Abstract
Pfizer and the Medicines Patent Pool (MPP) have reached a voluntary licensing agreement for Paxlovid (nirmatrelvir+ritonavir), a novel antiviral for coronavirus disease 2019 (COVID-19) taken orally in the first 5 days from symptom onset. The Pfizer-MPP deal enables 95 low- and middle-income countries (L/MICs) to access affordable biosimilars. Generics are delayed awaiting bioequivalence testing and may be ineffective in L/MICs with reduced testing capacity, which comprise only 10% of global diagnoses. Thirty-nine percent of diagnoses originate in MICs forced to pay high prices due to exclusion from the Pfizer-MPP deal. The cost-effectiveness of Paxlovid could be limited compared with the creation of sustainable vaccine infrastructure in these nations, delaying socioeconomic pandemic recovery. Furthermore, Paxlovid may not be cost-effective in vaccinated populations, and concerns remain over ritonavir drug interactions with COVID-19 comorbidity medications. We call for expanded coverage by the Paxlovid-MPP deal and greater access to testing.
Keywords: access to medicine, biosimilars, COVID-19, Paxlovid, test-and-treat
Global daily incidence of coronavirus disease 2019 (COVID-19) remains around 500 000 (08/03/2021) [1]. Global access to vaccination is drastically unequal due to corporate prioritization of profits. Meanwhile, continued spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increases emergence of highly transmissible variants like Delta and Omicron, which reduce vaccine efficacy. A test-and-treat approach is therefore crucial, and direct antivirals are key. Paxlovid (nirmatrelvir+ritonavir) is an oral antiviral developed by Pfizer for COVID-19. Pfizer’s EPIC-HR trial showed an 88% reduction in hospitalization and death compared with placebo in nonhospitalized high-risk adult patients [2].
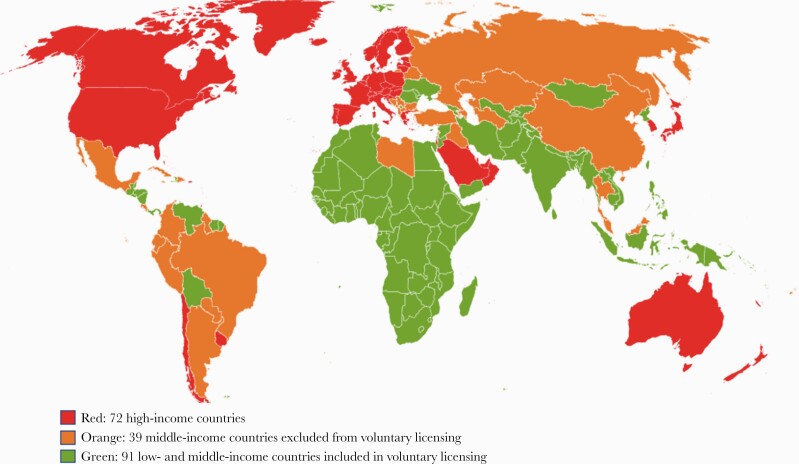
The active pharmaceutical ingredient (API) in Paxlovid is comprised of 3 subunits: (1) tert-leucine, (2) proline derivative, and (3) chiral pyrrolidone [3]. Tert-leucine is commonly used in drug candidates; however, the other 2 components currently have no commercial source and must be synthesized, meaning high initial prices for the API [4]. As an example, the US government has paid US$529 per 5-day treatment course [5]. However, Pfizer and the Medicines Patent Pool (MPP) have agreed upon a voluntary license for Paxlovid, enabling 95 low- and middle-income countries (L/MICs) to purchase generic versions from production sites anywhere in the world that qualify for a sublicense (Figure 1) [6]. This decentralized production is crucial for rapid widespread access. The deal is similar to the Merck-MPP agreement for Molnupiravir, another oral antiviral, but with a comparative net reduction of 10 countries able to procure generic Paxlovid. Countries can qualify for inclusion at the discretion of Pfizer based on gross domestic income; however, many MICs are excluded—Argentina, Brazil, Thailand, and Malaysia among them.
Figure 1.
World map of Pfizer voluntary licensing of Paxlovid.
Though these deals are revelatory in the context of the pharmaceutical industry, access will remain limited. The antivirals are only efficacious within 5 days of symptom onset; L/MICs with reduced testing availability will struggle to diagnose and treat cases quickly enough even when biosimilars become available. Conversely, high-income countries (HICs) with access to testing and Paxlovid have tiny high-risk populations due to vaccination rates around 75% on top of Omicron variant infection, reducing risk of hospitalization and death compared with Delta [7, 8].
This paper aims to elucidate the value of Paxlovid in the pandemic for countries at each income band with reference to the MPP deal.
METHODS
From Worldometer, a reference site that provides real-time COVID-19 statistics, we calculated mean daily diagnosis rates by country in 3 categories [9]: 72 high-income countries, as defined by the World Bank [10]; 91 low-income countries included in the Pfizer-MPP deal; and 39 middle-income countries judged too rich to benefit from pricing discounts [6]. To calculate mean daily diagnosis rates, we observed the sum of total reported cases in each country divided by the daily average of diagnosed cases in the past 7 days on November 18, 2021.
RESULTS
According to worldometer, there were 500 431 new COVID-19 diagnoses made per day worldwide in November 2021 [9]. Of these, only 51 373 (10%) diagnoses came from the 91 LICs in the MPP deal. However, 146 130 daily diagnoses (29%) came from the 39 middle-income countries ineligible for pricing discounts. For example, Thailand, not eligible for minimum prices in a similar Merck-MPP agreement for molnupiravir, is currently paying >$300 per treatment course as a result [11, 12]. A total of 302 928 or 61% of diagnoses came from high-income countries.
Table 1 compares testing capacity and vaccination rates for the 5 most impacted countries in each World Bank income level as of November 24, listed by inclusion in the Pfizer-MPP deal. Of note, the highest vaccination rate in LICs included in the table was 8.7% (Afghanistan). Sampled LICs had a low average test-to-population ratio of 1:72. MICs in the deal also have low test-to-population ratios. Nigeria (1:60) and Uganda (1:24) had especially low rates. Some nations not included in the deal have good testing rates (South Africa, 1:3).
Table 1.
Testing Capacity and Vaccination Rate for Selected Sample Countries, November 24, 2021, by Income Level and Inclusion in the Paxlovid-MPP Deal for Affordable Access
| Income | Country | MPP | New Diagnoses (7/12/21) | Tests: Pop [1, 2] | AVG | Vaccination Rate, % [3] |
|---|---|---|---|---|---|---|
| LICs | Afghanistana | Yes | 23 | 1: 52 | 1: 72 | 8.7 |
| Chada | 0 | 1: 115 | 0.4 | |||
| Haitia | 0 | 1: 88 | 0.6 | |||
| Malawia | 18 | 1: 45 | 3.1 | |||
| Nigera | 8 | 1: 134 | 1.9 | |||
| MICs | Paraguaya | Yes | 43 | 1: 4 | 1: 6.0 | 37 |
| Ugandaa | 19 | 1: 24 | 1.9 | |||
| Nigeriaa | 107 | 1: 60 | 1.8 | |||
| South Africa | 13 147 | 1: 3 | 24 | |||
| Indonesia | 261 | 1: 5 | 34 | |||
| Russia | No | 31 096 | 2: 1 | 1: 2.0 | 40 | |
| Thailand | 3614 | 1: 5 | 61 | |||
| Brazil | 10 250 | 1: 3 | 63 | |||
| Mexico | 752 | 1: 11 | 50 | |||
| Iraq | 625 | 1: 3 | 11 | |||
| HICs | US | No | 107 642 | 2: 1 | 2.4: 1 | 58 |
| UK | 45 691 | 5: 1 | 70 | |||
| France | 59 019 | 2: 1 | 70 | |||
| China | 94 | 1: 9 | 75 | |||
| Italy | 15 756 | 2: 1 | 73 |
DISCUSSION
Current data show that only 10% of global diagnoses come from LICs in the Pfizer-MPP deal. With 29% of diagnoses arising from MICs ineligible for affordable pricing, the list of countries included needs expanding.
Forty million more cases are reported in HICs compared with LICs due to significantly higher testing rates. The Democratic Republic of Congo shows the lowest testing rate, with 1 in 300 people being tested since the start of the pandemic [9]. Contrastingly, each Danish citizen has been tested 15 times on average [9]. The average test-to-population ratio across 5 sampled LICs was 1:72 (Table 1) [13]. LICs will struggle to treat infections if testing is not made widely available. This stands for some MICs in the deal also, as seen in Uganda and Nigeria (testing ratios, 1:24 and 1:60) (Table 1) [9]. MICs with the capacity to test and diagnose, like Argentina and Iraq (testing ratios, 1:2 and 1:3), may never reach treatment as they will be unable to procure biosimilars. These countries must prioritize effective vaccine programs, as in Iraq, excluded from the MPP deal, but with a vaccination rate of 11% (Table 1) [7].
Paxlovid may not be effective in HICs. To be eligible for Pfizer’s EPIC-HR trial, participants were required to be unvaccinated and over 60 years of age [14]. Vaccination rates are around 75% in HICs, with older populations prioritized; therefore, an accurate depiction of efficacy in vaccinated individuals is needed [9]. One such community study, the PANORAMIC trial, which has recruited 2500 of 17500 participants, is underway in the UK to validate industry-funded results for molnupiravir [15]. The less dangerous Omicron variant diminishes Paxlovid’s usefulness further in highly vaccinated HICs. The EPIC-SR trial showed an interim non–statistically significant 70% reduction in hospitalization, perhaps limiting cost-effectiveness in this population [2]. EPIC-SR was completed in November, but full results have not yet been reported. Randomized trials must be conducted by external bodies to validate industry-funded trials, and cost-benefit analysis is needed to justify continued purchase.
As L/MIC populations have low vaccination rates, they are at highest risk of severe COVID-19. However, generic production of Paxlovid is currently delayed by regulatory clinical trials testing bioequivalence [16]. Until Paxlovid biosimilars can be approved, L/MICs included in the Pfizer-MPP deal have no access to effective treatment, as Pfizer has promised only 10 million doses to L/MICs via the Global Fund [17]. Even then, for biosimilars to have good effect, testing inequality must be dramatically reduced to ensure treatment in the 5-day window. Instead of working to reduce these inequalities, Pfizer is prioritizing bilateral deals with HICs, countries where Paxlovid can make negligible contributions to severe case reduction; however, even these deals have not been upkept, necessitating wide uptake of molnupiravir despite comparatively low efficacy and theoretical side effects [18]. Furthermore, Paxlovid coformulations require approval by Pfizer rather than just independent regulatory bodies, meaning inevitable delays and potential blocks between successful trials and distribution.
Lastly, concerns remain about drug interaction. Five days of ritonavir can boost levels of drugs used for comorbidities such as stroke and hypertension [19]. Altering drug dosage requires specialist knowledge. Patients with any CYP3A4-dependent medications were excluded from the EPIC trials. Smaller interaction studies are ongoing. One study showed that 78% of COVID-19 patients (n = 125) had potential drug interactions with ritonavir [20].
CONCLUSIONS
We advocate for prioritization of bioequivalence studies and improved testing infrastructure in LICs to unlock biosimilars. The list of countries included in the Pfizer-MPP deal for Paxlovid should be expanded, as 29% of global diagnoses originate in currently excluded MICs. Further research is needed into the cost-effectiveness of Paxlovid in different settings, including drug interaction review.
Acknowledgments
Financial support. This study was funded by the International Treatment Preparedness Coalition/Make Medicines Affordable Campaign.
Potential conflicts of interest. There are no conflicts of interest to declare. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disc
Patient consent. This paper did not include factors necessitating patient consent.
Contributor Information
Toby Pepperrell, School of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, UK.
Leah Ellis, Faculty of Medicine, Imperial College London, London, UK.
Junzheng Wang, Faculty of Medicine, Imperial College London, London, UK.
Andrew Hill, Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool, UK.
References
- 1. World Health Organization. WHO coronavirus (COVID-19) dashboard with vaccination data. Available at: https://covid19.who.int/. Accessed 8 December 2021.
- 2. Pfizer. Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Accessed 23 December 2021.
- 3. Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S.. Supervised Molecular Dynamics (SuMD) insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332. J Enzyme Inhib Med Chem 2021; 36:1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bommarius AS, Schwarm M, Stingl K, Kottenhahn M, Huthmacher K, Drauz K.. Synthesis and use of enantiomerically pure tert-leucine. Tetrahedron Asymmetry 1995; 6:2851–88. [Google Scholar]
- 5. Pfizer. Pfizer and BioNTech announce an agreement with U.S. government for up to 600 million doses of mRNA-based vaccine candidate against SARS-CoV-2. 2020. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-agreement-us-government-600. Accessed 8 December 2021.
- 6. Pfizer. Pfizer and the Medicines Patent Pool (MPP) sign licensing agreement for COVID-19 oral antiviral treatment candidate to expand access in low- and middle-income countries. 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-medicines-patent-pool-mpp-sign-licensing. Accessed 8 December 2021.
- 7. Our World in Data. Coronavirus (COVID-19) vaccinations - statistics and research. Available at: https://ourworldindata.org/covid-vaccinations. Accessed 8 December 2021.
- 8. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Worldometer. COVID live update: 267,486,343 cases and 5,289,120 deaths from the coronavirus. Available at: https://www.worldometers.info/coronavirus/. Accessed 8 December 2021.
- 10. World Bank. High income | data. Available at: https://data.worldbank.org/country/XD. Accessed 8 December 2021.
- 11. Merck.com. The Medicines Patent Pool (MPP) and Merck enter into license agreement for molnupiravir, an investigational oral antiviral COVID-19 medicine, to increase broad access in low- and middle-income countries. 2021. Available at: https://www.merck.com/news/the-medicines-patent-pool-mpp-and-merck-enter-into-license-agreement-for-molnupiravir-an-investigational-oral-antiviral-covid-19-medicine-to-increase-broad-access-in-low-and-middle-income-countri/. Accessed 8 December 2021.
- 12. Bangprapa M. Cabinet approves purchase of molnupiravir. Bangkok Post. 9 November 2021.
- 13. Statista. COVID-19 testing rate by country. Available at: https://www.statista.com/statistics/1104645/covid19-testing-rate-select-countries-worldwide/. Accessed 8 December 2021.
- 14. EPIC-HR: study of oral PF-07321332/ritonavir compared with placebo in nonhospitalized high risk adults with COVID-19. ClinicalTrials.gov Identifier: NCT04960202. Available at: https://clinicaltrials.gov/ct2/show/NCT04960202?term=PF-07321332&draw=2&rank=5. Accessed 8 December 2021.
- 15. NIHR. NIHR funds community COVID-19 antiviral trial. Available at: https://www.nihr.ac.uk/news/nihr-funds-community-covid-19-antiviral-trial/29048. Accessed 8 December 2021.
- 16. Thacker T. Paxlovid: nod for Paxlovid’s Indian generic version to take longer. The Economic Times. 19 February 2022.
- 17. Erman M. Pfizer to provide 10 mln courses of COVID pill to developing countries—the Global Fund. Reuters. 2 March 2022.
- 18. Hillman A, Vaidya M.. Molnupiravir supplies dominate in times of Paxlovid scarcity. Pharmaceutical Technology. 11 February 2022.
- 19. NICE. Ritonavir | interactions. Available at: https://bnf.nice.org.uk/interaction/ritonavir-2.html. Accessed 8 December 2021.
- 20. Macías J, Pinilla A, Lao-Dominguez FA, et al. High rate of major drug-drug interactions of lopinavir-ritonavir for COVID-19 treatment. Sci Rep 2020; 10:20958. [DOI] [PMC free article] [PubMed] [Google Scholar]