Abstract
Introduction:
Gaps in HIV testing of children persist, particularly among older children born before the expansion of the prevention of mother-to-child transmission of HIV programs.
Methods:
The Counseling and Testing for Children at Home study evaluated an index-case pediatric HIV testing approach. Caregivers receiving HIV care at 7 health facilities in Kenya (index cases), who had children of unknown HIV status aged 0–12 years, were offered the choice of clinic-based testing (CBT) or home-based testing (HBT). Testing uptake and HIV prevalence were compared between groups choosing HBT and CBT; linkage to care, missed opportunities, and predictors of HIV-positive diagnosis were identified.
Results:
Among 493 caregivers, 70% completed HIV testing for ≥1 child. Most caregivers who tested children chose CBT (266/347, 77%), with 103 (30%) agreeing to same-day testing of an untested accompanying child. Overall HIV prevalence among 521 tested children was 5.8% (CBT 6.8% vs HBT 2.4%; P = 0.07). Within 1 month of diagnosis, 88% of 30 HIV-positive children had linked to care, and 54% had started antiretroviral treatment. For 851 children eligible for testing, the most common reason for having an unknown HIV status was that the child’s mother was not tested for HIV or had tested HIV negative during pregnancy (82%).
Conclusion:
Testing uptake and HIV prevalence were moderate with nonsignificant differences between HBT and CBT. Standardized offer to test children accompanying caregivers is feasible to scale-up with little additional investment. Linkage to care for HIV-positive children was suboptimal. Lack of peripartum maternal testing contributed to gaps in pediatric testing.
Keywords: HIV testing, pediatrics, index case testing, family testing, health system gaps, prevention of mother-to-child transmission
INTRODUCTION
Late HIV diagnosis in children results in high mortality,1,2 opportunistic infections,3 and long-term adverse sequelae.4,5 Scaled up prevention of mother-to-child transmission (PMTCT), early infant diagnosis (EID) programs,6–8 and improved access to antiretroviral treatment (ART)9,10 have decreased HIV-related mortality among children globally. However, pediatric treatment coverage remains low at 50% worldwide, primarily because of gaps in pediatric HIV diagnosis,10–12 such as testing known HIV-exposed infants within PMTCT programs13,14 and identifying newly HIV-exposed infants whose mothers acquired HIV during the peripartum period.15–17 Although current WHO guidelines recommend repeat HIV testing during pregnancy, this guidance is suboptimally operationalized.18 Gaps in repeat maternal testing, PMTCT, and EID have left a substantial number of HIV-infected older children undiagnosed.
To identify these older children, provider-initiated testing and counseling (PITC) is recommended by World Health Organization (WHO) and many countries’ national guidelines,19,20 but it remains suboptimally implemented.21,22 Community-based approaches, such as door-to-door testing, reach children outside health facilities23 but are relatively expensive and generally have low testing yield.19,24 Index case testing–testing the children of HIV-positive adults—is an efficient alternative also recommended by WHO and is delivered programmatically in several countries. This strategy has a relatively high HIV prevalence (8.4%)19 and can diagnose children while asymptomatic.19,25,26 However, index case testing uptake is also suboptimal, with a pooled uptake of 52% across 5 studies in sub-Saharan Africa (SSA).19
One innovation to increase uptake of index case testing is home-based testing (HBT).27 Few studies have quantified the differences in uptake and yield between HBT and clinic-based testing (CBT) strategies in index case testing for children,28 and such information could guide resource allocation in scale-up. Additionally, although factors associated with HIV-positive diagnosis in children are well described in PMTCT cohorts of HIV-exposed infants,29–31 less is known of children reached through index case testing. Understanding these factors could help in designing interventions that reach children at highest risk of HIV and improving future index case testing programs.
In a pilot study of pediatric index case testing in Kenya, we found that systematic referral of adult index clients for child testing resulted in a 4-fold increase in pediatric testing, and HIV prevalence was 7.4%.25 Kenya’s adult and pediatric HIV prevalence at the time was 5.6% and 0.9%, respectively.32 The present study expanded this model and assessed the additional benefit of offering HBT as a complement to CBT to increase uptake. We assessed the gaps in the PMTCT/EID cascade that contributed to lack of prior child HIV testing, compared uptake and HIV prevalence of HBT and CBT, described linkage to care for HIV-positive children, and assessed predictors of HIV-positive diagnosis.
METHODS
Study Design and Population
The Counseling and Testing for Children at Home (CATCH) study was a prospective cohort study conducted between 2013 and 2016 at 6 sites in Nairobi city and 1 in Kisumu city. The study recruited male and female HIV-positive caregivers receiving HIV care services (index clients) and offered testing for their children aged 0–12 years of unknown HIV status (eligible children). Children were considered of unknown status if they had never been tested for HIV or had tested negative during infancy but had not completed testing after the cessation of breastfeeding. Infants ≤6 weeks and those enrolled in EID programs were ineligible. Male caregivers were enrolled only if the biological mother of the eligible child was deceased, not involved with care of the child, or had provided consent to test the children, to avoid inadvertent disclosure of the mother’s HIV status.
Study Procedures
Recruitment and Enrollment
Clinic staff screened all adults attending the HIV clinic and offered eligible index clients referral to study staff. Some of the referred clients did not reach the study staff for secondary eligibility assessment, which included verification of child(ren)’s age and biological mothers’ HIV status. Caregivers provided sociodemographic, HIV testing and treatment information, PMTCT, and hospitalization history for all children. Caregivers chose whether and where to test their children, either CBT on the same day, CBT at a later date or HBT, within 3 months after enrollment.
HIV Testing and Linkage
Testing was conducted by trained HIV testing counselors. Following Kenyan HIV testing guidelines,20,33 children <18 months (or if ≥18 months old and still breastfeeding) received an HIV DNA polymerase chain reaction test processed at the Molecular and Virology Laboratory at the University of Nairobi34; results were delivered to caregivers at the clinic. Those who tested negative and were still breastfeeding were linked to facility EID programs. For children ≥18 months, a series of rapid HIV tests were done per HIV testing guidelines.20,33 Children who tested positive in clinic were linked to care in a clinic of the caregiver’s choice. For HBT, the study counselors were accompanied by a community health worker (CHW) who facilitated home tracing and linkage to care for children who tested positive.
Follow-up and Study Exit
HIV-positive children were followed up at home, clinic, or through a phone call at 1, 3, 6, 9, and 12 months after diagnosis. Caregivers selected the follow-up visit location and modality and provided information on linkage to care and ART initiation.
Ethical Considerations
Kenyatta National Hospital Ethics and Research Committee and University of Washington Institutional Review Board provided ethical approval. Caregivers provided oral consent for screening and written consent for enrollment, child testing, and follow-up. With the caregiver’s permission, study staff sought assent from children aged 7–12 years.
Statistical Analysis
To estimate the number of eligible index clients in the study clinics, we adjusted the overall number of caregivers referred by clinic staff to study staff by multiplying by the proportion of caregivers who were seen by the study staff and deemed eligible after secondary assessment. Sociodemographic characteristics and HIV history of index clients were described using counts, proportions, medians, and interquartile ranges.
The primary outcome was the proportion of caregivers who completed testing for ≥1 child at the different testing venues (uptake). We compared uptake and HIV prevalence for children tested at the different venues using χ2 tests. We compared the mean number of eligible and tested children per family at each testing venue, and mean age of children tested in each venue using Wilcoxon rank-sum test. For children who tested positive, we calculated the proportion that linked to care and that started ART after 1, 3, 6, 9 and 12 months after diagnosis.
Caregiver- and child-level characteristics associated with a child being HIV positive were selected a priori, including child age, maternal age during pregnancy, maternal years of education, months child was breastfed, child’s gender, having an HIV-positive sibling, having a deceased sibling, history of hospitalization, child’s history of HIV testing during infancy, child’s assumed HIV status by caregiver, caregiver gender, and maternal HIV status during pregnancy. Generalized linear models, accounting for clustering by site, with log link and poisson family were used to calculate prevalence ratios (PRs) and 95% confidence intervals (CIs).
Analysis of PMTCT gaps was restricted to children with female index clients because of high missingness from male respondents. The PMTCT experience of the mother–child pairs during pregnancies of children aged 0–5 years versus 6–12 years at enrollment were compared using χ2 tests. We considered the mother–child pair’s first incomplete step in the PMTCT cascade (maternal HIV testing, maternal ART, child’s ARV prophylaxis, and child HIV testing) to be the primary reason the child remained of unknown HIV status. Consistent with methods previously described elsewhere, each child contributed only once in this gap analysis.21 Analysis was conducted using R Studio (Version 1.1.456, 2009–2018) and Stata 15.1 (College Station, TX).
RESULTS
Caregiver Recruitment, Enrollment, and Demographics
The CATCH study screened 69,047 adults; of whom, 2096 (3%) were eligible, and 493 (24%) enrolled. Reasons for ineligibility (n = 66,951) included not having children (16%), having only child(ren) with known HIV status (52%), and having a child of unknown status age ≥13 years (32%). Reasons for declining to participate when eligible (n = 1603) were being in a hurry to leave the clinic (47%), having children who lived far from the clinic (16%), needing to discuss with partner before enrolling (13%), preferring to test children in a different clinic (8%), not being interested in joining the study (7%), or other (9%) (see Figure 1, Supplemental Digital Content, http://links.lww.com/QAI/B539). Of the 493 enrolled, 80% were enrolled in Nairobi sites and 20% in Kisumu sites; 84% were female and 74% were on ART (Table 1).
FIGURE 1.
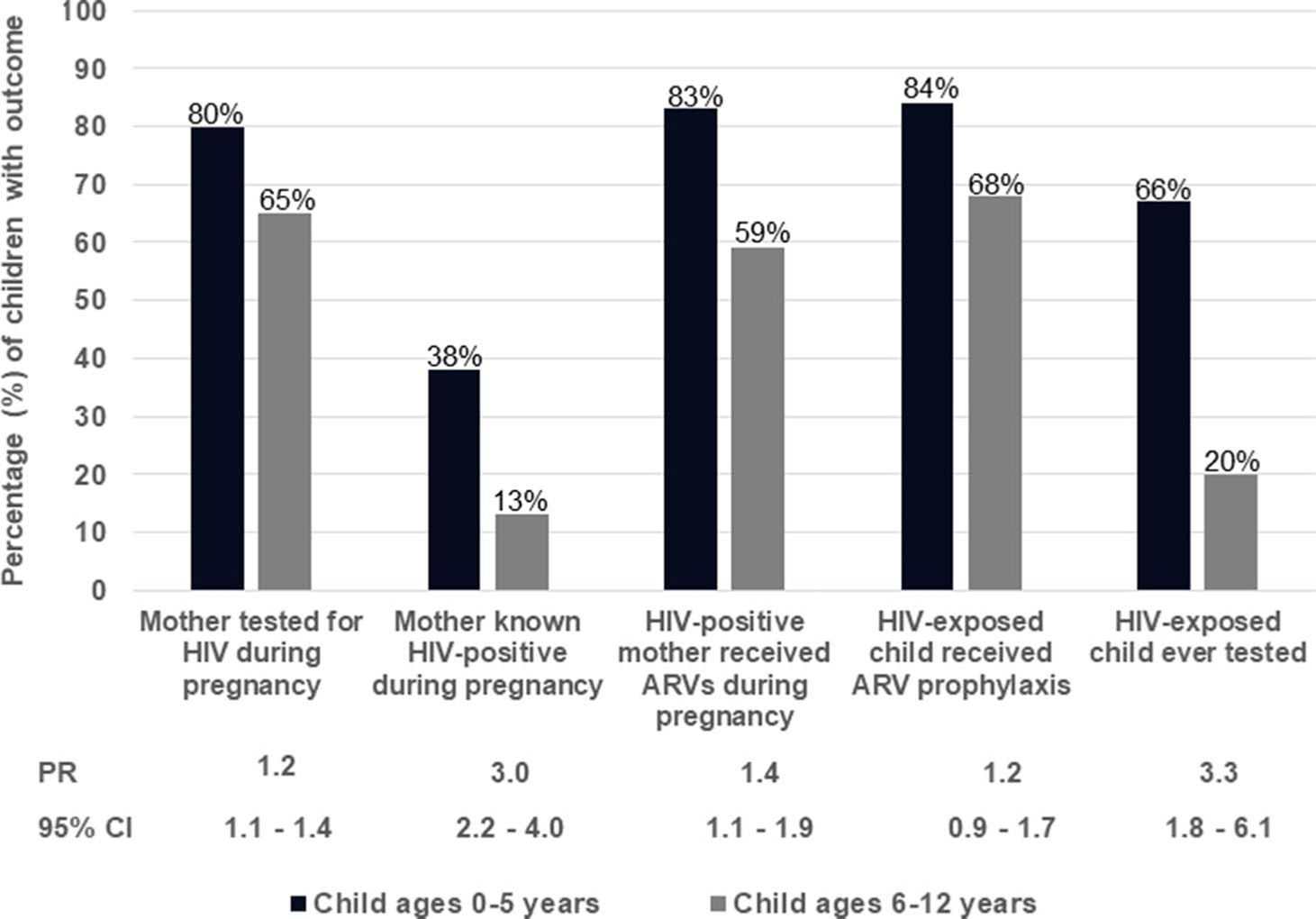
A comparison of PMTCT experience for mother–child pairs during and after pregnancy of children aged 0–5 years (n = 221) versus children aged 6–12 years (n = 410).
TABLE 1.
Index Client and Household Characteristics
| Characteristic | N | n (%) or Median (IQR) |
|---|---|---|
|
| ||
| Sociodemographic | ||
| Female | 493 | 414 (84) |
| Age, yr | 492 | 32 (28–38) |
| Years of school completed | 493 | |
| ≤ 8 yrs | 197 (40) | |
| 9–12 yrs | 207 (42) | |
| >12 yrs | 89 (18) | |
| Marital status | 493 | |
| Never married, no current partner | 36 (7) | |
| Previously married, no current partner | 145 (29) | |
| Currently married/steady partner | 312 (63) | |
| Partner HIV status | 312 | |
| Positive | 172 (55) | |
| Negative | 48 (15) | |
| Unknown | 92 (30) | |
| Has a paying job | 493 | 275 (56) |
| Monthly income ($) (among those with income) | 267* | 64 (43–128) |
| Crowding (≥3 people per room in home) | 493 | 267 (54) |
| HIV testing and treatment history | ||
| Years since HIV diagnosis | 493 | 1 (0–4) |
| Location of HIV diagnosis | 493 | |
| PMTCT | 142 (29) | |
| VCT | 140 (28) | |
| Hospital outpatient | 120 (24) | |
| Hospital inpatient | 75 (15) | |
| HBT | 16 (3) | |
| Reason tested for HIV | 493 | |
| Felt sick | 223 (45) | |
| PMTCT† | 137 (28) | |
| Routine VCT | 63 (13) | |
| Suspected partner was HIV positive | 30 (6) | |
| Partner diagnosed HIV positive | 27 (5) | |
| Child diagnosed HIV positive | 13 (3) | |
| Currently on ART | 492* | 367 (74) |
Denominators lower because of missing data.
Option relevant to only female index clients.
IQR, interquartile range; VCT, voluntary counseling and testing.
Completion of Testing at Home and Clinic
Overall, 347 of 493 caregivers (70%) completed testing for 521 of 851 eligible children (61%). Of 347 caregivers, 278 (80%) completed testing for 398 of 521 children (76%) in clinic; of whom, 145 (36%) tested on the same day in clinic. When considering test completion among all eligible index clients (N = 2096), only 17% completed testing for ≥1 child.
Of 493 caregivers, 115 caregivers (23%) initially preferred HBT, 105 (21%) CBT on the same day, 272 (55%) CBT at a later date, whereas 1 did not select a testing venue. The mean number of eligible children per caregiver was higher among caregivers who preferred HBT than those who preferred CBT on the same day (1.9 vs 1.6; P = 0.016) or CBT at a later date (1.9 vs 1.7; P = 0.012). Of the 115 who initially preferred HBT, 65 (57%) completed testing at home, 12 (10%) changed their mind and completed testing in clinic, and 38 (33%) did not complete testing. Among the 105 who initially preferred CBT on the same day, 103 (98%) tested in clinic, 1 (1%) tested at home, and 1 (1%) did not complete testing. Among the 272 who initially preferred CBT at a later date, 163 (60%) tested in clinic, 3 (1%) tested at home, and 106 (39%) did not complete testing. There was no difference in test completion between those who preferred HBT versus those who preferred CBT at a later date (67% vs 61%; P = 0.270) (Table 2).
TABLE 2.
Prevalence and Predictors of Uptake and HIV Diagnostic Yield for HBT Versus CBT
| HBT |
CBT |
||
|---|---|---|---|
| Characteristic | Mean (Range) or n (%) | P | |
|
| |||
| Caregivers’ initial preference of testing location (n = 492*) | 115 (23) | 377 (77) | |
| Eligible children per caregiver | 2 (1–6) | 2 (1–8) | 0.005§ |
| Caregivers with 1 child | 45 (18) | 205 (82) | |
| Caregivers with 2 children | 47 (28) | 118 (72) | |
| Caregivers with 3+ children | 23 (30) | 54 (70) | |
| Caregivers completing testing for at least 1 child (n = 347) | 69 (20)† | 278 (80)‡ | |
| Children tested per caregiver | 2 (1–5) | 1 (1–4) | 0.0003§ |
| Caregivers with 1 child | 33 (15) | 185 (85) | |
| Caregivers with 2 children | 24 (26) | 70 (74) | |
| Caregivers with 3+ children | 12 (34) | 23 (66) | |
| Children tested (n = 521) | 123 (24) | 398 (76) | |
| Child age (yr) | 7 (0–12) | 7 (0–12) | 0.282§ |
| Prevalence of HIV infection | 3 (2) | 27 (7) | 0.072∥ |
1 enrolled caregiver opted not to test their children.
4 of the 69 caregivers had initially chosen CBT, then switched to HBT
12 of the 278 caregivers had initially chosen HBT, then switched to CBT.
Wilcoxon rank-sum test comparing the age of children tested at home versus children tested in clinic.
χ2 test comparing proportion of children who tested HIV positive following HBT versus CBT.
The average age of children who were tested on the same day in clinic was younger than the average age of those tested at home (5.9 vs 7.1 years; P = 0.002) or in clinic at a later date (5.9 vs 7.2 years; P < 0.001). The mean number of children per family who were tested at home was greater than the number tested in clinic at a later date (1.8 vs 1.4; P = 0.001) or in clinic on the same day (1.8 vs 1.4; P = 0.003).
HIV Prevalence at Home and Clinic, Predictors of a Child Testing HIV Positive, and Follow-up After HIV Diagnosis
Overall HIV prevalence was 5.8%, with 30 of the 521 children testing positive. Among children who received HBT, HIV prevalence was 2.4% (3/123) compared with 6.9% (10/145; P = 0.09) for CBT on the same day and 6.7% (17/253; P = 0.08) for CBT at a later date. The HIV prevalence varied considerably by site, ranging from 1.9% to 8.9% (see Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B539).
Older child age was associated with lower likelihood of being HIV positive (PR = 0.8; 95% CI, 0.7 to 0.9). Compared with children whose mothers tested HIV negative during pregnancy, children whose mothers were known HIV positive before pregnancy were 5.8 times (95% CI, 1.8 to 18.5) more likely to be HIV positive (26.7% vs 4.6%). Children with HIV-positive siblings were 2.5 times (95% CI, 0.9 to 7.2) more likely to be HIV positive compared with those without a HIV-positive sibling; the association trending toward significance (13% vs 5%; Table 3).
TABLE 3.
Predictors of a Child Testing HIV Positive
| N | All (N = 521) |
HIV Negative (n = 491) |
HIV Positive (n = 30) |
Prevalence Ratio (95% CI) | P | |
|---|---|---|---|---|---|---|
| Median (IQR) or n (%) | ||||||
|
| ||||||
| Child characteristics | ||||||
| Child age | 521 | 7 (5–9) | 7 (5–9) | 5 (3–7) | 0.8 (0.7–0.9) | 0.001 |
| Female child | 521 | 292 (56) | 271 (55) | 21 (70) | 1.8 (0.8–4.0) | 0.129 |
| Has HIV-positive sibling | 521 | 30 (6) | 26 (5) | 4 (13) | 2.5 (0.9–7.2) | 0.085 |
| Has deceased sibling | 521 | 108 (21) | 105 (21) | 3 (10) | 0.4 (0.1–1.4) | 0.160 |
| Ever hospitalized | 365* | 24 (7) | 23 (7) | 1 (5) | 0.7 (0.1–5.7) | 0.774 |
| Any HIV testing during infancy | 444* | 46 (10) | 44 (11) | 2 (8) | 0.7 (0.2–3.2) | 0.691 |
| Months breastfed | 505* | 13 (8–24) | 13 (8–24) | 18 (12–24) | 1.0 (1.0–1.1) | 0.156 |
| Caregiver assumes child is HIV negative | 521 | 309 (59) | 293 (60) | 16 (53) | 0.8 (0.4–1.6) | 0.507 |
| Caregiver characteristics | ||||||
| Female caregiver | 521 | 439 (84) | 416 (85) | 23 (77) | 0.6 (0.3–1.4) | 0.258 |
| Maternal age during pregnancy (yr) | 439† | 23 (20–28) | 23 (20–28) | 24 (23–30) | 1.0 (1.0–1.1) | 0.183 |
| Maternal education (yr) | 439† | 9 (7–12) | 9 (8–12) | 9 (7–12) | 1.0 (0.9–1.2) | 0.786 |
| Maternal HIV status during pregnancy | 432*† | |||||
| Negative | 216 (50) | 206 (50) | 10 (45) | Reference | ||
| Unknown | 128 (30) | 121 (30) | 7 (32) | 1.2 (0.4–3.1) | 0.742 | |
| Positive, diagnosis during pregnancy | 73 (17) | 72 (18) | 1 (5) | 0.3 (0.04–2.3) | 0.246 | |
| Positive, diagnosis before pregnancy | 15 (3) | 11 (3) | 4 (18) | 5.8 (1.8–18.8) | 0.003 | |
Bolded P value <0.05 showing a significant association between the participant characteristic and HIV status.
Denominators vary because of missing data.
Denominator lower because it only includes female caregivers.
CI, confidence interval; IQR, interquartile range.
All (30/30, 100%) children linked to care within 6 months after diagnosis. Of 30, only 25 children were reached 1 month after diagnosis to ascertain linkage to care; of whom, 22 (88%) had linked to care. Of 30 children, we ascertained ART initiation for 26; 14 (54%) started ART within a month after diagnosis, 6 (23%) within 3 months, 4 (15%) within 6 months, 1 (4%) within 9 months, and 1 (4%) within 1 year.
Missed Opportunities for Child HIV Testing
The 493 enrolled caregivers had 851 children eligible for HIV testing. Of the 851 children, 630 (74%) had a female index client with information regarding maternal testing during pregnancy. Of the 630 children, 421 (67%) had a mother who was tested for HIV during pregnancy; of which 111 (26%) were HIV positive and 310 (74%) were HIV negative. Among the 209 of 630 (33%) children with mothers not tested during pregnancy, 26 (12%) had a mother diagnosed HIV positive before pregnancy. In total, 137 of 630 children (22%) were known to be HIV exposed at birth. Of the 137, 74% had mothers who received ARVs during pregnancy, 80% received ARV prophylaxis after birth, and 50% had an HIV test before cessation of breastfeeding. Only 43 (9%) of 483 children with information on hospitalization had ever been hospitalized.
Mothers of younger children (age 0–5 years) were more likely to have been tested for HIV during pregnancy than those of older children (age, 6–12 years; 80% vs 65%; PR = 1.2; 95% CI: 1.1 to 1.4). When HIV positive, mothers of younger children were also more likely to have received ARVs during pregnancy than mothers of older children (83% vs 59%; PR = 1.4; 95% CI: 1.1 to 1.9). Younger children were more likely to have received an HIV test before cessation of breastfeeding than the older children (67% vs 20%; PR = 3.3; 95% CI: 1.8 to 6.1; Fig. 1).
Relative Contribution of Each Gap in PMTCT Cascade to Missed HIV Testing of the Children
Of 630 children with mothers as index clients, 587 had complete PMTCT information. To establish the relative contribution each PMTCT gap had on pediatric HIV testing gaps, we considered the first step in the PMTCT cascade that the mother–child pair failed to complete as the primary reason the child was of unknown HIV status. Of 587 children, 31% had mothers who did not test for HIV during pregnancy, 51% had mothers who tested negative during pregnancy, 5% had known HIV-positive mothers who did not complete the PMTCT program, 3% were not followed up in an EID program after birth, and 2%—despite initiating ARV prophylaxis—never received any HIV testing. Finally, despite testing during infancy, the remaining 8% had no confirmatory testing after cessation of breastfeeding (Fig. 2).
FIGURE 2.
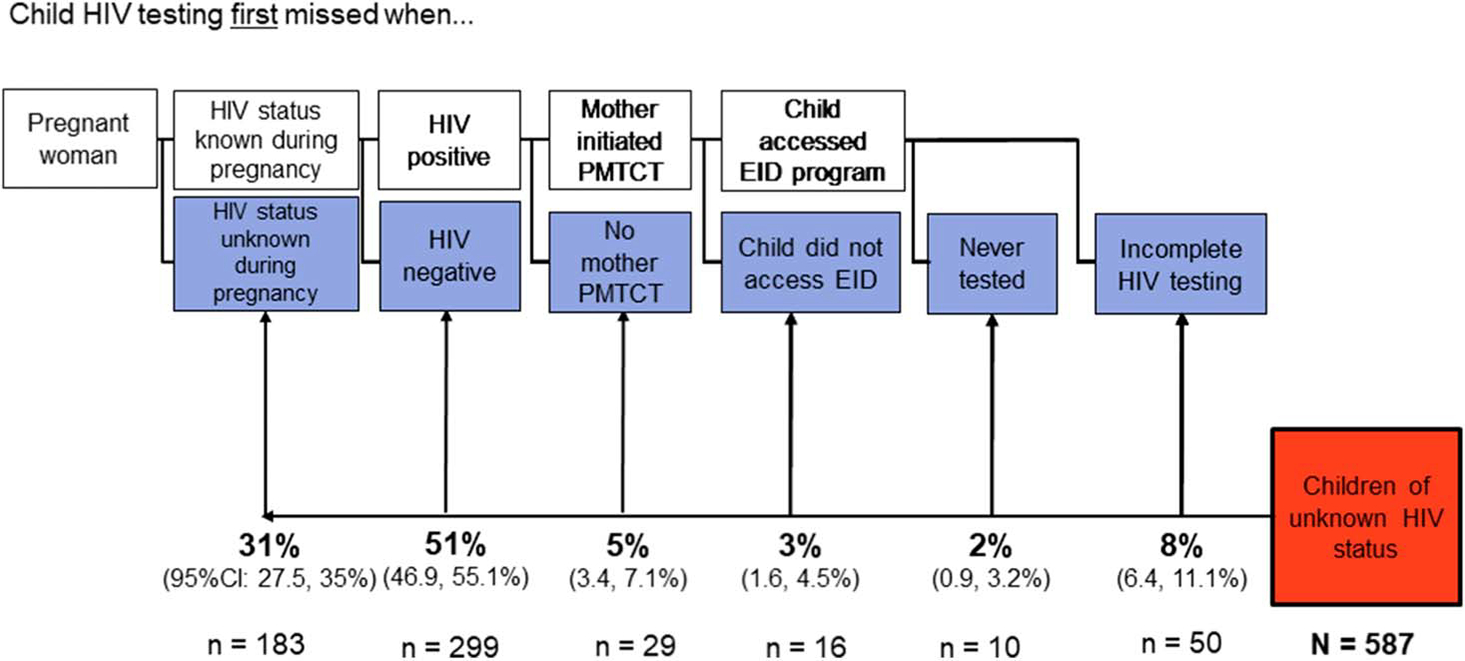
Health systems gaps in the PMTCT cascade that resulted in participants’ children remaining of unknown HIV status. ANC, antenatal care; ARV, antiretroviral; EID, early infant diagnosis.
DISCUSSION
In this study of index-based pediatric HIV testing, we found that 70% of enrolled caregivers accepted pediatric HIV testing and 80% of testers completed testing in clinic. Completion of testing was comparable between those who chose HBT and CBT. CBT resulted in a greater proportion of HIV-positive tests, whereas HBT resulted in testing more children per household. Within 1 month after diagnosis, 88% of the newly diagnosed HIV-positive children were linked to care and only slightly more than half were started on ART. Children who tested positive were younger, more likely to have mothers who were known to be HIV positive before pregnancy, and more likely to have an HIV-positive sibling. The PMTCT gaps associated with children having not received a definitive diagnosis of HIV status before enrollment were lack of maternal HIV testing during ANC or mothers testing HIV negative during pregnancy.
For enrolled caregivers, our study found a relatively high rate of test completion (70%); this rate is higher than in other index case testing studies targeting children in Kenya (57%),26,35 Uganda (40%),28 and Cameroon (57%)36 but lower than in Malawi (86%).27 However, when considering all eligible index clients screened, uptake of index case testing was low (17%), similar to the results of the CATCH pilot study.25 Variable findings across these studies of index case testing in SSA may be to the result of differences in study design. The CATCH study and the Cameroon study focused on testing children of index cases, whereas the other studies tested all household members. Furthermore, although some of the index case testing studies included both CBT and HBT,27,28,36 some exclusively employed CBT26 or HBT.35,37 Similar to Cameroon but unlike Malawi, caregivers in the CATCH study preferred CBT to HBT.36 Studies that offered a choice of testing venues27,36 had higher testing uptake overall than those offering a single venue.26,28,35 Those who preferred HBT in our study had similar test completion as those who preferred CBT (68% vs 72%), even after excluding caregivers who tested on the same day in clinic (68% vs 61%). Conversely, in the Ugandan study, HBT had significantly higher test completion than CBT (56% vs 11%).28 These results suggest that HBT may be an effective complement to CBT to modestly increase uptake of index case testing.
This study demonstrated an efficient but often missed opportunity for pediatric index case testing; 21% of caregivers had an untested accompanying child at their HIV care visit and agreed to same-day testing. Standardized offer of testing to untested children accompanying their caregivers attending HIV care had a high HIV prevalence (6.9%) and is feasible to scale up with little additional infrastructure or staffing investment.
The HIV prevalence of 5.8% that we observed is consistent with other pediatric index case testing studies in SSA (8.4%; range, 3.4%–13.5%).19 Overall, the HIV prevalence from our study was higher than what was reported following universal testing (1.3%; range, 0.4%–2.1%)19 but lower than inpatient PITC (15.4%; range, 5.0%–25.7%) and outpatient PITC (11.3%; range, 4.3%–18.3%).19 We found substantially, but not significantly, higher HIV prevalence with CBT than HBT (6.8% vs 2.4%). This finding is consistent with other index case testing studies comparing pediatric CBT and HBT,27,28,36 which may suggest that caregivers select the children at highest risk of being HIV positive to test at clinic, whereas in HBT, all children in the household are tested. Despite lower caregiver preference and HIV prevalence, HBT may be a relevant complement to CBT for caregivers with multiple untested children and could help reach more children within a household.
In the CATCH study, experienced HIV testing counselors were accompanied by CHW to facilitate identification of homes with untested children and to help link HIV-positive children to care. The CHW did not perform the testing themselves. To reduce costs in a programmatic setting, CHW could complete HBT as was done in Malawi and Came-roon.27,36 Linkage to care and ART initiation in this study was suboptimal, mirroring findings in other studies and data from HIV programs in SSA.27,36,38,39 The low proportion of children initiated on ART could be explained in part by the evolution of ART guidelines for children in Kenya. Before 2016, children older than 10 years did not initiate ART until they fulfilled clinical or immunological criteria.40,41 In the CATCH study, only 25% of those who did not initiate ART within a month of diagnosis were older than 10 years.
This study offers a historic view of gaps in PMTCT and pediatric testing; enrolled children were aged 0–12 years, reflecting caregivers’ experiences with PMTCT services between the years 2001–2016. During this period, Kenya expanded antenatal testing, ART provision during pregnancy, and EID,42,43 and this progress was reflected in this study as younger children (ages 0–5) were significantly more likely to be born to women who were tested for HIV or received ART during pregnancy, to have received infant ARV prophylaxis, and to be enrolled in EID programs than older children.
A majority of children in this study had mothers who were either not tested during pregnancy or tested HIV negative; the children born from these pregnancies may have been either HIV unexposed (if the mother did not acquire HIV until after this child had completed breastfeeding) or HIV exposed (if the mother acquired HIV during pregnancy or breastfeeding). Although it is not possible to determine from these data the relative contribution of acute maternal infection to pediatric HIV infections, other studies have found that as PMTCT programs continue to increase coverage and effectiveness for women who are known to be HIV positive, a growing proportion of new infant infections will be to the result of incident maternal infections.44
Strengths of our study include capture of detailed caregiver and child HIV and PMTCT histories, which enabled us to determine gaps in PMTCT and comprehensively describe the children reached by the interventions. Second, allowing caregivers to choose their testing location allowed us to assess whether CBT or HBT was preferred, providing a rationale for programmatic resource allocation for either testing approaches. Limitations of the study included the presence of pediatric HIV testing initiatives in Kenya over the period of the study, which may have affected the pool of eligible children; adoption of index case testing in the Kenyan national guidelines20 and short-term HIV testing campaigns45 could have reduced the number of eligible undiagnosed children. This is supported by findings from the 2018 population-based HIV assessment46 in Kenya. The large drop-off between recruitment and enrollment, which may have been a result of lengthy enrollment procedures, could have biased the sample to caregivers determined to complete testing for their children. However, this bias is unlikely to have differentially affected caregivers who preferred CBT or HBT. Finally, we may have underascertained testing uptake following enrollment in the study if caregivers tested their children outside of the study.
CONCLUSION
The CATCH approach to pediatric testing was effective, with moderate uptake and HIV prevalence, in reaching children of HIV-positive adults in care who previously missed HIV testing because of gaps in PMTCT. Optimizing maternal testing in pregnancy and the postpartum period could reduce gaps in pediatric HIV testing coverage. Although CBT was preferred and more efficient, HBT may be a useful complement, especially for caregivers with multiple children of unknown status. The study showed that younger children, those with HIV-positive siblings, and those whose mothers had been diagnosed before the pregnancy were at high risk of being HIV positive. This information could be useful to programs in prioritizing the caregivers in care, and the children in a household to target. To optimize this approach, however, additional interventions in linkage to care and ART initiation for HIV-positive children are needed. These approaches could be especially useful in countries with a high unmet need for pediatric testing, such as in West Africa.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the CATCH study caregivers and children without whom this research would not have been possible. The authors also thank participating site staff, County departments of health, the National AIDS and STI Control Programme for their support. The authors thank the CATCH study team members, Vincent Omondi, Verlinda Otieno, Margaret Nduati, Eliza Mabele, Florence Ayugi, and Mercy Atieno for their time and dedication. The authors also thank the University of Washington’s Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and University of Washington/Fred Hutch Center for AIDS Research for supporting the authors during preparation of this article.
The Counseling and Testing for Children at Home (CATCH) Study was funded by A83526 (University of Washington Royalty Research Fund, PI J.A.S.) and by R21 HD079637 (NIH, G.J.-S.). C.M. was supported by UWA83526 (UW RRF); during manuscript development, C.M. and I.N.N. were scholars in the International AIDS Research and Training Program, supported by the Fogarty International Center, National Institute on Drug Abuse, and National Institute of Mental Health (NIH grant D43 TW009580 and D43 TW009783 respectively). A.D.W. was supported by F31HD088204 (NIH) and subsequently K01MH121124 (NIH); I.N.N. and D.C.W. were supported by R01 HD023412 (NIH); I.N.N. was supported additionally by D43TW009783; G.J.-S. was supported by R01 HD023412 and K24 HD054314 (NIH); J.A.S. was supported by K01 AI087369. The authors were also supported by the University of Washington’s Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and University of Washington/Fred Hutch Center for AIDS Research during preparation of this article.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Wamalwa D, Benki-Nugent S, Langat A, et al. Survival benefit of early infant antiretroviral therapy is compromised when diagnosis is delayed. Pediatr Infect Dis J. 2012;31:729–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner A, Slyker J, Langat A, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luma HN, Jua P, Donfack OT, et al. Late presentation to HIV/AIDS care at the Douala general hospital, Cameroon: its associated factors, and consequences. BMC Infect Dis. 2018;18:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benki-Nugent S, Wamalwa D, Langat A, et al. Comparison of developmental milestone attainment in early treated HIV-infected infants versus HIV-unexposed infants: a prospective cohort study. BMC Pediatr. 2017;17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo C, Akwara P, Ngongo N, et al. Global progress in PMTCT and paediatric HIV care and treatment in low-and middle-income countries in 2004–2005. Reprod Health Matters. 2007;15:179–189. [DOI] [PubMed] [Google Scholar]
- 7.Weiler G Global Update on HIV Treatment 2013: Results, Impact and Opportunities World Health Organization; 2013. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 8.Kiragu K, Collins L, Von Zinkernagel D, et al. Integrating PMTCT into maternal, newborn, and child health and related services: experiences from the global plan priority countries. J Acquir Immune Defici Syndr. 2017;75:S36–S42. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Pediatric AIDS. Increasing antiretroviral drug access for children with HIV infection. Pediatrics. 2007;119:838–845. [DOI] [PubMed] [Google Scholar]
- 10.Penazzato M, Amzel A, Abrams EJ, et al. Pediatric treatment scale-up: the unfinished agenda of the global plan. J Acquir Immune Defici Syndr. 2017;75:S59–S65. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. AIDSInfo treatment cascade. UNAIDS. Available at: http://aidsinfo.unaids.org/. Accessed February 3, 2019. [Google Scholar]
- 12.Bekker L-G, Siberry GK, Hirnschall G. Ensuring children and adolescents are not left behind. J Acquir Immune Defici Syndr. 2018;78:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. UNAIDS Data 2019. 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf. Accessed July 21, 2019.
- 14.Essajee S, Bhairavabhotla R, Penazzato M, et al. Scale-up of early infant HIV diagnosis and improving access to pediatric HIV care in global plan countries: past and future perspectives. J Acquir Immune Defici Syndr. 2017;75:S51–S58. [DOI] [PubMed] [Google Scholar]
- 15.Rogers AJ, Akama E, Weke E, et al. Implementation of repeat HIV testing during pregnancy in southwestern Kenya: progress and missed opportunities. J Int AIDS Soc. 2017;20:e25036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Consolidated Guidelines on HIV Testing Services. 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/179870/9789241508926_eng.pdf. Accessed July, 21, 2019. [PubMed]
- 17.Abuogi LL, Humphrey JM, Mpody C, et al. Achieving UNAIDS 90–90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J Virus Eradication. 2018;4(suppl 2): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake AL, Thomson KA, Quinn C, et al. Retest and treat: a review of national HIV retesting guidelines to inform elimination of mother-to-child HIV transmission (EMTCT) efforts. J Int AIDS Soc. 2019;22: e25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govindasamy D, Ferrand RA, Wilmore SM, et al. Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18:20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health. The Kenya HIV Testing Guidelines. Ministry of Health; 2015. Available at: https://aidsfree.usaid.gov/sites/default/files/hts_policy_kenya_2015.pdf. Accessed July, 21, 2019. [Google Scholar]
- 21.Njuguna IN, Wagner AD, Cranmer LM, et al. Hospitalized children reveal health systems gaps in the mother-child HIV care cascade in Kenya. AIDS Patient Care STDS. 2016;30:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayieko J, Chamie G, Balzer L, et al. Mobile, population-wide, hybrid HIV testing strategy increases number of children tested in rural Kenya and Uganda. Pediatr Infect Dis J. 2018;37:1279–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherutich P, Bunnell R, Mermin J. HIV testing: current practice and future directions. Curr HIV/AIDS Rep. 2013;10:134–141. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AD, Mugo C, Njuguna IN, et al. Implementation and operational research: active referral of children of HIV-positive adults reveals high prevalence of undiagnosed HIV. J Acquir Immune Defic Syndr. 2016;73: e83–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis Kulzer J, Penner JA, Marima R, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 2012;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed S, Sabelli RA, Simon K, et al. Index case finding facilitates identification and linkage to care of children and young persons living with HIV/AIDS in Malawi. Trop Med Int Health. 2017;22:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lugada E, Levin J, Abang B, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr. 2010;55:245–252. [DOI] [PubMed] [Google Scholar]
- 29.Yengopal V, Kolisa Y, Thekiso MD, et al. The child and adolescent with HIV in resource poor countries. Oral Dis. 2016;22(suppl 1):25–34. [DOI] [PubMed] [Google Scholar]
- 30.Liu JF, Liu G, Li ZG. Factors responsible for mother to child transmission (MTCT) of HIV-1—a review. Eur Rev Med Pharmacol Sci. 2017;21(4 suppl):74–78. [PubMed] [Google Scholar]
- 31.Woldesenbet SA, Jackson DJ, Lombard C, et al. Structural level differences in the mother-to-child HIV transmission rate in South Africa: a multilevel assessment of individual-, health facility-, and provincial-level predictors of infant HIV transmission. J Acquir Immune Defici Syndr. 2017;74:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National AIDS and STI Control Programme (NASCOP). Kenya AIDS Indicator Survey 2012: Final Report. Government of Kenya; 2014. Available at: https://nacc.or.ke/wp-content/uploads/2015/10/KAIS-2012.pdf. Accessed September 15, 2018. [Google Scholar]
- 33.Ministry of Public Health and Sanitation. National Guidelines for HIV Testing and Counseling in Kenya. Government of Kenya; 2010. Available at: http://guidelines.health.go.ke:8000/media/National_Guidelines_for_HTC_in_Kenya_2010_dWuc0Rr.pdf. Accessed September 15, 2018. [Google Scholar]
- 34.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37:350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vreeman RC, Nyandiko WM, Braitstein P, et al. Acceptance of HIV testing for children ages 18 months to 13 years identified through voluntary, home-based HIV counseling and testing in western Kenya. J Acquir Immune Defic Syndr. 2010;55:e3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yumo HA, Kuaban C, Ajeh RA, et al. Active case finding: comparison of the acceptability, feasibility and effectiveness of targeted versus blanket provider-initiated-testing and counseling of HIV among children and adolescents in Cameroon. BMC Pediatr. 2018;18:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defici Syndr. 2006;43:91–95. [DOI] [PubMed] [Google Scholar]
- 38.Phelps BR, Ahmed S, Amzel A, et al. Linkage, initiation and retention of children in the antiretroviral therapy cascade: an overview. AIDS. 2013; 27:S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruzagira E, Baisley K, Kamali A, et al. Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2017;22:807–821. [DOI] [PubMed] [Google Scholar]
- 40.Ministry of Health Kenya. Guidelines for Antiretroviral Therapy in Kenya. 2011. Available at: http://guidelines.health.go.ke:8000/media/Final_guidelines_re_print_11-09-2012.pdf. Accessed July, 21, 2019.
- 41.Ministry of Health Kenya. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. 2016. Available at: https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf. Accessed July, 21, 2019.
- 42.Pricilla RA, Brown M, Wexler C, et al. Progress toward eliminating mother to child transmission of HIV in Kenya: review of treatment guidelines uptake and pediatric transmission between 2013 and 2016—a follow up. Matern Child Health J. 2018;22:1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joint United Nations Programme on HIV/AIDS. On the Fast-Track to an AIDS-free Generation. Geneva, Switzerland: UNAIDS Web site; 2016. Available at: https://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf. Accessed July 21, 2019. [Google Scholar]
- 44.Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defici Syndr. 2012;59: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Center for Health Solutions Kenya. Countrywide 100-day Rapid Results Initiative for HIV Testing and Treatment. CHS Kenya; 2016. Available at: https://www.chskenya.org/media_centre/countrywide-100-day-rapid-results-initiative-for-hiv-testing-and-treatment/. Accessed July, 21, 2019. [Google Scholar]
- 46.Ministry of Health, Kenya. Kenya population-based HIV impact assessment (KENPHIA). National AIDS & STI Control Programme; 2018. Available at: https://www.nascop.or.ke/kenphia-report/. Accessed April 20, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


