Abstract
Osmoregulated periplasmic glucans (OPGs) of Escherichia coli are anionic oligosaccharides that accumulate in the periplasmic space in response to low osmolarity of the medium. Their anionic character is provided by the substitution of the glucosidic backbone by phosphoglycerol originating from the membrane phospholipids and by succinyl residues from unknown origin. A phosphoglycerol-transferase-deficient mdoB mutant was subjected to Tn5 transposon mutagenesis, and putative mutant clones were screened for changes in the anionic character of OPGs by thin-layer chromatography. One mutant deficient in succinylation of OPGs was obtained, and the gene inactivated in this mutant was characterized and named mdoC. mdoC, which encodes a membrane-bound protein, is closely linked to the mdoGH operon necessary for the synthesis of the OPG backbone.
Osmoregulated periplasmic glucans (OPGs) are a family of oligosaccharides found in the periplasmic space of gram-negative bacteria. Their common features are the presence of glucose as the sole constituent sugar and their increased levels in low-osmolarity media.
Nevertheless, they are very different in structure. Rhizobiaceae and related bacteria synthesize cyclic or branched cyclic glucans, whereas Enterobacteriaceae and related bacteria synthesize branched linear glucans. These structural differences are correlated with genes implicated in the synthesis of the glucose backbone. These genes can be divided into two classes which have no homology. The first class is represented by the very similar genes of Agrobacterium tumefaciens (chvA and chvB) and Sinorhizobium meliloti (ndvA and ndvB [7]), while the second class contains the very similar genes of Escherichia coli (mdoG and mdoH) and Pseudomonas syringae (orfI and hrpM [20]).
These glucans may be substituted with various residues, depending on the species considered. Surprisingly, there is no correlation between the glucosidic structure of OPGs and the nature of substituents. Cyclic OPGs of Brucella abortus (7), Ralstonia solanacearum, Xanthomonas campestris (28), and linear glucans of P. syringae (27) are devoid of substituents. OPGs of Azospirillum brasilense (cyclic glucans [1]) and Erwinia chrysanthemi (linear glucans [30]) are substituted only with succinyl residues, and cyclic OPGs of Bradyrhizobium japonicum are substituted only by phosphocholine (24). Cyclic glucans originating from S. meliloti and A. tumefaciens are substituted with both succinyl and phosphatidylglycerol residues (7), while cyclic OPGs of Rhodobacter sphaeroides are substituted with succinyl and acetate residues (29). OPGs originating from E. coli (linear glucans) are the only ones known to be substituted with three different substituents. These OPGs are substituted with phosphatidylglycerol and phosphatidylethanolamine derived from membrane phospholipids and by succinyl residues of unknown origin (17).
In E. coli, the glucosidic backbones are synthesized by the products of the mdoGH operon. They appear to contain 6 to 12 glucose residues per mole, with the principal species containing 8 to 9 glucose units (17). MdoG is a periplasmic protein of unknown function (which is nevertheless required for synthesis of glucans [18]), while MdoH is a transmembrane β-1,2 glucosyl transferase acting on the cytoplasmic side of the inner membrane and is probably involved in the transfer of these glucans to the periplasmic space (10). The first characterized gene implicated in substitution was mdoB encoding the phosphoglycerol transferase I of E. coli (12, 16). This enzyme can transfer phosphoglycerol residues to an artificial acceptor, the β-glucoside arbutin. Since arbutin cannot enter the cytoplasm, the transfer should occur on the periplasmic face of the inner membrane. Thus, one can conclude that glucan backbones are substituted after their translocation to the periplasmic space (6). Similar conclusions were proposed for the substitution of cyclic glucans in S. meliloti (7). This transfer leads to the formation of diacylglycerol, which is toxic for cells and immediately phosphorylated by the diacylglycerol-kinase (encoded by the dgk gene) to yield phosphatidic acid.
When a dgk strain was grown in presence of arbutin, diacylglycerol formation was stimulated and its subsequent accumulation stopped the growth of the dgk strain. This was the basis of the selection of mdoB mutants in which phosphatidylglycerol turnover was slowed sufficiently to allow growth (16).
More recently, Breedveld et al. (8) isolated a mutant unable to transfer phosphoglycerol to OPGs in S. meliloti. They used a thin-layer chromatographic screening protocol because the genetic selection described above was inefficient in this bacterium (8a).
In this paper, we describe the isolation of a new gene required for the substitution of OPGs by succinate in an E. coli mdoB strain by using a similar thin-layer chromatographic screening protocol.
MATERIALS AND METHODS
Bacterial strains and media.
The E. coli strains and phage used in this study are described in Table 1. Bacteria were grown at 37°C with vigorous shaking in Luria broth (LB) or in minimal medium 63 supplemented with the required metabolites and glucose as the carbon source (21). Solid media were obtained by adding agar (15 g · l−1). When low-osmolarity medium was required, LB without NaCl or low-osmolarity medium was used (19).
TABLE 1.
Bacterial strains and phage used in this study
| Strain or phage | Relevant genotype | Construction, source, or reference |
|---|---|---|
| Bacterial strains | ||
| JM83 | ara Δ(lac-pro) thi rpsL (φ80 ΔlacZ ΔM15) | 32 |
| DF214 | his pgi::Mu Δ(zwf-edd)1 eda-1 rpsL | D. Fraenkel via E. P. Kennedy |
| PT227 | F−thr-1 leuB6 Δ(gpt-proA)62 hisG4 argE3 thi-1 ara-14 lacY1 galK2 xyl-5 mtl-1 mdoB214::Tn10 rpsL31 glnV44 | 16 |
| NFB732 | JM83 mdoB214::Tn10 | JM83 + P1 (PT227) |
| NFB734 | DF214 mdoB214::Tn10 | DF214 + P1 (PT227) |
| NFB1864 | JM83 mdoB214::Tn10 mdoC1::Tn5 | Tn5 mutant |
| NFB1897 | JM83 mdoB214::Tn10 mdoC1::Tn5 | NFB732 + P1 (NFB1864) |
| NFB1919 | DF214 mdoC1::Tn5 | NFB732 + P1 (NFB1864) |
| NFB1933 | DF214 mdoB214::Tn10 mdoC1::Tn5 | NFB734 + P1 (NFB1864) |
| Bacteriophage | ||
| Phage λNK467 | b221 rex::Tn5 cI857 Oam29 Pam80 | N. Kleckner via A. Pugsley |
Antibiotics in media were used at the following concentrations: ampicillin, 50 μg · ml−1; kanamycin, 50 μg · ml−1; tetracycline, 25 μg · ml−1. In addition, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used in media at a concentration of 20 μg · ml−1.
Transduction and transformation.
Transduction with phage P1vir was carried out according to the method of Miller (21). E. coli cells were made competent and transformed by the calcium chloride technique (26).
Transposon mutagenesis.
Random integration of Tn5 into the chromosome was obtained by using λNK467 (Table 1) according to standard procedures (26).
Restriction enzymes, modification enzyme, and ligase.
Restriction endonucleases (Eurogentec), the large (Klenow) fragment of DNA polymerase I, and ligase of T4 phage (Gibco BRL) were used according to manufacturer’s recommendations.
Plasmid construction.
For the sequencing of a fragment of the mdoC gene, a genomic DNA fragment of the mdoC1::Tn5 strain NFB1897 was cloned in the BamHI site of plasmid pUC8. This restriction enzyme cut once in the Tn5 DNA outside the gene, conferring kanamycin resistance to the transposon. Kanamycin-resistant clones harboring a plasmid containing a 16-kb DNA insert were isolated on plates containing kanamycin and ampicillin.
For the complementation tests, a 3-kb PstI fragment containing mdoC originating from pNF239 (19) (see Fig. 3) was subcloned into the PstI site of pBGS18 or pUC19 to yield pNF245 or pNF418, respectively.
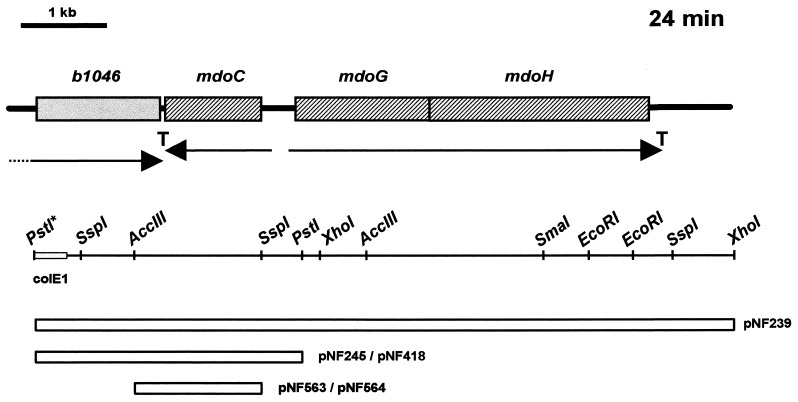
FIG. 3.
Genetic organization of the mdoC region of the E. coli chromosome (top) and restriction map of the 8-kb insert of pNF239 and its derivatives (bottom). Only relevant restriction sites are shown; arrows and T indicate transcriptional units and transcriptional terminators, respectively. PstI* and colE1 are part of the insert originating from the colE1 vector (9). Horizontal empty bars indicate the structure of the various inserts of the plasmids.
For the sequencing of the SspI-PstI fragment (0.4 kb) lying downstream from mdoC, pNF245 was cut by SspI (one site localized downstream from mdoC) and HindIII (one site derived from the polycloning site of pBGS18), and a 0.4-kb fragment was cloned into the SmaI and HindIII sites of M13mp18 and M13mp19.
For the translational fusion between the lac operon and the mdoC gene (lacZ-mdoC), pNF245 was cut by SspI and AccIII. A 1.4-kb DNA fragment containing the putative coding region of mdoC (see Fig. 3) was purified and cloned into pUC19 cut first by SalI, filled in by using the Klenow fragment of DNA polymerase I, and then cut by XmaI to give pNF563. This plasmid was cut at the unique HindIII site (derived from the polycloning site of pUC19), filled in, and ligated in order to disrupt the reading frame of the fusion (pNF564).
DNA purification.
Standard procedures (26) were used for genomic DNA extraction, large scale plasmid isolation, and rapid analysis of recombinant plasmids.
DNA sequencing.
DNA sequences were determined with the Sequenase version 2.0 kit (US Biochemical Corporation) except for the Tn5 insertion point, where the oligonucleotide 5′-CATGGAAGTCAGATCCTGG-3′ (Eurogentec), corresponding to the end of the two IS50 of Tn5, was used as a primer.
The DNA sequences and deduced amino acid sequences were analyzed by using computer programs made available from Infobiogen (15a).
Thin-layer chromatographic screening method.
Each Tn5 mutant generated from NFB732 was screened as described by Breedveld et al. (8) and modified as follows: 4 ml of an overnight culture of E. coli grown in LB without NaCl was centrifuged and washed with 1 ml of water, and the pellet was resuspended in 250 μl of 80% ethanol (vol/vol). The cell suspension was then heated for 30 min at 70°C and centrifuged for 10 min at 15,000 × g at room temperature, and the supernatant (containing OPGs) was dried under a vacuum.
Dried supernatant was then resuspended in 15 μl of water, and 5 μl was spotted onto 0.2-mm-thick aluminum silica gel 60 plates (Merck). Oligosaccharides were separated in two runs, with an intermediate drying step, in an ethanol-butanol-water (5:5:4) solvent. Glucans were detected by spraying dried plates with 0.2% orcinol in 20% sulfuric acid followed by heating at 110°C for 10 min.
Extraction of OPGs.
Cultures (200 ml) of E. coli were grown overnight in LB without NaCl. OPGs were extracted by the charcoal adsorption procedure (19). Pyridine extract obtained by this procedure was chromatographed on a Biogel P4 column (Bio-Rad). The column (1.5 by 68 cm) was equilibrated with 0.5% acetic acid and eluted at a rate of 15 ml · h−1 in the same buffer. Fractions (2.5 ml) containing oligosaccharides were pooled, concentrated by rotary evaporation, and desalted on a Biogel P2 column (Bio-Rad), and fractions containing OPGs were pooled and lyophilized. Sugar content was determined colorimetrically by using the phenol-sulfuric acid reagent procedure (11).
Determination of succinate content from OPGs.
One milligram of OPG was dissolved in 200 μl of 0.5 M NaOH and incubated at 100°C for 30 min to liberate the succinyl residues from OPG. Glucosidic backbones were removed by absorption on 50 mg of charcoal, and the charcoal was then washed three times with 0.5 ml of water. The four supernatants (2.5 ml) were pooled, concentrated by rotary evaporation, and desalted on a minicolumn of Dowex AG 50 × 8, 20- to 50-mesh (Bio-Rad) on H+ form.
After lyophilization, samples were dissolved in 1 ml of water, and succinic acid content was determined with a succinic acid kit (Boehringer Mannheim).
Determination of neutral and anionic characteristics of OPGs.
Strains were grown in low-osmolarity medium (5 ml) supplemented with 0.24 mM d-[U-14C]glucose (125 MBq mmol−1). Glucans extracted as described above were desalted on a PD10 column (Pharmacia) equilibrated with a Tris-HCl 10 mM [pH 7.4] buffer. OPG-containing fractions were pooled and chromatographed on a DEAE-Sephacel column (1.5 by 38 cm; Pharmacia) equilibrated with 10 mM Tris-HCl [pH 7.4] and eluted with the same buffer containing concentrations of NaCl ranging from 0 to 0.2 M by steps of 0.05 M. A volume of 60 ml was used for each NaCl concentration, and the volume of each collected fraction was 4 ml.
RESULTS AND DISCUSSION
Isolation of a mutant with altered phenotype of OPGs.
A random Tn5 mutagenesis was performed in the mdoB strain NFB732 (deficient for the phosphoglycerol transferase I). In mdoB strains, OPGs lack phosphoglycerol substituents and succinyl residues are the only anionic substituents. Mild alkali treatment of OPGs removes selectively these succinyl residues. Glucans of NFB732 devoid of anionic substituents were substituted only with phosphoethanolamine residues and can be easily distinguished by a thin-layer chromatographic method (described in Materials and Methods). As expected, migration of glucans is greatly reduced after mild alkali treatment (Fig. 1, lane 3) compared to that of untreated glucans (Fig. 1, lane 1). Glucans extracted from 996 random Tn5 insertion mutants of NFB732 were analyzed by this chromatographic screening.
FIG. 1.
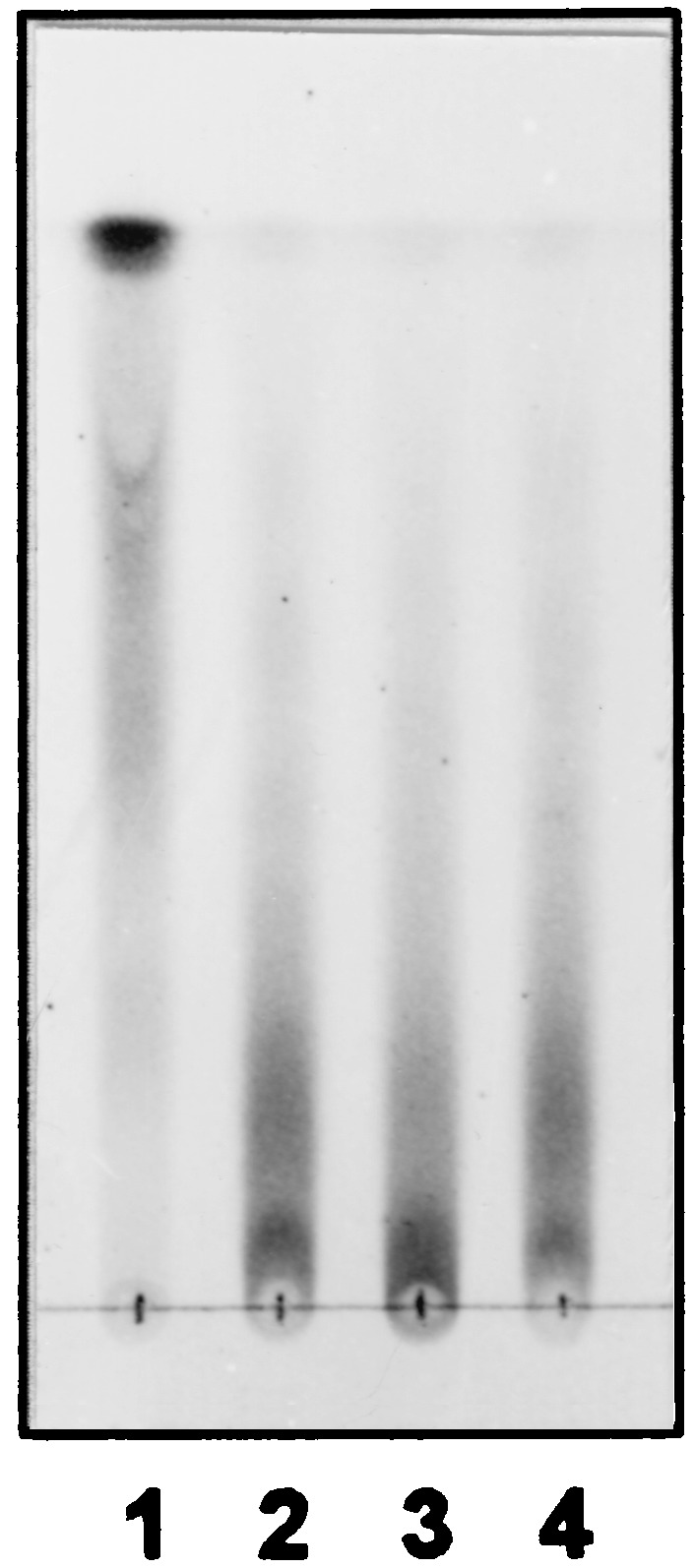
Thin-layer chromatographic analysis of OPGs extracted from NFB732 (mdoB, lanes 1 and 2) or NFB1864 (mdoB mdoC, lanes 3 and 4). Extracts were applied directly to thin-layer chromatography plates (lanes 1 and 3) or first subjected to mild alkali treatment to remove succinyl substituents (lanes 2 and 4).
OPGs from clone 214, called NFB1864, showed the same reduced migration pattern with (Fig. 1, lane 4) or without alkali treatment (Fig. 1, lane 2), as seen with OPGs extracted from NFB732 and subjected to the mild alkali treatment (Fig. 1, lane 3).
Glucans from NFB1864 and NFB732 were subsequently analyzed for their succinyl content. Succinyl ester residues were hydrolyzed from glucans by alkali treatment, glucose backbones were removed, and succinic acid quantities were measured. No detectable succinate was found in the glucans extracted from NFB1864, while an average of 35 μg of succinyl per mg of OPGs was found in glucans extracted from NFB732. On the other hand, the glucan amount per cell was shown to be identical in the two strains, indicating that the loss of succinyl residues was not the consequence of a reduction in the glucose backbone synthesis. Thus, this mutant synthesized glucans devoid of succinyl residues, and the gene interrupted by the Tn5 insertion was called mdoC.
The Tn5 insertion is responsible for the altered phenotype of OPGs.
The Tn5 mutation from NFB1864 was transferred by P1 phage generalized transduction into strains DF214 and JM83 and their mdoB derivatives. After selection of transductants for kanamycin resistance, OPGs were extracted and subjected to the thin-layer chromatographic analysis. Strains containing both mdoB and mdoC mutations produced glucans with the same migration pattern as those of NFB1864, indicating that the Tn5 insertion was responsible for the altered phenotype of OPGs (data not shown).
OPGs isolated from the mdoC mutant are neutral.
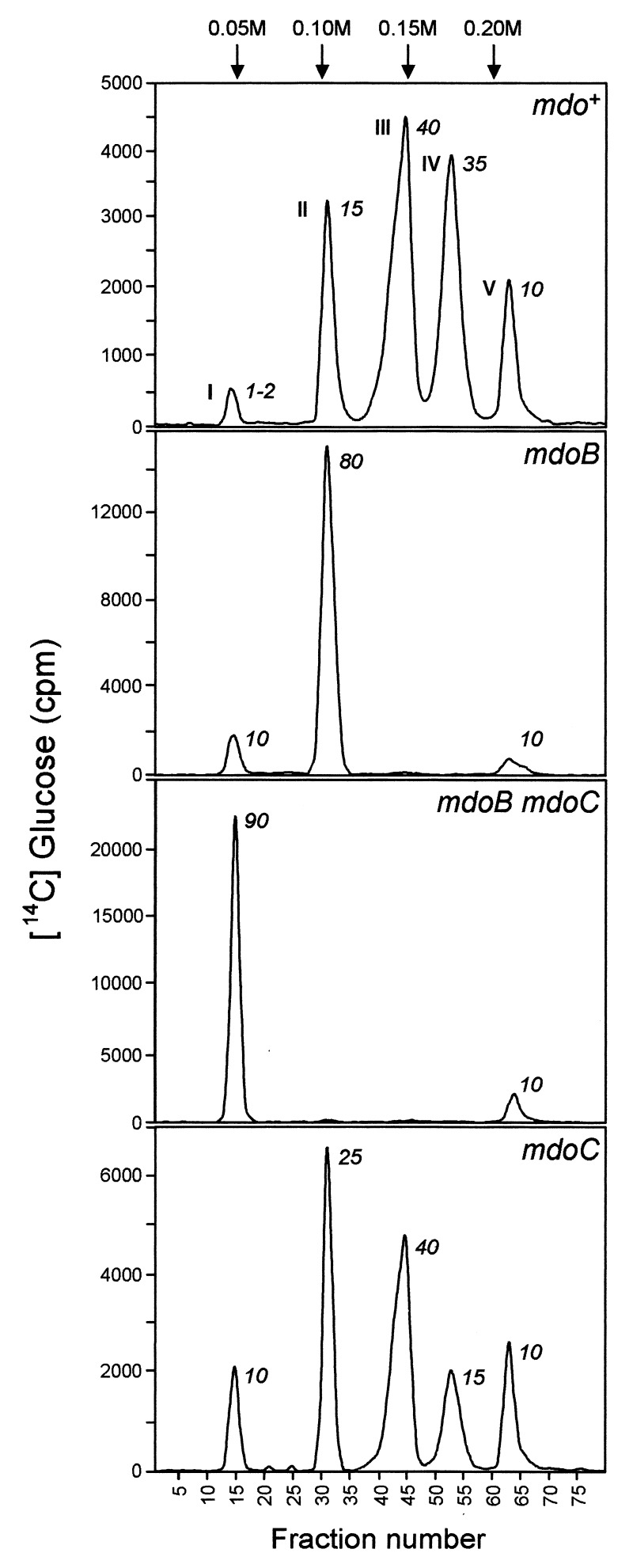
Specific labeling of OPGs was obtained by growing cells of strain DF214 (pgi, zwf) and its derivatives, NFB734 (mdoB), NFB1919 (mdoC), and NFB1933 (mdoB, mdoC), in the presence of radioactive glucose. They produced the same amount of OPGs when grown in a low-osmolarity medium and a similar reduction (six to seven times) was observed when the osmolarity was increased by the addition of 0.3 M NaCl. The anionic or neutral character of OPGs was confirmed by a DEAE-Sephacel chromatographic analysis of radiolabeled glucans. OPGs extracted from DF214 (mdo+, Fig. 2) were separated into five subfractions, named I to V (III, IV, and V correspond to A, B, and C, respectively, in Van Golde et al. [31]). Subfractions II to V were eluted at increasing concentrations of NaCl and correspond to anionic replaced glucans. Phosphoglycerol and phosphoethanolamine substitutions of each subfraction were previously analyzed by using radiolabeled precursors (22, 31). Phosphoglycerol and phosphoethanolamine were present in subfractions III to V, and low levels of phosphoethanolamine were detected in subfractions I and II, while phosphoglycerol was hardly detected in subfraction II. Subfractions III and IV were further characterized (31). Both were heterogeneous and contained succinyl ester substituents. At pH 7.4, the anionic character is conferred only by phosphoglycerol and succinyl substituents. If OPGs were homogeneously sized, one could consider that subfraction II to V correspond to increasing negative charges (−1, −2, −3, and −4) attached to the glucosidic backbone. However, the OPG backbones are heterogeneous in size, with a degree of polymerization ranging from 6 to 12. Thus, subfraction II to V should correspond to various charge-to-mass ratios due to various levels of substitution of heterogeneously sized backbones.
FIG. 2.
DEAE-Sephacel anion exchange column chromatography profiles of [U-14C] glucose labeled OPGs from strains DF214 (mdo+), NFB734 (mdoB), NFB1933 (mdoB mdoC), and NFB1919 (mdoC). Ionic strength was increased by steps of 0.05 M NaCl at fractions indicated by the arrows. Fractions (4 ml) were collected and aliquots were counted. Peak I, neutral glucans; peaks II to V, anionic glucans. The number on the right of each peak refers to the percentage of the total for the corresponding subfraction.
This interpretation was confirmed by the analysis of OPGs produced by various mutant strains. In OPGs extracted from NFB734 (mdoB; Fig. 2), subfraction II became prominent (80% of the total), subfractions III and IV disappeared, and subfraction I increased to 10%. In glucans extracted from NFB1933 (mdoB mdoC; Fig. 2), 90% of the OPGs were found in the neutral subfraction I. These results clearly indicated that mdoC is involved in the succinyl substitution of OPGs. In strain NFB1919 (mdoC; Fig. 2), subfraction II was increased while subfraction IV decreased. The simplest interpretation is that parts of the OPGs in the subfractions II, III, or IV change their charge-to-mass ratio since they are no longer succinylated in the mdoC strain, and are eluted with subfractions I, II, or III, respectively.
The increase in subfraction I of the mdoB and the mdoC strains indicates that part of the molecules are substituted by only one kind of anionic residue. This is consistent with the observation that about 50% of the subfraction III of the wild-type strain contains only phosphoglycerol substituents (17). However, since the increase observed in the mutant cells was lower than expected, one can conclude that a defect of one kind of substitution is compensated, at least partially, by increasing the level of the other.
In this context, the nature of the subfraction V remains obscure; the possibility exists that it contains non-OPG materials since low levels of labeled materials were observed by Sephadex G25 chromatography of extracts of a mdoG mutant (18).
Tn5 insertion lies within a gene adjacent to the mdoGH operon.
The mdoC1::Tn5 mutation was cloned into the pUC8 plasmid vector from the genomic DNA by selecting for the kanamycin resistance conferred by the transposon. The DNA sequence read from the resulting plasmid started within the IS50 DNA and continued in the mdoC DNA. Comparison of this sequence and the E. coli genomic DNA data (5, 23) allowed us to localize mdoC as the previously unassigned gene, named b1047, just upstream from the mdoGH operon (19).
The mdoC gene is entirely contained between two PstI sites in plasmid pNF239, which was previously said to contain the mdoGH operon (19). However, the comparison of the pNF239 restriction map with the sequence data now available revealed a large discrepancy of a set of restriction sites located upstream from mdoG (19). The PstI site present on the left of Fig. 3 does not appear to be of chromosomal origin. To elucidate the PstI origin, the 0.4-kb PstI-SspI (Fig. 3) fragment was cloned and sequenced; 75% of this sequence is from the ColE1 vector used to obtain plasmid pLC25-18 (9) from which pNF239 was derived (19).
The localization of mdoC was confirmed by a complementation test with plasmid pNF418. This plasmid was constructed by subcloning the 3-kb PstI fragment from pNF239 (b1046 is inactivated in plasmid pNF418; Fig. 3). Thin-layer chromatography of OPGs from NFB1135 (pNF418/NFB1897) showed that they recovered their anionic character and therefore their succinyl substituents (data not shown).
Nucleotide sequence analysis of the mdoC gene.
The Tn5 was found inserted at nucleotide 1107787 (5) (GenBank accession no. U00096). It is 377 nucleotides downstream from a putative start codon ATG (nucleotide 1108164). The corresponding open reading frame, encoding a 385-amino-acid polypeptide, ends at a TAA codon (nucleotide 1107007). Fifty-five nucleotides downstream from the TAA, two inverted repeats followed by a stretch of six Ts characteristic of an intrinsic transcription terminator are found.
The function of this open reading frame was confirmed as follows. A translational fusion lacZ-mdoC (pNF563) was obtained at the SspI site (Fig. 3) lying 27 nucleotides downstream from the first ATG of the open reading frame of mdoC. OPGs extracted from NFB1447 (pNF563/NFB1864) were analyzed on thin-layer chromatography and showed anionic character, indicating that this fusion was able to complement the mutation (data not shown). This fusion was disrupted by an insertion of 4 nucleotides at the HindIII site of the polylinker (pNF564). OPGs extracted from NFB1448 (pNF564/NFB1864) presented a neutral character, indicating that the mdoC1::Tn5 mutation was not complemented by this plasmid.
Recent release of sequence data from Salmonella typhi (24a) revealed a strong conservation of the mdoC (82% identity), mdoG (94% identity), and mdoH (95% identity) coding sequences. The ATG of the mdoGH operon is separated from the putative ATG of the mdoC gene by 393 nucleotides in E. coli while in S. typhi, a deletion of 120 nucleotides reduces this region to 273 nucleotides. Nevertheless, this noncoding sequence is highly conserved (82% identity). A putative promoter is present in the conserved region: the −35 region is separated by 16 nucleotides from the −10 region located 170 nucleotides before the start codon. This putative mdoC promoter would be separated from the mdoGH promoter by only 102 nucleotides. Thus, the two operons may be coregulated since regulatory sites are generally found within 100 nucleotides upstream from the promoter (14).
Amino acid sequence analysis of MdoC.
The MdoC protein (385 amino acids) exhibits stretches of hydrophobic amino acids over its entire length: 10 transmembrane segments are predicted by the TopPred2 program (26a, 33). The exoH gene, encoding a succinyl-transferase of 370 amino acids, is implicated in the synthesis of exopolysaccharides and is essential for the nodulation process of Rhizobium meliloti. ExoH, like MdoC, is essentially hydrophobic, and 7 or 11 transmembrane segments were proposed (3, 13). Nevertheless, no amino acid similarities between these two proteins were found.
The deduced amino acid sequence encoded by mdoC was compared to protein sequence data banks by using the BLAST2 software (2). Similarities were found with only one other bacterial protein, the product of nolL. nolL is a host-specificity gene encoding an acetyl-transferase protein of 373 amino acids implicated in the synthesis of the lipo-oligosaccharide Nod signal molecules (4) essential for the nodulation process of Mesorhizobium loti (25). The degree of similarity between MdoC and NolL, determined by using the LALIGN program (15), is 24.2% in 149 amino acid with no gaps in alignment except for three residues (two in MdoC and one in NolL). These similarities extended from amino acid 45 to 192 for MdoC and from residue 53 to 201 for NolL. The NolL protein, like ExoH and MdoC, is hydrophobic, and nine transmembrane segments are predicted. Thus, these three proteins are probably membrane associated. The origin of the succinyl residues remains unknown but one can postulate that they came from the succinyl-coenzyme A pool. It was clearly demonstrated that phosphoglycerol substituents, originating from the membrane phospholipids, are transferred on OPGs in the periplasmic space (6). Thus, one can suggest that the other substituents are also transferred in this cellular compartment. Then, the membrane-associated MdoC protein could catalyze the transfer of succinyl residues from the cytoplasmic side of the membrane to the nascent glucan backbones on the periplasmic side of the membrane.
In conclusion, closely linked to the operon encoding the two proteins necessary for OPG glucosidic backbone synthesis, mdoC is a gene encoding a membrane-spanning protein necessary for the substitution of these backbones. This gene organization could allow a common genetic control which remains to be elucidated.
ACKNOWLEDGMENTS
J.-M.L. and E.L. contributed equally to this work.
This research was supported by grants from the Centre National de la Recherche Scientifique (UMR111) and the Ministère de l’Education nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Altabe S G, Talaga P, Wieruszeski J-M, Lippens G, Ugalde R, Bohin J-P. Periplasmic glucans of Azospirillum brasilense. In: Elmerich C, Kondorosi A, Newton W E, editors. Biological nitrogen fixation for the 21st century. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. p. 390. [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker A, Kleickmann A, Arnold W, Puhler A. Analysis of the Rhizobium meliloti exoH/exoK/exoLfragment: ExoK shows homology to excreted endo-β-1,3-1,4-glucanases and ExoH resembles membrane proteins. Mol Gen Genet. 1993;238:145–154. doi: 10.1007/BF00279541. [DOI] [PubMed] [Google Scholar]
- 4.Berck S, Perret X, Quesada-Vincens D, Promé J-C, Broughton W J, Jabbouri S. NolL of Rhizobium sp. strain NGR234 is required for O-acetyltransferase activity. J Bacteriol. 1999;181:957–964. doi: 10.1128/jb.181.3.957-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunket III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coliK-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Bohin J-P, Kennedy E P. Regulation of the synthesis of membrane-derived oligosaccharides in Escherichia coli. Assay of phosphoglycerol transferase I in vivo. J Biol Chem. 1984;259:8388–8393. [PubMed] [Google Scholar]
- 7.Breedveld M W, Miller K J. Cyclic β-glucans of the family Rhizobiaceae. Microbiol Rev. 1994;58:145–161. doi: 10.1128/mr.58.2.145-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld M W, Hadley J A, Miller K J. A novel cyclic β-1,2-glucan mutant of Rhizobium meliloti. J Bacteriol. 1995;177:6346–6351. doi: 10.1128/jb.177.22.6346-6351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Breedveld, M. W., J. A. Hadley, and K. J. Miller. Personal communication.
- 9.Clarke L, Carbon J. A colony bank containing synthetic ColE1 hybrid plasmids representative of the entire Escherichia coligenome. Cell. 1976;9:91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- 10.Debarbieux L, Bohin A, Bohin J-P. Topological analysis of the membrane-bound glucosyltransferase, MdoH, required for osmoregulated periplasmic glucan synthesis in Escherichia coli. J Bacteriol. 1997;179:6692–6698. doi: 10.1128/jb.179.21.6692-6698.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois M, Gilles K A, Hamilton J K, Smith F. Colorimetric method for determination of sugars and related substances. Anal Biochem. 1956;28:350–356. [Google Scholar]
- 12.Fiedler W, Rotering H. Characterization of an Escherichia coli mdoB mutant strain unable to transfer sn-1-phosphoglycerol to membrane-derived oligosaccharides. J Biol Chem. 1985;260:4799–4806. [PubMed] [Google Scholar]
- 13.Gluckman M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gralla G D, Collado-Vides J. Organization and function of transcription regulatory elements. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1232–1245. [Google Scholar]
- 15.Huang X, Miller W. A time-efficient, linear-space local similarity algorithm. Adv Appl Math. 1991;12:337–357. [Google Scholar]
- 15a.Infobiogen Website. 5 February 1998, revision date. [Online.] http://www.infobiogen.fr/. [5 February 1998, last date accessed.]
- 16.Jackson B J, Bohin J-P, Kennedy E P. Biosynthesis of membrane-derived oligosaccharides: characterization of mdoBmutants defective in phosphoglycerol transferase I activity. J Bacteriol. 1984;160:976–981. doi: 10.1128/jb.160.3.976-981.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy E P. Membrane-derived oligosaccharides (periplasmic beta-d-glucans) of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1064–1074. [Google Scholar]
- 18.Lacroix J-M, Loubens I, Tempête M, Menichi B, Bohin J-P. The mdoA locus of Escherichia coliconsists of an operon under osmotic control. Mol Microbiol. 1991;5:1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix J-M, Tempête M, Menichi B, Bohin J-P. Molecular cloning and expression of a locus (mdoA) implicated in the biosynthesis of the membrane-derived oligosaccharides in Escherichia coli. Mol Microbiol. 1989;3:1173–1182. doi: 10.1111/j.1365-2958.1989.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 20.Loubens I, Debarbieux L, Bohin A, Lacroix J-M, Bohin J-P. Homology between a genetic locus (mdoA) involved in the osmoregulated biosynthesis of periplasmic glucans in Escherichia coli and a genetic locus (hrpM) controlling the pathogenicity of Pseudomonas syringae. Mol Microbiol. 1993;10:329–340. doi: 10.1111/j.1365-2958.1993.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Miller K J, Kennedy E P. Transfer of phosphoethanolamine residues from phosphatidylethanolamine to the membrane-derived oligosaccharides of Escherichia coli. J Bacteriol. 1987;169:682–686. doi: 10.1128/jb.169.2.682-686.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima T, Aiba H, Baba T, Fujita K, Hayashi K, Honjo A, Ikemoto K, Inada T, Itoh T, Kajihara M, Kanai K, Kashimoto K, Kimura S, Kitawaga M, Makino K, Masuda S, Miki T, Mizobuchi K, Mori H, Motomura K, Nakamura Y, Nashimoto H, Nishio Y, Saito N, Horiuchi T, et al. A 718-kb DNA sequence of the Escherichia coliK-12 genome corresponding to the 12.7-28.0 min region on the linkage map. DNA Res. 1996;3:137–155. doi: 10.1093/dnares/3.3.137. [DOI] [PubMed] [Google Scholar]
- 24.Rolin D B, Pfeffer P E, Osman S F, Szwergold B S, Kappler F, Benesi A J. Structural studies of a phosphocholine substituted β-(1,3);(1,6) macrocyclic glucan from Bradyrhizobium japonicumUSDA 110. Biochem Biophys Acta. 1992;1116:215–225. doi: 10.1016/0304-4165(92)90014-l. [DOI] [PubMed] [Google Scholar]
- 24a.Sanger Centre Website. 2 February 1998, revision date. Sequences. [Online.] http://www.sanger.ac.uk/Projects/S_typhi/. [2 February 1998, last date accessed.]
- 25.Scott D B, Young C A, Collins-Emerson J M, Terzaghi E A, Rockman E S, Lewis P E, Pankhurst C E. Novel and complex chromosomal arrangement of Rhizobium lotinodulation genes. Mol Plant-Microbe Interact. 1996;9:187–197. doi: 10.1094/mpmi-9-0187. [DOI] [PubMed] [Google Scholar]
- 26.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 26a.Stockholm University Theoretical Chemistry Protein Prediction Servers Website. 11 May 1997, revision date. Software. [Online.] http://www.biokemi.su.se/s̃erver/toppred2/. [17 April 1998, last date accessed.]
- 27.Talaga P, Fournet B, Bohin J-P. Periplasmic glucans of Pseudomonas syringaepv. syringae. J Bacteriol. 1994;176:6538–6544. doi: 10.1128/jb.176.21.6538-6544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talaga P, Stahl B, Wieruszeski J-M, Hillenkamp F, Tsuyumu S, Lippens G, Bohin J-P. Cell-associated glucans of Burkholderia solanacearum and Xanthomonas campestrispv. citri. J Bacteriol. 1996;178:2263–2271. doi: 10.1128/jb.178.8.2263-2271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talaga, P., J.-M. Wieruszeski, V. Cogez, A. Bohin, G. Lippens, and J.-P. Bohin. Unpublished results.
- 30.Talaga, P., V. Cogez, and J.-P. Bohin. Unpublished results.
- 31.Van Golde L M G, Schulman H, Kennedy E P. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci USA. 1973;70:1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira J, Messing J. The pUC plasmids, an M13 mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 33.Von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]