 0.05. Full data set shown in Supplementary Data 4. The statistical tests were two-sided.
Next, we investigated the expression of genes that drove the repositioning of Tet+ cells with cardiovascular disease in HIV− participants. Using Seurat, we determined the significantly (adjusted p-value < 0.05) differentially expressed genes (DEGs) between Tet+ cells and Tregs, Th1, and Tmem. In sCVD− participants, IL7R was significantly higher expressed in Tet+ cells and DUSP4 was significantly lower expressed than in Tregs from the same sCVD− participants (Figure 5E). Proliferating CD4 T cells were very recently shown to express MHC-II.22 The main human MHC-II alleles are in HLA-DR, DP and DQ. We found that HLA-DPB1 was downregulated in Tet+ cells compared to Treg in sCVD− participants (Figure 5E), but not in sCVD+ participants. This gene is related to Antigen Presentation Pathway (p=0.004). This suggests the intriguing possibility that some CD4 T cells may be antigen-presenting in the context of CVD.23 In sCVD+ participants, NELL2, THEMIS, and CTSL were significantly more highly expressed in Tet+ cells than in Tregs. THEMIS plays a central role in late thymocyte development by controlling both positive and negative T-cell selection. CTSL encodes Cathepsin L, which is associated with the risk of cardiovascular mortality.24 MMP28 was also upregulated in Tet+ cells compared to Treg, which is related to the hypoxia-inducible factors (HIF) pathway (p=0.03). HIF in CD4 T cells is related to humoral immunity, promotes CD40L expression and restrains FoxP3-positive CD4 T cells.25 MMP28 may also play a role in tissue homeostasis and repair.26
In CVD− women, Tet+ cells expressed significantly more TYMS and CENPA than Th1 and Tmem cells from the same participants (Figure 5E). Thymidylate cyclase, the product of the TYMS gene, is involved in mitochondrial thymidylate biosynthesis.27 CENPA encodes a histone H3-like protein found in centromeric nucleosomes.28 Moreover, Tet+ cells expressed significantly more GZMK and WDFY3 than Tmem cells from the same HIV−sCVD− participants (Figure 5E, Supplementary Data 4). GZMK encodes Granzyme K, a member of a group of related serine proteases from the cytoplasmic granules of cytotoxic lymphocytes. WDFY3 encodes a phosphatidylinositol 3-phosphate-binding protein that functions as a master conductor for aggregate clearance by autophagy.
In women with CVD, protein-coding genes including IL17D, PTGIR, LURAP1, and CLNK were significantly higher in Tet+ cells than in Th1 and Tmem cells from the same HIV−CVD+ subjects (Figure 5F). Most notably, IL17D encodes interleukin IL-17D, also known as IL-27. This pro-inflammatory cytokine induces expression of IL-8 and GM-CSF, known drivers of atherosclerosis.29 PTGIR encodes the prostacyclin receptor, a very important vasodilator and inhibitor of thrombosis.30 LURAP1 acts as an activator of the canonical NF-kappa-B pathway and drives the production of proinflammatory cytokines.31 CLNK encodes a protein which enhances CD3-triggered activation of T cells and subsequent IL-2 production.32 Also Tet+ cells expressed more APOBEC3B, encoding APOB mRNA Editing Enzyme Catalytic Subunit 3B (Figure 5F). In HIV+ participants, these DEGs were not detected (Extended Data Fig. 6, Supplementary Data 5).
Taken together, these data suggest that ApoB-specific T cells move away from Treg transcriptomes towards Tmem transcriptomes, analogous to Tregs becoming exTregs.3 exTregs can be tracked in mice,3,33 but not in humans. To directly test whether exTregs are pro-atherogenic, we sorted exTregs from lineage tracker Apoe−/− mice immunized with ApoB peptide p6,9 or irrelevant MOG peptide (Figure 6A). The frequency of exTregs (RFP+GFP−, Extended Data Fig. 7A) ranged from 2-6% (average, 4%) in spleens and lymph nodes and was not different in both groups of mice. Vaccination mediated induction of antigen-specific memory CD4 T cells was validated in an in vitro restimulation assay, where CD44+CD4 T cells from immunized mice responded to their cognate peptides (Figure 6B, upper panel). ApoB-p6 induced TNF and IFNγ production was observed in ApoB-p6-immunized mice, but not in MOG immunized control mice (Figure 6B, lower panel), confirming the specificity of the p6-vaccination regime in generating p6-specific memory CD4 T cells. Stimulated exTregs produced more IFNγ and TNF as detected by intracellular straining (Figure 7A, Extended Data Fig. 7B and C). 5 weeks after adoptive transfer from ApoB-p6-immunized mice, about 0.1% of FoxP3-negative CD4 T cells were exTregs in the blood of recipient mice. Apoe−/− mice receiving exTregs from ApoB-p6-immunized donors showed significantly larger atherosclerotic lesions in the thoracic and abdominal aortas than vehicle-treated mice (Figure 7Bi and ii). Apoe−/− mice receiving exTregs from MOG-immunized mice showed smaller lesions than mice receiving exTregs from ApoB-p6 immunized mice (p=0.057). Plasma IFNγ was significantly higher in mice receiving exTregs from ApoB-p6 immunized mice than from MOG-immunized mice (Figure 7Diii), suggesting that adoptive transfer of ApoB-specific exTregs enhances a Th1 response in Apoe−/− mice. Thus, exTregs from ApoB-p6 immunized mice exacerbate atherosclerosis in immunocompetent Apoe−/− mice.
Figure 6. Antigen-specific response in mice immunized with ApoB-p6.

A, Schematic of exTreg adoptive transfer experiment. Lineage tracker mice were injected with an emulsion composed of p6 or MOG peptides (2 mg/ml) with an equal volume of CFA (prime). A total of 0.1 ml of the emulsion was given i.m (0.05 ml per quadriceps femoris muscle). 2 weeks later, a boost of p6 or MOG peptide emulsified in IFA was injected i.m.. Lymph nodes and spleens were harvested 2 weeks later. Cells from lymph nodes and spleens from pooled p6- or MOG-immunized lineage tracker mice were enriched for CD4 T cells. 2*105 exTregs were injected retro-orbitally in 100uL of PBS 1X in 8-10 weeks old 6Gy irradiated female Apoe−/− mice that had been on western diet (WD) for 3 weeks and antibiotics for 3 days before and 14 days after adoptive transfer. Control mice were injected with 100uL of PBS as vehicle control. Recipient mice continued on WD for 5 more weeks. B, Splenocytes from p6 and MOG immunized mice were stimulated for 6h and cytokine production was analyzed using intracellular staining and flow cytometry. Representative FACS plot (i) and the corresponding quantification (ii) of %TNF+CD44+ CD4 T cells in MOG or p6 peptide stimulated sets and unstimulated (“no stim”) controls. Pairwise statistical comparisons between unstimulated and individual peptide stimulated sets were performed using paired student’s t-test (p=0.0085 and 0.0005, respectively). (iii) Representative FACS plots (left) and quantification (right) of p6-induced %TNF+CD44+ and (iv) %IFNγ+CD44+ CD4 T cells in stimulated CD4 T cells from p6 and MOG immunized mice (n=5 and 3, respectively). Bars represent mean values with standard error of mean (SEM). Comparison of mean responses between two different sets of immunized mice was done using unpaired student’s t-test with Welch’s correction (p=0.0087 for iii, and p=0.019 for iv). *p<0.05, **p<0.01, ***p<0.001. The statistical tests were two-sided.
Figure 7. Analysis of exTregs from ApoB-p6 immunized mice in intracellular staining and adoptive transfer study.
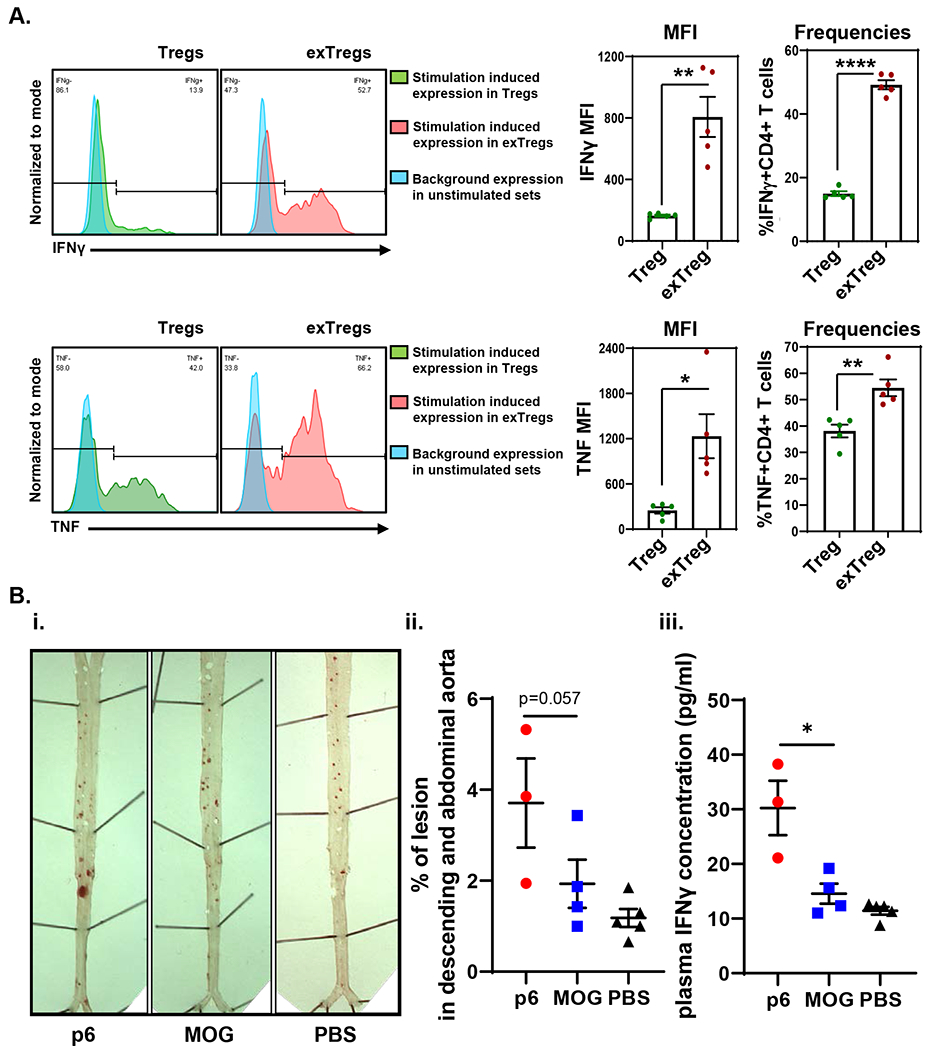
A, Representative histogram plots showing IFNγ (upper left panel) and TNF (bottom left panel) production in CD4+ Tregs (green) and exTregs (red) stimulated with PMA and ionomycin. Blue peaks denote background expression of the cytokines in both cell types in unstimulated control sets. Quantification of median fluorescence intensities (MFI) of cytokine expression in each subset and frequencies of cytokine+ Tregs and exTregs are shown (IFNγ, upper right; TNF, lower right) (n=5). Bars represent mean values with standard error of mean (SEM). Statistical comparisons between mean expressions and frequencies of cytokine responses in the two subsets were performed using unpaired student’s t-test with Welch’s correction (two-sided). p=0.0078 for MFI, and p<0.0001 for frequencies for IFNγ. p=0.027 for MFI, and p=0.004 for frequencies for TNF. *p<0.05, **p<0.01, ****p<0.0001. Bi, ii, Atherosclerotic lesions in the aorta were visualized by Sudan-IV (i) and quantified as area (% of lesion in descending and abdominal aorta) (ii). Biii, Plasma IFNγ concentration (pg/ml) of the recipient mice. p6: exTreg transferred from p6-immunized mice (n=3), MOG: exTregs from MOG-immunized mice (n=4), PBS: vehicle control (n=5). One-tailed Mann-Whitney test was performed (p=0.028). *p<0.05. Bars represent mean values ± SEM.
Transcriptomes of human CD4 T cells with the same TCRβ
Finally, we combined the human scRNA-Seq data with the human TCR-Seq data to analyze gene expression in cells with the same TCRβ clonotype (Figure 8). As shown in Figure 3A, some TCRVβJβ clonotypes were shared between the four groups of CD4 T cells. Some Treg signature genes including FOXP3, TIGIT, IL2RA (CD25), IL10RA and CTLA4 were downregulated in Tet+ cells compared to Tregs (Figure 8A, Supplementary Data 6). Comparing Tet+ cells and Th1 with shared TCRVβJβ clonotypes, COL18A1, TMIGD2, and MMP28 were more highly expressed in Tet+ cells compared to Th1 (Figure 8B). COL18A1 encodes collagen XVIII, which has anti-angiogenic properties.34 TMIGD2 encodes a CD28 homolog, which costimulates TCR-mediated T cell activation.35 TXN, S100A4, KLF6, and ANXA1 were downregulated in Tet+ compared to Th1. TXN may prevent the initiation of atherosclerosis by attenuating adhesion molecule expression.36 S100A4 is involved in instability of atherosclerotic plaques.37 KLF6 encodes a protein which involved in cardiac fibrosis.38 ANXA1 may be involved in a protective activity for atherosclerosis.39 DCHS1 and ZNRF3 are highly expressed in Tet+ cells compared to Tmem. DCHS1 encodes a calcium-dependent cell-adhesion protein, acting in the regulation of cell migration in heart valves.40 ZNRF3 encodes a protein involved in signaling by GPCR and Wnt.41 KLF6 was downregulated in Tet+ cells compared to Tmem (Figure 8C).
Figure 8. Differentially expressed genes between Tet+ cells and the other cell types sharing the same TCRβ clonotypes as shown in Figure 3A.

Volcano plots comparing gene expression in single cells of Tet+ cells compared to Treg (A), Th1 (B), and Tmem (C), which shared the TCRβ clonotypes (Figure 3A, Tet+ 70 cells vs Treg 174 cells, Tet+ 89 cells vs Th1 1013 cells, and Tet+ 88 cells and Tmem 1098 cells, respectively). Differential expression analysis was performed using Seurat’s non-parametric Wilcoxon rank-sum test to extract marker genes. Significant markers were selected based on Bonferroni-adjusted P-Values <0.05. Colored dots (upregulated genes in red, and downregulated genes in blue) indicate significantly differentiated expressed genes (adjusted p-value <0.05). Dashed line indicates adjusted p-value of 0.05. Full data set shown in Supplementary Data 6.
DISCUSSION
This is the first scRNA-Seq study of APOB-specific Tet+ cells in individuals with and without sCVD. We show that a recombinant APOB-p18 DRB1*07:01 tetramer is useful for detecting the naturally occurring population of CD4 T cells in humans that recognize a human APOB peptide, determining their transcriptomes and TCRα and β sequences.
Many APOB-p18 DRB1*07:01 tetramer+ cells2 expressed FoxP3, suggesting that they were Tregs. Tregs control immune homeostasis and prevent exacerbated immune responses. Consistent with a previous mouse study,2 human APOB-specific Tet+ cells move further away from Tregs and towards Tmem in participants with sCVD. The present transcriptomic data suggest that, even in participants without sCVD, Tet+ are not true Tregs, although they share some genes with the Treg transcriptome. Based on these data, we propose to call APOB-specific Tet+ cells in healthy individuals Treg-like, rather than Tregs.
By cosine distance, the transcriptomes of Tet+ cells were more similar to Tmem and Th1 than Tregs. However, the order of V and J combinations was more similar between Tet+ cells and Tregs and significantly different from Th1 and Tmem. These findings suggest that APOB-specific Tet+ cells may derive from Tregs, but may have lost the typical Treg transcriptome. It is known that induced Tregs (iTregs) derived from conventional CD4 T cells are unstable.4,42 Our findings support the concept that Tet+ cells retain some transcriptomic features with iTregs in women without CVD, and lose these features in women with CVD.
In mouse studies of bulk Tregs (not APOB-specific), some Tregs showed plasticity and acquired traits of Th17,3 Th1,3 or TFH.43 Here, we show that adoptive transfer of exTregs from ApoB-p6-immunized mice exacerbates aortic atherosclerosis in immunocompetent Apoe−/− recipient mice. The mechanistic mouse data and the transcriptomes of human APOB-specific CD4 T cells suggest that conversion from Treg-like cells to exTregs promotes atherosclerosis in mice and humans. In the only study of APOB-specific CD4 T cells in mice,3 identified by mouse ApoB-p6 tetramer, the tetramer+ cells showed more effector and central memory markers compared to non-tetramer+ cells, which was further exacerbated by atherosclerosis (in Apoe−/− mice). Consistent with this, we show here that the transcriptomes of Tet+ cells in women with sCVD are significantly closer to Tmem than to Treg transcriptomes. Mouse Tet+ cells also showed more proliferation than tetramer− cells. Although proliferation of Tet+ cells cannot be measured directly (too few cells), we found centromere and kinetochore genes enriched in Tet+ cells, suggesting potential proliferation. Based on our data, we propose that Tet+ cells in humans start out Treg-like and move significantly closer to Tmem cells in humans with sCVD. Our previous study3 suggested that APOB-specific Tregs are modestly but not strongly atheroprotective. These aspects of antigen-specific CD4 T cells (both Tregs and exTregs) need to be addressed in more detail and with larger numbers of animals and human subjects.
Our clonotype analysis showed that Tmem has the highest clonality index, probably reflecting the fact that this population contains clones of memory cells that had responded to previous infections.44 Some chronic viral infections like cytomegalovirus (CMV) induce a large and stable memory CD8 and CD4 T cell response.44 Surprisingly, the APOB-specific cells have a lower clonality index, suggesting a diverse population.
TCR sequences reported here are consistent with a previous report,20 which showed that there are no notable differences in the overall profiles of the V-J gene utilization of T cell repertoires in the peripheral blood between participants with or without atherosclerotic plaques. The same study also showed that several T cell clonotypes (Vβ29-1Jβ2-1, Vβ20-1Jβ1-6, Vβ6-3Jβ2-7 and Vβ11-2Jβ2-2) are increased in the atherosclerotic plaques compared to PBMCs from healthy subjects and subjects with atherosclerotic plaques.20 Interestingly, this local clonotype expansion is not reflected in APOB-specific Tet+ cells in the blood as studied here. Another study showed a strong bias toward clonotypes Vβ3-1 and −2, and Jβ2-1 in peripheral blood of subjects with CVD,45 but we did not see that in our APOB-specific Tet+ cells from participants with sCVD.
Cardiovascular risk in people living with HIV is elevated more than 2-fold compared to uninfected controls.17 Although we had some samples from HIV+ participants, this study was not powered to discern the HIV effect on sCVD (Extended Data Fig. 7). However, in HIV+ participants with sCVD, the distance of Tet+ cells from Treg was decreased, and the distance to Tmem and Th1 was significantly increased. The interaction between sCVD and HIV remains to be investigated by scRNA-Seq.
A strength of the present study is that we successfully assembled TCRαβ pairs in ~80-90% of CD4 T cells. TCRα and β sequences can inform engineered Tregs, which can be used as cell therapy to dampen autoimmune diseases.46,47 We identified 110 different TCRαβ pairs that specifically recognize APOB-p18 presented by DRB1*07:01. These TCRs are candidate sequences that could be used to engineer such targeted Tregs to dampen the autoimmune aspect of atherosclerosis.5 Such an effort would require experimental evidence to determine which TCRαβ pairs are best suited for this purpose. Limitations of this present study include the small number of individuals (8) and the small number of Tet+ cells (113) investigated. The number of individuals was limited by the availability of DRB1*07:01+ samples. The number of Tet+ T cells probably reflects the limited size of the natural autoimmune response to one single epitope in ApoB. Restimulation assays4 can analyze multiple ApoB epitopes and thus may yield more cells.
In conclusion, we report the first single cell transcriptomes of Tet+ cells in humans. Although the Tet+ cells show similar V and J usage as Tregs, their transcriptomes were different from Tregs even in women without cardiovascular disease and moved significantly further away from Tregs and closer to Tmems in women with sCVD. Mouse experiments show that these ApoB-specific CD4 T cells are likely pro-atherogenic.
METHODS
Our research complied with all relevant ethical regulations; MWCCS committee approved the study for the experiment using human samples (W16021), and all mouse experiments were conducted in accordance with the institutional guidelines at the La Jolla Institute for Immunology animal facility (the protocol number: AP00001019).
Human restimulation assays
Fresh blood samples were collected from healthy adult participants through La Jolla Institute for Immunology’s Normal Blood Donation Program. PBMC were isolated using Ficoll-Paque (Millipore Sigma) density-gradient sedimentation. For in vitro expansion, 2x106 PBMCs/ml were cultured in serum-free medium (TexMACS, Miltenyi Biotech) in the presence of twenty MHC Class-II restricted 15-mer peptides (>70% purity, A&A labs) derived from human APOB (APOB20 peptide pool, 5 μg/ml). These peptides have been experimentally validated to bind a broad range human MHC-II alleles.3 10 U/ml human IL-2 (Invitrogen) was added to the media at Days 4, 7 and 10. On Day 14, cells were washed and re-plated in 96-well ELISpot plates (Millipore) coated with either mouse anti-human IFNγ (clone 1-D1K). PBMCs were treated with APOB20 pool (5 μg/ml) or p18 peptide (20 μg/ml) and incubated for 24h. Secreted cytokine responses from untreated and peptide-stimulated cells were detected using mouse anti-human IFNγ (clone 7-B6-1). Wells were imaged and spot-forming cells were quantified using the Zeiss KS ELISPOT reader. HLA typing was done at Murdoch University’s Institute for Immunology and Infectious Diseases (IIID), Australia.
Human PBMCs
Blood samples for scRNA-Seq were collected between 2003 and 2005 as part of WIHS.48 All participants provided informed consent at each site participating in WIHS, and each site obtained institutional review committee approval. PBMCs were processed and stored in liquid nitrogen at 6 million cells per aliquot or more.48 The analyzed samples came from the WIHS vascular substudy, in which subclinical atherosclerosis was measured by carotid artery intima-media thickness using high-resolution B-mode carotid artery ultrasound.19 Presence of lesions was defined as a focal intima-media thickness >1.5 mm in any of the imaged segments.49 There were 778 WIHS participants with serial carotid artery plaque data between 2004 and 2012.50 Among these, 758 had HLA genotyping available at the time of the analysis,51 with 106 identified as DRB1*07:01+ (55 sequenced genotypes and 51 imputed genotypes). Excluding HIV+ participants meeting clinical or immunologic (CD4+ count <200 cells/uL) criteria for AIDS, as well as participants with carotid plaque who had a history of statin use, resulted in 69 participants in the following 4 groups defined by HIV and/or sCVD (defined as at least one plaque assessed during the study): HIV−sCVD+ (N=9), HIV−sCVD− (N=22), HIV+sCVD+ (N=9), and HIV+sCVD− (N=29). From these 69 participants, we selected 2 quartets (8 participants in total), with each quartet containing a participant from each HIV/sCVD group matched by age, race, smoking status and menopause status. HLA Genotyping were performed as previously described.52
APOB-p18 DRB1*07:01 Tetramer
Recombinant HLA-DR proteins were generated as described.53 Briefly, HLA-DR was purified from the supernatants of transfected insect cells, biotinylated, and dialyzed into 0.1 mol/L phosphate buffer. Biotinylated monomer was loaded with 0.2 mg/mL of SLFFSAQPFEITAST peptide by incubating at 37°C for 72 hours in the presence of 2.5 mg/mL n-octyl-β-d-glucopyranoside and 1 mmol/L Pefabloc SC protease inhibitor (Sigma-Aldrich) and then conjugated using phycoerythin- or allophycocyanin-conjugated streptavidin (Biosource International) at a molar ratio of 8 to 1.
T cell Media
Each aliquot (40ml) of RPMI 1640 including glutamin (with 25mM HEPES) was supplemented with 7.2mL pooled human serum (Gemini Bio), 0.48mL penicillin/streptomycin, filtered through a 0.2μm bottle top filter and stored at 4°C.
APOB-p18 DRB1*07:01 tetramer staining
We suspended CD4 T cells in T cell medium at 2-10 million/ 50 μl and added 2μl of Fc blocking reagent (Miltenyi Biotec) at room temperature (RT) for 5min. Dasatinib (STEMCELL Technologies) was added at a final concentration of 50 nM and incubated at 37°C for 15 minutes. APOB-p18 DRB1*07:01 tetramer (2 μl each of PE− and APC-conjugated) was added into each FACS tube and incubated in the dark at RT for 2 hours. The cells were washed with 2 mL sorting buffer (2%FBS containing PBS). All APC+PE+ cells were gated based on mismatched controls (DRB1*07:01-negative) and sorted.
Human antibody staining and sorting
After live dead aqua (1:1000, Thermo Fisher Scientific) staining (in PBS, 4°C for 20min), cells were stained with antibodies (listed in Supplementary table 3) in T cell media on ice in the dark for 30min. Cells were washed twice with 2 mL flow buffer (PBS with 2%FBS, Gemini Bio). The cells were sorted by the FACS sorter (FACSAria, BD) into 4 Eppendorf tubes (DNA low bind), each of which contained 100μl flow buffer. Some surplus of the sorted Th1 and Tmem were discarded. We used human FOXP3 antibody (206D, Biolegend) and FoxP3/Transcription Factor Staining Buffer Set (eBioscience) for FOXP3 staining, and the data was acquired on LSR II flow cytometer (BD Biosciences) using the FACSDiva software (BD Biosciences) and analyzed with FlowJo software (BD Biosciences and FlowJo LLC) in some experiment.
Confocal Imaging & Processing
Blood CD4 T cells (after enrichment) from a donor with DRB1*701 were stained with ApoB:MHC (APC and PE), TCR-αβ (AF488, clone IP26, Biolegend), and live-dead (aqua) and sorted into the indicated cell populations (TCRαβ+tet-APC and PE+, and TCRαβ+tet-APC and PE−, separately) on an Aria cytometer (BD, San Diego, USA). Nozzle size was 70nM. Cells were collected in FACS media (2%FBS-PBS),stained with Hoechst for nuclei, fixed in 4%PFA, re-suspended with Prolong Diamond and spun onto glass slides and cover with a coverslip. 3D image stacks were acquired with a Zeiss laser scanning confocal microscope (LSCM) 880 Airyscan using a 63x (1.4na) objective and the 32-channel GaAsP-PMT area detector. All 16 bit image stacks (on average 30 slices) were acquired with Nyquist resolution parameters using a 0.159 μm step size and optimal frame size of 2100x2100 pixels. Airyscan images were then processed in Zen 3.0 (Zeiss Inc), where they underwent pixel reassignment and deconvolution and then presented as maximum intensity projections (MIPs) in figures.
Staining for hashtag oligo (HTO) antibody
Each single cell suspension in a 1.5mL sterile Eppendorf tube containing no more than 1 million cells in 50 μL of staining buffer (2% FBS in 1X PBS) was incubated with 2.0 μL of unique hashtag oligonucleotide antibody (1:1 ratio of β2-microglobulin and CD298, Biolegend) to each sample (Treg, Th1 and Tmem). For the rare cell (Tet+ cell) population, we prepared a single cell suspension containing no more than 1000 cells in 50 μL of staining buffer, and added 2 μL of 1:20 diluted hashtag oligonucleotide antibody. TotalSeq™-C0251 anti-human Hashtag 1 Antibody (barcode sequence: GTCAACTCTTTAGCG), Hashtag 2 Antibody (TGATGGCCTATTGGG), Hashtag 3 Antibody (TTCCGCCTCTCTTTG), Hashtag 4 Antibody (AGTAAGTTCAGCGTA), Hashtag 5 Antibody (AAGTATCGTTTCGCA) were used (all from Biolegend). All samples were incubated on ice for 30 minutes. We transferred the bulk samples to 5mL Eppendorf tubes using 3.5 mL of staining buffer as the first wash, centrifuged the bulk samples at 400xg for 5 minutes at 4°C, carefully decanted the supernatant. We washed the cells of Th1, Tmem and Tregs with 3.5mL of staining buffer and centrifuged at 400xg for 5 minutes at 4°C followed by carefully decanting and dabbing two more times for a total of three washes, counted the cells in each sample with the Moxi mini (ORFLO Technologies) and pooled Th1, Tmem and Tregs. 500 μL of staining buffer was added to each Tet+ sample. We centrifuged the bulk samples at 400xG for 5 minutes at 4°C. We added ~12,000 cells of the Th1, Tmem and Treg mix to the entire rare cell sample (Tet+ cell tube) and resuspended them, ensuring that there was no leftover staining buffer in the pipette tip. We again centrifuged the mixed samples at 400xg for 5 minutes at 4°C, aspirated the supernatant, and resuspended the tube to a concentration of ~1,000 cells/uL. We added the cells to the 10x Genomics Step 1 master mix and proceeded with the 10x Genomics 5’ protocol to completion. A graphical summary from sorting to the end of HTO staining and the following procedures is shown in Extended Data Fig. 2.
Quality control after sequencing and data processing
After demultiplexing using Cell Ranger (www.10xgenomics.com), we retrieved 1368 to 3097 cells (median 1948 cells) per a sample, and totally 21,411 cells with transcriptomes (Supplementary table 2). Among all the cell types, we retrieved 10-41 Tet+ cells (median 20 cells), 13-535 Treg cells (median 89 cells), 510-1438 Th1 cells (median 634), and 539-1531 Tmem cells (median 756 cells) per a sample (Supplementary table 2). After doublet (DoubletFinder v3,54) and low viability (mitochondrial gene content >10%) removal and batch effect correction, 16,644 cells remained. Overall, 33,538 genes were detected. The median number of genes per cell was 1,678 based on UMI (unique molecular identifier) counts. Median UMI counts per cell was 5784. Doublets, low quality and proliferating cells were removed and batch effects were controlled by Harmony,55 taking advantage of spiked-in PBMCs from the same donor sequenced on each run. The data is log2 normalized, and we used Seurat v4.0 for differentially expressed genes and Dotplots, and Seaborn v0.9.0 in Python for plotting the heatmaps. Ingenuity Pathway Analysis (QIAGEN) was used for the pathway analysis.
Data availability
Data is available on NCBI GEO database (the accession number: GSE199103).
TCR analysis
TCRs for the cells were assembled using Cell Ranger v3.1.0 vdj module based on the Ensembl reference genome GRCh38 v3.1.0 available from 10X Genomics. We discarded cells which had less than 500 genes. Among a total of 13,769 high quality cells, TCRα was observed in 11,534 cells (fraction of total number of cells, 83%), TCRβ was assembled in 12,491 cells (90%), and either TCRα or TCRβ are in 12,598 (91%). Clonality index was defined as 1-H/ loge(n) [H, Shannon index, the negative value of the sum of pi loge(pi); i, from 1 to n].56
Cell type calling
Cell types (Tet+, Th1, Treg and Tmem) was based on HTO expression (Extended Data Fig. 8). The expression of HTO1 (added to Tet+ cells) was observed in all the cells, as expected (Extended Data Fig. 8A). Tet+ cells were called based on expression of HTO1 and no expression of HTO2-5 (Extended Data Fig. 8F). 19,615 cells were assigned to Th1, Tmem, Treg, Tet+ or bulk CD4 T cells.
Distance Calculation
For each of the donors, we calculated the distance between the tetramer+ cells and the pseudobulk transcriptomes (calculated median transcriptomes) of the three other cell types, namely Tmem, Th1, and Treg. The cosine distance of these pseudobulk transcriptomes from each tetramer+ cell was calculated as 1-(u・v)/(llull2llvll2) (the cosine distance between u and v, u・v is the dot product of u and v). The cosine distance measure is robust to the outliers.
Lineage-tracker mice
For fate mapping of exTregs, Foxp3eGFP-Cre-ERT2 (Jackson; #016961) mice were crossed to B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Jackson; #007914) and B6.129P2-Apoetm1Unc/J (Jackson; #002052) to obtain the lineage tracker Apoe−/− mice (Foxp3eGFP-Cre-ERT2;Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J;129P2-Apoetm1Unc/J, ROSA26-SORT-CAG/FoxP3-eGFP-ERT-Cre/Apoe−/−). Mice received tamoxifen injections (75 mg/kg body weight; i.p) for 5 days at 8 weeks old and again one week before the experiment to induce Cre, resulting in GFP and RFP expression in Foxp3+ cells. These animals were used to distinguish current Tregs (GFP+RFP+) from exTregs (GFP-RFP+). 8-10 weeks old female B6.129P2-Apoetm1Unc/J mice (Apoe−/−) were used as recipients for adoptive transfer experiments. The housing conditions for these mice were as follows; lights on at 6am and off at 6pm, ambient temperature between 69-75 degrees, humidity within 30%-70% (average 40%, outdoor environment can affect humidity inside).
Immunizations
Mouse peptides mApoB-100978–992, designated as ApoB-p6 (TGAYSNASSTESASY, TC peptide labs), and MOG35–55 (MEVGWYRSPFSRVVHLYRNGK, R&D sytems), were resuspended in dimethyl sulfoxide (Sigma Aldrich) at 20 mg/ml concentration. Lineage-tracker Apoe−/− mice were immunized intramuscularly with ApoB-p6 or MOG peptides (100μg per mouse) emulsified in in 50% CFA (BD Biosciences). After two weeks, each mouse received a 100μg booster dose of the priming peptide emulsified in 50% IFA (incomplete Freund’s adjuvant, BD Biosciences), also administered intramuscularly. Two weeks later mice were euthanized and blood, spleen, lymph nodes, aorta and heart were collected.
Adoptive Transfer Studies
Cells from lymph nodes and spleens from pooled p6- or MOG-immunized lineage tracker mice were extracted and enriched for CD4 T cells (StemCell). exTreg were sorted by FACS ARIA II (BD Biosciences) gating on lymphocyte morphology, single cells and live cells (DAPI−)CD4+TCRb+GFP−RFP+. Anti-mouse CD4 antibody (clone GK1.5, Biolegend), and anti-mouse TCRβ antibody (clone H57-597, Biolegend) were used. 2.105 exTreg were injected retro-orbitally in 100uL of PBS 1X in 8-10 weeks old 6Gy irradiated female Apoe−/− mice that had been on WD (WD; 42% kcal from fat, 0.2% cholesterol) for 3 weeks and antibiotics 3 days before and for 14 days. The other mice were injected with 100uL of PBS as negative control. Recipient mice continued on WD for 5 more weeks. At the end of the experiment, blood and aortas were harvested to determine engraftment, plasma IFNγ (Invitrogen ELISA) and atherosclerotic lesions (Sudan-IV), respectively.
Mouse restimulation assay of antigen-specific CD4+T cells
Single cell suspension of mouse splenocytes were plated in 96-well round-bottom wells at a density of 2x106 cells per conditions. Restimulation was performed in L-glutamine containing RPMI 1640 (Gibco) medium supplemented with 10% FBS (Gemini Bio), 10 mM HEPES, 1X MEM Non-Essential Amino Acids Solution, 50μM 2-mercaptoethanol (all from Gibco) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were stimulated with either ApoB-p6 or MOG peptide (25 μg/ml) or with phorbol 12-myristate 13-acetate (PMA) and ionomycin (1X; Cell Stimulation Cocktail, eBioscience) and incubated for 6h at 37°C with 5% CO2. Unstimulated cells served as negative control. Last 4h of incubation was performed in the presence of protein transport inhibitors brefeldin and monensin (1X; Protein transport inhibitor cocktail, eBioscience). After the stimulation period, cells were washed with FACS buffer (PBS w/o Ca/Mg, 2% FBS) and resuspended in staining master mix containing anti-human Fc-Block (Biolegend), fixable Ghost 510 viability dye (Tonbo Biosciences) and fluorochrome-conjugated antibodies against the following cell surface markers: anti-TCRβ-AF700, H57-597; anti-CD4-BV785, GK1.5); anti-CD8b-APC-Cy7, YTS156.7.7; and anti-CD44-PeCy7, IM7. For intracellular staining of cytokines and detection of Foxp3 transcription factor, cells were fixed and permeabilized with the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) according to manufacturer’s instructions. Cells were stained with anti-Foxp3-AF488, MF14; anti-TNF-APC, MP6-XT22; and anti-IFNγ-BV421, XMG1.2. All anti-mouse antibodies were purchased from Biolegend and were used at a final dilution of 1:50 (for cytokines and transcription factor) and 1:100 (for surface markers). Data was acquired on LSR II flow cytometer (BD Biosciences) using the FACSDiva software (BD Biosciences) and analyzed with FlowJo software (BD Biosciences and FlowJo LLC). Live single CD4+CD8− T cells from mice immunized with ApoB-p6 or MOG were restimulated by ApoB-p6 or MOG, and the percentage of activated (CD44+TNF+ or CD44+IFNγ+) cells were determined by FACS. Crossover experiments (cells from ApoB-p6-immunized mice restimulated with MOG or MOG immunized mice with ApoB-p6) were used as specificity controls. PMA-stimulated Tregs and exTregs from these mice were analyzed by intracellular staining for TNF and IFNγ (gating details are in Extended Data Fig. 7B and C).
Extended Data
Extended Data Fig. 1. MHC-II tetramer DRB1*07:01 APOB-p18 validation.
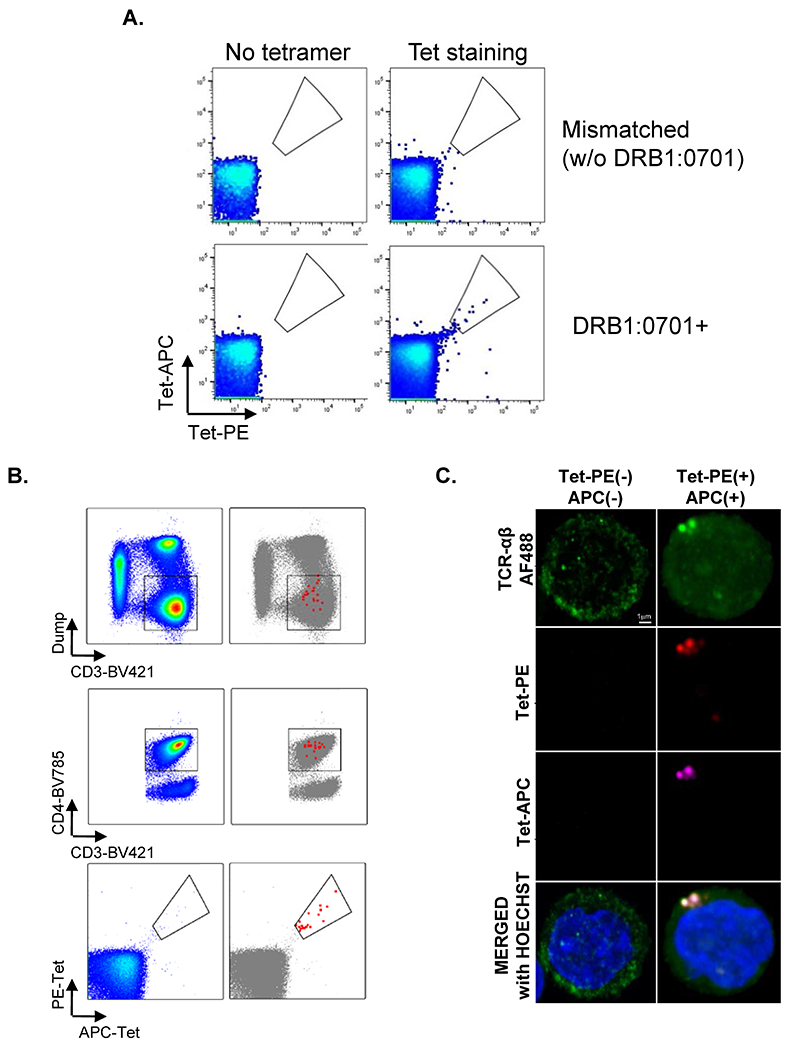
Peripheral blood mononuclear cells from healthy donors with DRB1:701 and with other DRB1 rather than DRB1:0701 were gated on CD3+CD4+TCRαβ+ CD4+. A, From the gated cells, we detected tetramer-PE and tet-APC double positive cells in the donor with DRB1:0701+ (right bottom), while no Tet+ cells were detected in no tetramer staining (left column) and mismatched controls (without DRB1*0701, right top). B, Backgating showed that tet-PE and tet-APC positive cells were in CD3+CD4+Dump−. To estimate the false positive rate of tetramer binding, we calculated the combinational specificity of tetramer binding. Let the fraction of APC-single positive cells in CD4 T cells be p(APC), the fraction of PE-single positive cells be p(PE), and the fraction of double positive cells be p(DP), then specificity can be calculated. Non-specific binding would randomly produce APC+PE− or APC-PE+ (single positive) cells. If all tetramer binding were non-specific, the fraction of DP would be expected to be equal to the product of p(APC) times p(PE). The fraction of true specific binding of tetramer APC and PE double positive cells in CD4 T cells can be calculated by p(DP)-p(APC)*p(PE). [p(DP)-p(APC)*p(PE)]/p(DP) was above 99.99% in all experiments (99.99782452%, 99.9998338%, 99.99946356% and 99.99800718%). Thus, there is negligible false positive staining. C, Confocal microscopy of human CD4+ T cells from donors with DRB1:701 after incubation with PE− and APC-labeled apoB: MHC-II tetramer DRB1*07:01 APOB-p18 and anti-TCR-β-FITC. The result was repeated once.
Extended Data Fig. 2. Schematic summary of sorting and hashtag oligo (HTO) staining.

Tet+ cells, Th1 cells, Treg, and CXCR3-memory T cells (Tmem) were sorted into 4 different tubes, and were stained with antibodies with hash tag oligo (HTO1-4). Treg, Th1, and Tmem were pre-gated for CD45RA−. After staining of HTO antibody, tet+ cells weren’t washed, not to lose any cells, because one wash would lose almost half of the cells. Other cells (Th1, Treg and Tmem) were washed three times following to the manufacturer’s instruction. The volume of each HTO antibody had previously been titrated. The cell number of these three cell types (Th1, Treg and Tmem) was appropriately adjusted, and they are merged into the tet+ cell tube. At the same time, bulk CD4 T cells from a healthy donor were merged into the tube for the following batch correction. After that, the sample proceeded to barcording, cDNA amplication, library preparation, and sequencing.
Extended Data Fig. 3. The analysis of the usage of TCRα and β, separately.

Separate TCR clonotypes of the TCRα and β sequences and VDJ usage. A-E, Pie chart for all the TCRβ clonotypes from all the cells (A) and each 4 cell type (B, Tet+; C, Treg; D, Th1; E, Tmem), with clonality index. F-J, Pie chart for all the TCRα clonotypes from all the cells (F) and each 4 cell type (G, Tet+; H, Treg; I, Th1; J, Tmem), with clonality index. Clonotypes with more than 1 clone exploded in the graph. Top 5 clonotypes with more than 1 clone are as shown. ct, clonotype. Clonotype3326, which was expanded in Tet+ cells, are shared with other cell types, and highlighted in red.
Extended Data Fig. 4. UMAPs of Th1 and Tmem, and signature genes and molecule expressions.
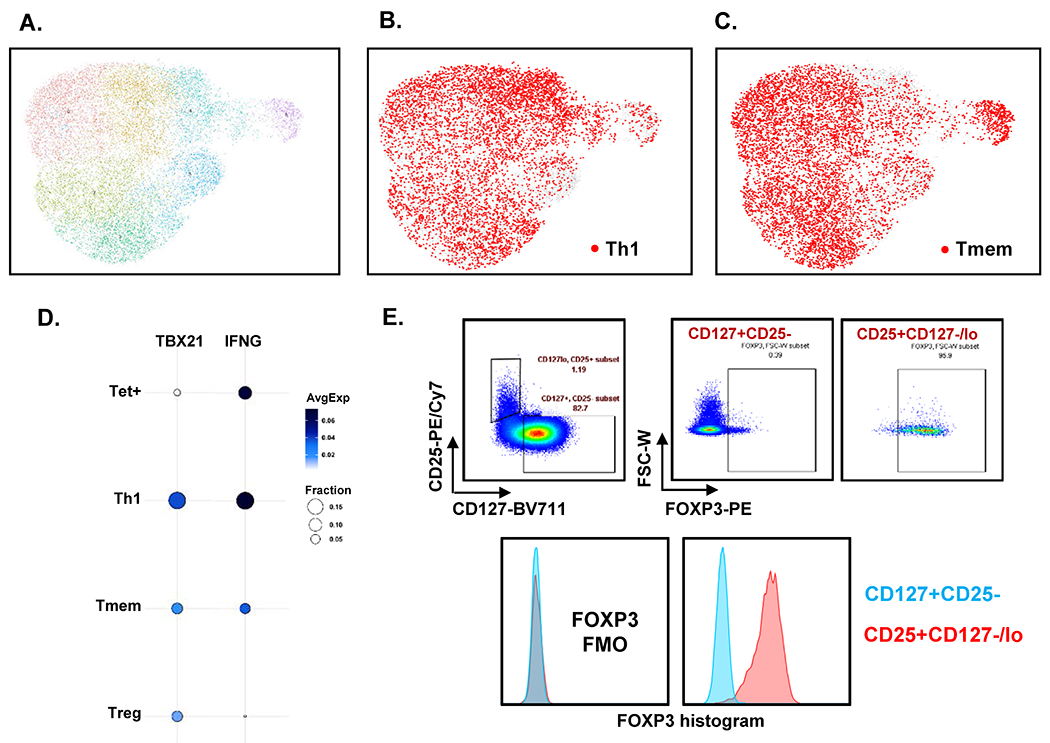
A, UMAP with Louvain clustering of all 16,644 cells. B, C, UMAP of Th1 (B) and Tmem (C) highlighted in red. Other cells light grey. D, Expression levels of Th1 signature genes on 4 cell types. TBX21 and IFNG expressions are shown. Dot plot: fraction of cells in cluster expressing each gene shown by size of circle and level of expression shown from white (=0) to dark blue (=max, log2 scale). E, We checked FoxP3 expression in CD3+CD4+CD127−CD25+ cells and the percentage was 91.3±4.06% (Mean±SD), The representative image of plots and the histogram of FoxP3 expression was shown.
Extended Data Fig. 5. UMAPs of tet+ cells from sCVD− and sCVD+ participants without HIV.

A, B, APOB-p18 DRB1*07:01 tetramer positive cells (tet+ cells, solid circles) are plotted in UMAP of cell from HIV−sCVD− participants (A), from HIV−sCVD+ participants (B). Treg, Th1 and Tmem distribution are shown as contour plots of density.
Extended Data Fig. 6. The analysis of the similarity of tet+ cells to other cell types in HIV+.
APOB-p18 DRB1*07:01 tetramer positive cells (Tet+ cells, solid circles) are plotted in UMAP of cell from HIV+sCVD− participants (A), from HIV+sCVD+ participants (B). Treg, Th1 and Tmem distribution are shown as contour plots of density. C, Cumulative histogram of the distances of each Tet+ cells against Tmem, Treg and Th1 in HIV-. D-F, Cumulative histograms of the distances of each of the Tet+ cells against Treg (D), Th1 (E), and Tmem (F) cells, separately for sCVD+ (yellow) and sCVD− (purple) and HIV+ in the first 6 PCA components. Significance by Kolmogorov-Smirnov test. G, A Ternary plot of relative median positions of Tet+ cells relative to pseudobulk mean of Tregs, Th1 and Tmem in the first 6 PCA components in HIV+. H, I, Volcano plots comparing gene expression in single cells of tet+ cells compared to Treg, Th1, and Tmem in HIV+sCVD− (H), and HIV+sCVD+ (I). Differential expression analysis was performed using Seurat’s non-parametric Wilcoxon rank-sum test to extract marker genes. Significant markers were selected based on Bonferroni-adjusted P-Values <0.05. Colored dots (upregulated genes in red, and downregulated genes in blue) indicate significantly differentiated expressed genes (adjusted p-value <0.05). Dashed line indicates adjusted p-value of 0.05. Full data set shown in Supplemental Excel File 5.
Extended Data Fig. 7. exTreg gating strategy and frequency of exTreg among CD4T cells in blood.

A, exTreg gating strategy of exTreg adoptive transfer experiment. Cells from lymph nodes and spleens from pooled p6- or MOG-immunized lineage tracker mice were extracted and enriched for CD4 T cells. exTreg were sorted gating on lymphocyte morphology, single cells and live cells (DAPI−)CD4+TCRb+GFP-RFP+. B, C, Gating strategy of CD4T cell (B) and exTreg among them (C) in restimulation assay. D, Engraftment of exTreg gating strategy. Single, DAPI−CD4+TCRb+GFP-RFP+ were checked. E, Frequency of exTreg among CD4T cells in blood. 5 weeks after adoptive transfer. p6, the recipient mice of exTreg from p6-immunized mice (n=3); PBS, PBS injected mice (n=5). Kruskal-Wallis and Dunns’s multiple comparisons test was performed (p=0.0073, two-sided). **, p<0.01. Bars represent mean values with standard error of mean (SEM).
Extended Data Fig. 8. Hashtag oligo (HTO) expressions for cell type identification.

A-E, HTO expressions (HTO1-5) on UMAP from HTO expressions (A, HTO1; B, HTO2; C, HTO3; D, HTO4; E, HTO5). F, Cell calling based on HTO expressions. Doublets were removed.
Supplementary Material
ACKNOWLDGEMENTS
Data in this manuscript were collected by WIHS, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
SOURCES OF FUNDING
Supported by Japan society for the promotion of science overseas research fellowship, and the Uehara Memorial Foundation research fellowship to R.S., NIH HL 136275, 145241, 148094 to K.L., K01HL137557 to D.B.H. The Zeiss LSM 880 Airyscan Confocal was funded by the NIH S10OD021831 grant.
Footnotes
DISCLOSURES
There are no conflicts of interest.
REFERENCES
- 1.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, Jenkins MK, James EA, Kwok WW, Hanna DB, Kaplan RC, Strickler HD, Durkin HG, Kassaye SG, Karim R, Tien PC, Landay AL, Gange SJ, Sidney J, Sette A, Ley K. Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule–Restricted Peptide Epitopes of Apolipoprotein B. Circulation. 2018;138:1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf D, Gerhardt T, Winkels H, Michel NA, Pramod AB, Ghosheh Y, Brunel S, Buscher K, Miller J, McArdle S, Baas L, Kobiyama K, Vassallo M, Ehinger E, Dileepan T, Ali A, Schell M, Mikulski Z, Sidler D, Kimura T, Sheng X, Horstmann H, Hansen S, Mitre LS, Stachon P, Hilgendorf I, Gaddis DE, Hedrick C, Benedict CA, Peters B, Zirlik A, Sette A, Ley K. Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4+ T-Regulatory Cells. Circulation. 2020;142:1279–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobiyama K, Ley K. Atherosclerosis: A Chronic Inflammatory Disease With an Autoimmune Component. Circ Res. 2018;123:1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. [DOI] [PubMed] [Google Scholar]
- 7.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of Naturally Occurring CD4+CD25+ Regulatory T Cells in Experimental Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. [DOI] [PubMed] [Google Scholar]
- 8.Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DFJ, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Lüscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw MK, Tse KY, Zhao X, Welch K, Eitzman DT, Thipparthi RR, Montgomery PC, Thummel R, Tse HY. T-Cells Specific for a Self-Peptide of ApoB-100 Exacerbate Aortic Atheroma in Murine Atherosclerosis. Front Immunol. 2017;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Robertson A-KL, Hjerpe C, Hansson GK. Adoptive Transfer of CD4 + T Cells Reactive to Modified Low-Density Lipoprotein Aggravates Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. [DOI] [PubMed] [Google Scholar]
- 11.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. [DOI] [PubMed] [Google Scholar]
- 12.Stubbington MJT, Lönnberg T, Proserpio V, Clare S, Speak AO, Dougan G, Teichmann SA. T cell fate and clonality inference from single-cell transcriptomes. Nat Methods. 2016;13:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto C, Bombardi RG, Kozhevnikov M, Sinkovits RS, Chen EC, Branchizio A, Kose N, Day SB, Pilkinton M, Gujral M, Mallal S, Crowe JE. High Frequency of Shared Clonotypes in Human T Cell Receptor Repertoires. Cell Rep. 2020;32:107882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. [DOI] [PubMed] [Google Scholar]
- 15.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M-C, Tien PC, Kassaye SG, Anastos K, Cohen M, Minkoff H, Wingood G, Ofotokun I, Fischl MA, Gange S. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza G, Golub ET, Gange SJ. The Changing Science of HIV Epidemiology in the United States. Am J Epidemiol. 2019;188:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, Rajagopalan S, Kottilil S, Nair H, Newby DE, McAllister DA, Mills NL. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation. 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung M, Parrinello CM, Xue X, Mack WJ, Anastos K, Lazar JM, Selzer RH, Shircore AM, Plankey M, Tien P, Cohen M, Gange SJ, Hodis HN, Kaplan RC. Echolucency of the Carotid Artery Intima-Media Complex and Intima-Media Thickness Have Different Cardiovascular Risk Factor Relationships: The Women’s Interagency HIV Study. J Am Heart Assoc. 2015;4:e001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Qian S, Gong Y, Ren J, Zhao L, Wang D, Wang X, Zhang Y, Wang Z, Zhang Q. Deep sequencing of the T cell receptor β repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget. 2017;8:99312–99322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X, Li X. CD4+CD25+CD127low/− T Cells: A More Specific Treg Population in Human Peripheral Blood. Inflammation. 2012;35:1773–1780. [DOI] [PubMed] [Google Scholar]
- 22.Tippalagama R, Singhania A, Dubelko P, Lindestam Arlehamn CS, Crinklaw A, Pomaznoy M, Seumois G, deSilva AD, Premawansa S, Vidanagama D, Gunasena B, Goonawardhana NDS, Ariyaratne D, Scriba TJ, Gilman RH, Saito M, Taplitz R, Vijayanand P, Sette A, Peters B, Burel JG. HLA-DR Marks Recently Divided Antigen-Specific Effector CD4 T Cells in Active Tuberculosis Patients. J Immunol. 2021;207:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin C, Mohanta SK, Srikakulapu P, Weber C, Habenicht AJR. Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity. Front Immunol. 2016;7:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldreich T, Carlsson AC, Risérus U, Larsson A, Lind L, Ärnlöv J. The association between serum cathepsin L and mortality in older adults. Atherosclerosis. 2016;254:109–116. [DOI] [PubMed] [Google Scholar]
- 25.Cho SH, Raybuck AL, Blagih J, Kemboi E, Haase VH, Jones RG, Boothby MR. Hypoxia-inducible factors in CD4+ T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc Natl Acad Sci U S A. 2019;116:8975–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J Biol Chem. 2001;276:10134–10144. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DD, Quintero CM, Stover PJ. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc Natl Acad Sci U S A. 2011;108:15163–15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan KF, Hechenberger M, Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol. 1994;127:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregersen I, Sandanger Ø, Askevold ET, Sagen EL, Yang K, Holm S, Pedersen TM, Skjelland M, Krohg-Sørensen K, Hansen TV, Dahl TB, Otterdal K, Espevik T, Aukrust P, Yndestad A, Halvorsen B. Interleukin 27 is increased in carotid atherosclerosis and promotes NLRP3 inflammasome activation. PloS One. 2017;12:e0188387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu WW, Wang H, Chen XH, Fu SW, Liu ML. miR-34b-3p May Promote Antiplatelet Efficiency of Aspirin by Inhibiting Thromboxane Synthase Expression. Thromb Haemost. 2019;119:1451–1460. [DOI] [PubMed] [Google Scholar]
- 31.Jing Z, Yuan X, Zhang J, Huang X, Zhang Z, Liu J, Zhang M, Oyang J, Zhang Y, Zhang Z, Yang R. Chromosome 1 open reading frame 190 promotes activation of NF-κB canonical pathway and resistance of dendritic cells to tumor-associated inhibition in vitro. J Immunol Baltim Md 1950. 2010;185:6719–6727. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Riou C, Davidson D, Minhas R, Robson JD, Julius M, Arnold R, Kiefer F, Veillette A. Synergistic regulation of immunoreceptor signaling by SLP-76-related adaptor Clnk and serine/threonine protein kinase HPK-1. Mol Cell Biol. 2001;21:6102–6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsov YP, Niec R, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W, Taube JM, Zheng L, Luo L, Zhu G, Chen J, Chen L. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen B, Wang W, Shen T, Qi R. Thioredoxin1 downregulates oxidized low-density lipoprotein-induced adhesion molecule expression via Smad3 protein. PloS One. 2013;8:e76226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakic A, Chaabane C, Ambartsumian N, Klingelhöfer J, Lemeille S, Kwak BR, Grigorian M, Bochaton-Piallat M-L. Neutralization of S100A4 induces stabilization of atherosclerotic plaques: role of smooth muscle cells. Cardiovasc Res. 2022;118:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawaki D, Hou L, Tomida S, Sun J, Zhan H, Aizawa K, Son B-K, Kariya T, Takimoto E, Otsu K, Conway SJ, Manabe I, Komuro I, Friedman SL, Nagai R, Suzuki T. Modulation of cardiac fibrosis by Krüppel-like factor 6 through transcriptional control of thrombospondin 4 in cardiomyocytes. Cardiovasc Res. 2015;107:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X, Zhang S, Guo Z, Xing D, Chen W. The crosstalk of ABCA1 and ANXA1: a potential mechanism for protection against atherosclerosis. Mol Med Camb Mass. 2020;26:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durst R, Sauls K, Peal DS, deVlaming A, Toomer K, Leyne M, Salani M, Talkowski ME, Brand H, Perrocheau M, Simpson C, Jett C, Stone MR, Charles F, Chiang C, Lynch SN, Bouatia-Naji N, Delling FN, Freed LA, Tribouilloy C, Le Tourneau T, LeMarec H, Fernandez-Friera L, Solis J, Trujillano D, Ossowski S, Estivill X, Dina C, Bruneval P, Chester A, Schott J-J, Irvine KD, Mao Y, Wessels A, Motiwala T, Puceat M, Tsukasaki Y, Menick DR, Kasiganesan H, Nie X, Broome A-M, Williams K, Johnson A, Markwald RR, Jeunemaitre X, Hagege A, Levine RA, Milan DJ, Norris RA, Slaugenhaupt SA. Mutations in DCHS1 cause mitral valve prolapse. Nature. 2015;525:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao H-X, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. [DOI] [PubMed] [Google Scholar]
- 42.Ali AJ, Makings J, Ley K. Regulatory T Cell Stability and Plasticity in Atherosclerosis. Cells. 2020;9:2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, Sorci-Thomas MG, Hedrick CC. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Picarda G, Benedict CA. Cytomegalovirus: Shape-Shifting the Immune System. J Immunol Baltim Md 1950. 2018;200:3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-Cell Proliferation and Plaque Instability in Acute Coronary Syndromes. Circulation. 2000;101:2883–2888. [DOI] [PubMed] [Google Scholar]
- 46.Mukhatayev Z, Ostapchuk YO, Fang D, Le Poole IC. Engineered antigen-specific regulatory T cells for autoimmune skin conditions. Autoimmun Rev. 2021;20:102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssens I, Cools N. Regulating the regulators: Is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cell Immunol. 2020;358:104236. [DOI] [PubMed] [Google Scholar]
- 48.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women’s Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clin Vaccine Immunol. 2005;12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, Anastos K, Tien PC, Sharrett AR, Hodis HN. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men: AIDS. 2008;22:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, Anastos K, Gange SJ, Landay AL, Lazar JM, Palella FJ, Tien PC, Witt MD, Xue X, Young MA, Kaplan RC, Kingsley LA. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuniholm MH, Xie X, Anastos K, Xue X, Reimers L, French AL, Gange SJ, Kassaye SG, Kovacs A, Wang T, Aouizerat BE, Strickler HD. Human leucocyte antigen class I and II imputation in a multiracial population. Int J Immunogenet. 2016;43:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuniholm MH, Kovacs A, Gao X, Xue X, Marti D, Thio CL, Peters MG, Terrault NA, Greenblatt RM, Goedert JJ, Cohen MH, Minkoff H, Gange SJ, Anastos K, Fazzari M, Harris TG, Young MA, Strickler HD, Carrington M. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology. 2010;51:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:R63–R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGinnis CS, Murrow LM, Gartner ZJ. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019;8:329–337.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fast Korsunsky I., sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L, Cham J, Paciorek A, Trager J, Sheikh N, Fong L. 3D: diversity, dynamics, differential testing – a proposed pipeline for analysis of next-generation sequencing T cell repertoire data. BMC Bioinformatics. 2017;18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on NCBI GEO database (the accession number: GSE199103).


