Abstract
In an attempt to develop natural product-based anticancer agents, a series of novel piperazine-linked bergenin heterocyclic hybrids bearing arylthiazolyl (5a–e), benzothiazolyl (10a–i), and arylsulfonyl (13a–o) were synthesized using the classical Mannich reaction and evaluated for their anticancer activity. All the synthesized derivatives were assessed for in vitro cytotoxic activity against a panel of human cancer and normal cell lines and the results showed that most of the compounds exhibited significant cytotoxic activity against cancer cells and mild cytotoxicity against normal cells. In particular, the compounds 5a, 5c, 10f, and 13o showed potent cytotoxic activity against tongue and oral cancer cell lines compared to the parent compound (<100 μM). Considering the efficacy, the compounds 5a, 5c, 10f, and 13o were subjected to cell cycle analysis and the results indicated that the compounds mitigated the cell cycle progression at the G0/G1 phase in the tongue and oral cancer cell lines. Subsequently, the annexin V/PI staining assay demonstrated that the compounds 5a, 5c, 10f, and 13o induced early and late apoptosis against tongue cancer and necrosis against oral cancer. Further, gene expression analysis revealed that 5a, 5c, and 13o treatment regulated the BAX and BcL-2 expression and also the selected compounds significantly reduced the expression level of vimentin, oct-4, and nanog. In addition, molecular docking studies revealed that the selected derivatives have strong binding energy with the BcL2 protein and downregulates the expression. Taken together, the study results implied that these compounds are promising anticancer candidates by modulating the epithelial to mesenchymal transition axis and could be considered for further development of novel anticancer drugs.
Novel piperazine-linked bergenin derivatives were synthesized and the compounds 5a, 5c, 10f, and 13o showed excellent cytotoxic activity. These compounds arrest the cell cycle and induce apoptosis by regulating the Bax/BcL2 expression.
1. Introduction
Over the past few decades, substantial developments in cancer therapy have been evolved, such as chemotherapy, surgery, radiotherapy, and biotherapy, and a plethora of anticancer agents were approved for this purpose.1 However, multidrug resistance along with fatal side effects, as well as low specificity of the existing anticancer agents, highlights a need for the discovery of novel anticancer candidates with different mechanisms of action.2 In addition, epithelial to mesenchymal transition (EMT) and stemness play a greater role in metastasis and tumor relapse.3 In this context, nature can be viewed as a remarkable source for biologically active leads and therefore, the synthesis of natural product-based libraries of these lead compounds is an important area of research in modern drug discovery.4 Vincristine, camptothecin and paclitaxel are the best examples of natural product-based anticancer agents which play a significant role in cancer chemotherapy.5
During the past two decades, great effort of our group has been devoted to the exploration of traditional flora and structural modification of natural product leads for finding more effective analogs with novel mechanisms of action, which resulted in a series of structurally intriguing NP derivatives with potential biological activities.6–8 In the course of our continuous efforts on exploring traditional Indian flora, we have isolated and identified bergenin (5) as a major lead phytochemical from fruits of Mallotus philippensis which is reported to have versatile biological activities including anticancer, anti-hepatotoxic, antioxidant, anti-HIV, anti-inflammatory and neuroprotective properties.9,10 Moreover, recent studies also have demonstrated that bergenin can induce apoptosis by regulating the AKT signaling pathway in human bladder cancer.11 Despite the wide biological activities, bergenin has poor solubility with weak permeation leading to low absorption and poor bioavailability,12 which attracted medicinal chemists to focus on its structural modifications to enhance their physical properties. Consequently, few approaches have been devised for either derivatization or synthesis of related compounds to optimize them as lead molecules.13,14
Recently, the concept of molecular hybridization emerged as a valuable structural modification perception, which involves the design of new hybrid compounds with the combination of two or more pharmacophoric units.15 These hybrids are claimed to have increased biological affinity and to reduce undesirable effects compared to their parent molecules. In this context, piperazines linked to arylthiazolyl, benzothiazolyl, and arylsulfonyl moieties have gained importance due to their lipophilicity, which is supposed to aid in enhancing pharmacokinetic properties,16 and also many commercially available drugs share a similar skeleton to the piperazine linked scaffold (Fig. 1). Based on this hypothesis and in continuation of work on bergenin analogues, the present study was focused to modify bergenin by incorporation of piperazinyl linked heterocyclic hybrids to obtain new drug candidates for the treatment of cancer diseases. Herein, we reported the synthesis, anticancer activity and molecular docking studies of piperazine tethered bergenin analogues.
Fig. 1. Representation of commercially available drugs with a similar skeleton to bergenin derivatives.
2. Results and discussion
2.1. Chemistry
A series of schemes (Schemes 1–3) describe the convergent synthetic methods to prepare the novel bergenin analogues by addition of various substituents at the C-7 position of bergenin using the Mannich reaction. The synthetic route for the preparation of bergenin derivatives attached with N-(thiazol-2-yl)-2-(substituted amino)acetamide (5a–5e) is illustrated in Scheme 1. Initially, the cyclization of substituted phenacyl bromides (1a–1e) was performed with thiourea at 0 °C–rt to afford a good quantity of 2-amino-4-substituted-thiazoles (2a–2e). Subsequent acylation of the 2-aminothiazoles (2a–2e) with chloroacetyl chloride in the presence of triethylamine afforded 2-(2-chloroacetamido)thiazoles in good yield (3a–3e), which were further reacted with piperazine in the presence of TEA and 1,4-dioxane to afford the key intermediates, N-(substituted thiazol-2-yl)-2-(piperazine-1-yl)acetamides (4a–4e), in good yields.17,18 The 1H NMR spectra of the intermediates (3a–3e) indicated the presence of signals at δ 3–4 ppm, due to CH2 of the chloroacetamide group, and at δ 7–8 ppm assigned to the 2-amino-4-phenylthiazole ring along with their molecular weight determination from mass spectra which confirmed the structures of the N-(substituted thiazol-2-yl)-2-(piperazine-1-yl)acetamides (4a–4e). Finally, the Mannich reaction was employed to treat bergenin (5) with the as-prepared substituted arylthiazolylpiperazines (4a–4e) in the presence of formaldehyde in DMSO to afford the desired final products (5a–e). All bergenin derivatives were purified using a Sephadex LH-20 column chromatograph by eluting with methanol to get the desired products and were characterized using NMR and HRMS analysis.
Scheme 1. Reagents and conditions: (i) thiourea, EtOH, 80 °C; (ii) chloroacetyl chloride, TEA, THF, 0 °C-rt, 2–6 h; (iii) piperazine, TEA, 1,4-dioxane, 110 °C, 2–6 h; (iv) DMSO, 37% formaldehyde, 50 °C, 12 h.
Scheme 2. Reagents and conditions: (i) potassium thiocyanide, bromine in acetic acid, 3 h; (ii) chloroacetyl chloride, K2CO3, benzene, reflux, 6–12 h; (iii) piperazine, TEA, 1,4-dioxane, 110 °C, 2–6 h; (iv) DMSO, 37% formaldehyde, 50 °C, 12 h.
Scheme 3. Reagents and conditions: (i) piperazine, DIPEA, THF, 0 °C, 2 h; (ii) DMSO, 37% formaldehyde, 50 °C, 12 h.
The synthetic route for the target compounds (10a–i) is shown in Scheme 2. The key intermediates (9a–9i) were prepared by reacting the respective ortho- and para-substituted aryl anilines and potassium thiocyanate dissolved in acetic acid with bromine to afford 2-amino-substituted benzothiazoles (7a–i) using a precipitation method. The structure of the benzothiazoles was characterized using NMR analysis and corresponded with those in previous reports.19 Subsequently, the 2-aminobenzothiazoles were reacted with chloroacetyl chloride in benzene under reflux conditions, which afforded N-(benzo[d]thiazol-2-yl)-2-chloroacetamides (8a–i) in good yield.20 The resulting chloroacetamides were reacted with piperazines in the presence of TEA and 1,4-dioxane to afford the key intermediates N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl) acetamide (9a–i) in moderate yield.211H NMR showed the presence of CH2 group signals of piperazine acetamide at δ 3–4 ppm and also the existence of benzothiazole was observed at δ 7–8.0 ppm. Finally, bergenin was electrophilically substituted with the N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl)acetamides at the C-7 position in the presence of formaldehyde using the Mannich reaction, which affords the desired final products (10a–i).
As illustrated in Scheme 3, the target bergenin derivatives (13a–o) were prepared by attaching different arylsulfonylpiperazines.22,23 Initially, the substituted benzenesulfonyl chlorides were reacted with piperazine in the presence of DIPEA, which afforded piperazine substituted benzosulfonyl chlorides (12a–o) in good yield. Then, bergenin was electrophilically substituted with the prepared compounds (12a–o) at the C-7 position using the Mannich reaction in the presence of formaldehyde, which affords the desired final products (13a–o). The series of bergenin derivatives substituted with piperazine benzosulfonyl chlorides at the C-7 position were purified and characterized by 1H and 13C NMR, and HRMS analysis. All bergenin derivatives were purified using silica gel and a Sephadex LH-20 column chromatograph eluting with methanol to get the desired products and the structures of the target compounds were characterized using NMR and HRMS analysis.
2.2. Anticancer activity
2.2.1. In vitro cytotoxic activity
Bergenin and its analogues were dissolved in sterile DMSO to attain 10 mg ml−1 stock concentrations and were assessed for in vitro anticancer activity against a panel of human cancer cell lines, including tongue (CAL27), oral (SCC09), colorectal (HCT-15), breast (MCF7), and lung (A549) cancer cells, and normal lung epithelial cells (BEAS-2B). Doxorubicin was used as a standard drug and DMSO (0.01%) was used as vehicle control in all the cell lines. As shown in Table 1, the mean IC50 values revealed that the substitution of different heterocyclic moieties at C-7 of bergenin significantly increased their cytotoxic activity against selected cancer cells.
In vitro cytotoxic activity of the bergenin derivatives against a panel of cancer and normal cells and the values are represented as IC50 values (μM).
| Compound | HCT-15a | CAL-27b | SCC09c | A549d | MCF7e | BEAS-2Bf |
|---|---|---|---|---|---|---|
| 5 | >100 | >100 | >100 | >100 | >100 | >100 |
| 5a | 70.5 ± 9.33 | 16.03 ± 0.075 | 32.43 ± 1.78 | >100 | >100 | >100 |
| 5b | >100 | 46.57 ± 1.125 | >100 | 49.89 ± 0.23 | 37.33 ± 0.19 | >100 |
| 5c | 69.7 ± 0.16 | 20.71 ± 0.04 | 34.4 ± 3.03 | >100 | >100 | >100 |
| 5d | 86.21 ± 4.5 | 57.2 ± 0.3 | 65.5 ± 1.75 | >100 | >100 | 83.45 ± 6.75 |
| 5e | >100 | 42.7 ± 4.06 | >100 | 40.62 ± 1.545 | 25.25 ± 0.09 | >100 |
| 10a | >100 | >100 | >100 | >100 | >100 | >100 |
| 10b | >100 | 91.23 ± 7.13 | 64.8 ± 8.715 | >100 | >100 | 97.29 ± 14.81 |
| 10c | 85.94 ± 2.7 | 30.48 ± 1.16 | 37.60 ± 8.805 | >100 | >100 | 58.55 ± 10.5 |
| 10d | 30.2 ± 0.64 | 15.41 ± 0.5 | 17.41 ± 1.255 | 22.46 ± 0.06 | 19.59 ± 0.40 | 21.73 ± 0.68 |
| 10e | 97.56 ± 6.3 | >100 | 32.6 ± 9.09 | 65.61 ± 1.91 | 64.09 ± 0.47 | 44.16 ± 8.5 |
| 10f | >100 | 63.72 ± 2.62 | 91.9 ± 2.6 | 90.64 ± 0.39 | 74.54 ± 1.42 | >100 |
| 10g | >100 | 81.20 ± 1.065 | >100 | >100 | >100 | >100 |
| 10h | >100 | 83.73 ± 7.5 | >100 | >100 | >100 | >100 |
| 10i | >100 | 53.03 ± 2.64 | >100 | >100 | >100 | >100 |
| 13a | >100 | 56.75 ± 0.46 | >100 | >100 | >100 | 51.62 ± 6.07 |
| 13b | >100 | >100 | >100 | >100 | >100 | 22.4 ± 0.71 |
| 13c | >100 | >100 | >100 | >100 | >100 | >100 |
| 13d | >100 | 85.48 ± 2.97 | >100 | >100 | >100 | >100 |
| 13e | >100 | 74 ± 2.65 | >100 | >100 | >100 | >100 |
| 13f | >100 | >100 | >100 | >100 | >100 | >100 |
| 13g | >100 | >100 | >100 | >100 | >100 | >100 |
| 13h | >100 | >100 | >100 | >100 | >100 | >100 |
| 13i | >100 | 92.93 ± 1.25 | >100 | >100 | >100 | >100 |
| 13j | >100 | >100 | >100 | >100 | >100 | >100 |
| 13k | >100 | >100 | >100 | >100 | >100 | >100 |
| 13l | >100 | >100 | >100 | >100 | >100 | >100 |
| 13m | >100 | 68.6 ± 6 | >100 | >100 | >100 | >100 |
| 13n | >100 | 91.56 ± 1.09 | >100 | >100 | >100 | >100 |
| 13o | >100 | 41.62 ± 4.59 | >100 | >100 | >100 | >100 |
| Doxorubicin | 0.8 ± 0.2 | 0.4 ± 0.1 | 0.39 ± 0.12 | 0.8 ± 0.2 | 1.1 ± 0.13 | 0.8 ± 1.1 |
Colorectal cancer.
Tongue cancer.
Oral cancer.
Breast cancer.
Lung cancer.
Normal lung epithelial cells.
As shown in Table 1, the bergenin derivatives (5a–e) substituted with different N-(substituted thiazole-2-yl)-2-(piperazine-1-yl)acetamides displayed cell-specific cytotoxic activity against tongue cancer cells compared to bergenin. Moreover, a few derivatives showed moderate activity against breast and lung cancer cells and a few derivatives did not show any activity against colorectal, lung, and breast cancer cells. At first inspection, derivatives 5b and 5e showed moderate activity against breast and lung cancer cells and did not show any activity against colorectal cancer cells. In particular, the addition of N-(4-(4-methoxyphenyl)thiazole-2-yl)-2-(piperazine-1-yl)acetamide (5a) or N-(4-phenylthiazol-2-yl)-2-(piperazin-1-yl)acetamide (5c) at the 7th position enhanced the activity of bergenin significantly compared with that of other arylthiazolyl piperazine derivatives. It is important to mention that no cytotoxic activity was observed at higher concentrations (100 μM) for all the derivatives (5a–e) against normal lung epithelial cells, indicating that these derivatives are safer to use for further studies.
The anticancer activities of the benzothiazolylpiperazine-bearing bergenin hybrids (10a–i) against cancer cells revealed that most of the substituted N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl)acetamide derivatives showed moderate to poor cytotoxic activity and very few derivatives (10c, 10d, 10e, and 10f) displayed significant activity. From these results, it is clear that the addition of halides in the aromatic ring of benzothiazoles (10a–e) significantly enhanced the activity of bergenin. Further, the addition of nitro, halogen (–Cl), and methoxy groups as substituents (10f–h) on the aromatic ring significantly enhanced the cytotoxic activity against tongue cancer cells. Moreover, these derivatives (10f–h) did not show any cytotoxic activity against normal cells.
Concerning the piperazine linked arylsulfonyl–bergenin hybrids (13a–o), the derivatives substituted at the 2nd, 3rd and 4th positions of bergenin displayed cell-specific activity. As shown in Table 1, these derivatives showed excellent cytotoxic activity against tongue cancer compared with bergenin while there was no activity observed against the colorectal, oral, lung and breast cancer cells. In addition, these derivatives did not show any cytotoxic activity against human normal lung epithelial cells, indicating that the derivatives can be used for further studies. The cell-specific activity of these derivatives is due to the substitution of hydrogen and methyl at any of the positions and the poor activity of these derivatives is due to the substitution of halide and methoxy groups on arylsulfonylpiperazine. Among the derivatives, compound 13o showed moderate cytotoxic activity compared to the parent molecule due to the presence of a halide (I) which has a lower electronegativity than the other halides.
Overall, from the results of in vitro cytotoxic activity, derivatives 5a, 5c, 10f, and 13o showed excellent activity against the tongue cancer cell line and 5a, 5c, and 10f against oral cancer. These derivatives were selected to study their molecular mechanism of cytotoxicity to cancer cells via cell cycle analysis and induction of apoptosis using flow cytometry analysis and gene expression studies using qRT-PCR analysis.
2.2.2. Cell cycle analysis
Owing to the potential in vitro cytotoxic activity of the bergenin derivatives against CAL-27 and SCC09 cells, the effect of these derivatives on the cell cycle progression was examined after 24 h treatment at different concentrations. As shown in Fig. 2 and Table S2,† increasing the concentration of compound 5a (16 to 32 μM) increased the cell population at the G0/G1 phase while compound 5c (10 to 34 μM) increased both G0/G1 and S phases compared with the control CAL-27 cells. Additionally, the cells treated with 31.5 μM 10f arrest the population at the G0/G1 phase but an increased concentration of 10f (63 μM) arrests the cell population at the G2/M phase compared with the control. As compared with the control (76.4%), the cell population at the G0/G1 phase of CAL-27 cells was increased after treatment with 21 μM (83.3%) and 42 μM (83.6%) 13o, indicating that compound 13o arrests the cell cycle at the G0/G1 phase, while there are no significant changes in the population of cells at the S phase and G2/M phase.
Fig. 2. Cell cycle analysis using flow cytometry against CAL27 cells. The CAL27 cells were treated with the vehicle (a), doxorubicin (b), 16 μM (c) and 32 μM (d) 5a, 10 μM (e) and 34 μM (f) 5c, 31.5 μM (g) and 63 μM (h) 10f, and 21 μM (i) and 42 μM (j) 13o. The treated cells were analysed for cell cycle arrest using flow cytometry.
Furthermore, the bergenin derivatives (5a, 5c, and 10f) also showed excellent cytotoxic activity against SCC09 cells and the effect of these derivatives on the cell cycle progression was assessed. As shown in Fig. 3 and Table S3,† the treatment with compound 5a (16.2 μM) and 5c (17 μM) arrests the cell cycle at both G0/G1 and S phases while increasing the concentration of 5a (32.4) and 5c (34 μM) arrests the cell cycle progression at the G0/G1 phase itself, indicating the cytotoxic efficiency of the compounds. In addition, the activity of compound 10f clearly reveals that an increase in the concentration of the compounds enhanced the cell population at the G0/G1 phase compared with control CAL-27 cells. Therefore, the obtained results in the cell cycle analysis directed us to study the induction of apoptosis which could provide a link between the losses of viability during cell cycle arrest.
Fig. 3. Cell cycle analysis using flow cytometry against SCC09 cells. The SCC09 cells were treated with the vehicle (a), doxorubicin (b), 16.2 μM (c) and 32.4 μM (d) 5a, 17 μM (e) and 34 μM (f) 5c and 46 μM (g) and 92 μM (h) 10f. The treated cells were analysed for cell cycle arrest using flow cytometry.
2.2.3. Apoptosis assay
The cytotoxic activity of drugs leads to either induction of apoptosis, cell cycle arrest or a combination of both approaches.24 In the present study, the annexin V/PI staining assay has been employed to determine the apoptotic effect of the bergenin derivatives using flow cytometry analysis. As shown in Fig. 4 and Table S4,† the percentage of apoptotic cells was increased in a concentration-dependent manner during the treatment with 5a, 5c, 10f, and 13o. The CAL-27 cells treated with 16 μM 5a showed 2.7% early apoptotic cells which increased up to 2.8% at a 32 μM concentration, which is higher than that in the control cells. Moreover, the treatment with different concentrations (10 μM and 34 μM) of compound 5c significantly increased the apoptosis in CAL-27 cells than in the control cells. In addition, the percentage of early apoptotic cells was also increased during the treatment with 10f (8.3% to 11.7%) and 13o (1.1% to 1.7%) and the percentage of late apoptotic cells also increased during the treatment of CAL-27 with 10f and 13o. These results indicated that the compounds 10f and 13o showed an excellent apoptotic effect and 5a showed a moderate apoptotic effect as compared with control CAL-27 cells.
Fig. 4. Apoptosis effect of compounds against CAL27 cells. The CAL27 cells were treated with the vehicle (a), doxorubicin (b), 16 μM (c) and 32 μM (d) 5a, 10 μM (e) and 34 μM (f) 5c, 31.5 μM (g) and 63 μM (h) 10f, and 21 μM (i) and 42 μM (j) 13o. The treated cells were analysed for the apoptosis effect using the annexin V–FITC staining assay.
Further, the apoptotic effect of bergenin derivatives against SCC09 cells was also determined and the results are shown in Fig. 5 and Table S5.† After 24 h treatment of SCC09 cells with different concentrations of 5a, 5c, and 10f, a decrease in the percentage of live cells was observed. Additionally, a significant increase in late apoptotic cells (40.8%) compared to control cells (1.2%) was observed in the cells treated with 16.2 μM 5a and increasing the concentration of 5a up to 32.4 μM did not increase the apoptosis effect. Among the other compounds, the 5c treatment showed an excellent apoptotic effect against SCC09 cells by inducing late apoptosis in a concentration-dependent manner. A lower concentration of 5c (17 μM) induced 43% late apoptosis and increasing the concentration to 34 μM also increased the late apoptosis (47.2%) in SCC09 cells compared to that in the control cells (1.2%). In another experiment, the treatment of SCC09 cells with compound 10f induced a necrotic (37.6%) effect compared with control cells (0.1%). Together, the results indicated that the cytotoxic effect of the bergenin derivatives might be involved in the induction of apoptosis.
Fig. 5. Apoptosis effect of compounds against SCC09 cells. The SCC09 cells were treated with the vehicle (a), doxorubicin (b), 16.2 μM (c) and 32.4 μM (d) 5a, 17 μM (e) and 34 μM (f) 5c and 46 μM (g) and 92 μM (h) 10f. The treated cells were analysed for the apoptosis effect using the annexin V–FITC staining assay.
2.2.4. Effect of compounds on gene expression
To determine the mechanism of action of the bergenin derivatives (5a, 5c, and 13o), anticancer potential apoptosis related genes, stemness and epithelial to mesenchymal transition (EMT) marker expression were analysed using qRT-PCR. Stemness and EMT play a key role in the progression of diseases and initiation of metastasis,25 and targeting these genes along with apoptosis genes may be useful to mitigate cancer progression. In CAL27 cells, the levels of vimentin, Oct-4, Nanog and BcL-2 genes were down regulated after treatment with compounds 5a and 13o and the expression of Bax and E-cad was upregulated in 13o treated CAL27 cells. However, the cells treated with 5c did not show any significant changes (Fig. 6). It was previously reported that the up-regulation of E-cad and down-regulation of vimentin, oct-4 or Nanog lead to the suppression of cell migration/invasion and stemness properties of cancer cells26,27 and apoptosis was induced upon responding to the upregulation of the Bax and BcL-2 genes.28,29 In the case of SCC09 cells, the compounds 5a and 13o showed the significant upregulation of Bax and E-cad and downregulation of genes such as vimentin, Oct-4, Nanog and BcL-2, which leads to the abrogation of the EMT–stemness axis and induction of apoptosis (Fig. 7). Overall, the results indicate that the compounds 5a and 13o have excellent anticancer activity by downregulating the expression of oncogenes and upregulating the expression of apoptosis genes.
Fig. 6. Effect of compounds on gene expression in CAL27 cells. The CAL27 cells treated with different concentrations of compounds (5a, 5c, and 13o) and the relative expressions of the vimentin (a), E-cad (b), Nanog (c), Oct-4 (d), Bax (e) and BcL-2 (f) genes were analysed using qRT-PCR analysis.
Fig. 7. Effect of compounds on gene expression in SCC09 cells. The SCC09 cells were treated with different concentrations of compounds (5a, 5c) and the relative expressions of the vimentin (a), E-cad (b), Nanog (c), Oct-4 (d), Bax (e) and BcL-2 (f) genes were analysed using qRT-PCR analysis.
2.3. Molecular interaction analysis
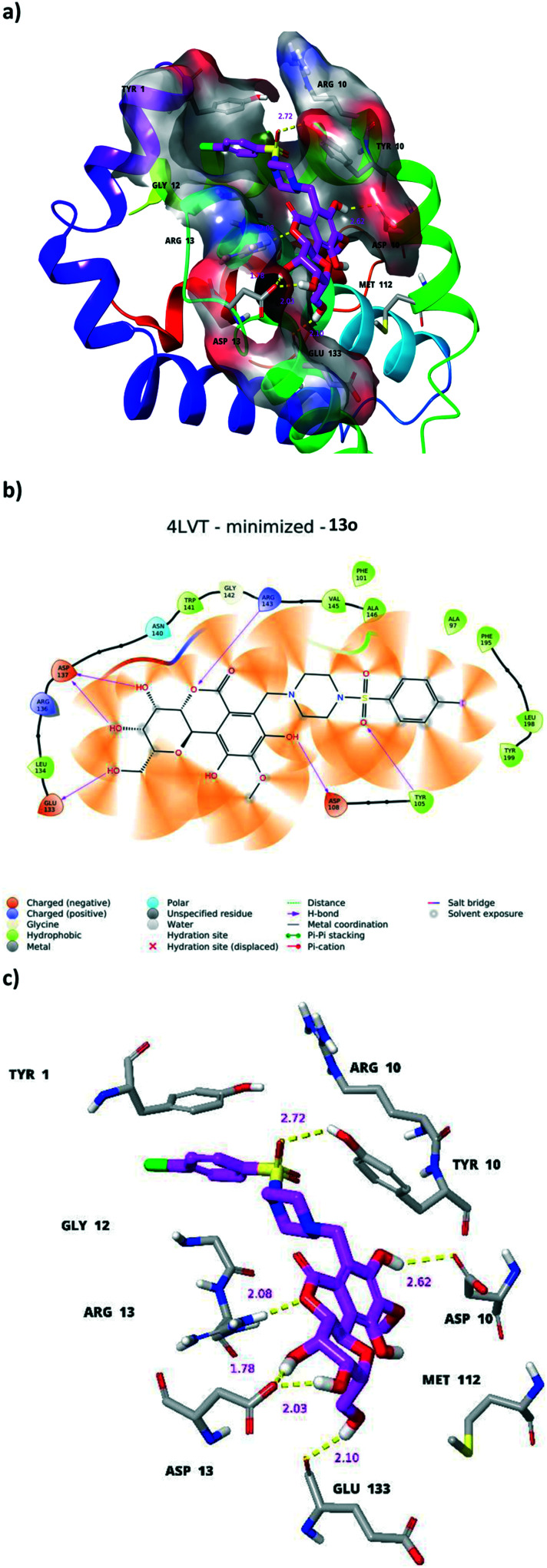
The bergenin analogues are putatively docked in the active site of the navitoclax binding cavity present in the BcL2 protein. The alkaloid core of ligands including 5a, 5c and 13o was identified to be accommodated by a nearby P4 subsite residue Trp199 (Fig. S127†). Another ligand 13o was observed to occupy in the opposite orientation and its alkaloid core formed a hydrogen bond network in binding mode with Asp137, Arg143, Glu133 and Asp108. The sulfonamide group showed another hydrogen bond with Tyr105. The overall binding mode of the bergenin docked poses showed them as preferential ligands towards BcL2 binding (Fig. 8). Moreover, the lipophilicity of the bergenin derivatives 5a, 5c and 13o was determined, and plays a key role in the binding with proteins and bioavailability of drugs. Variations in the physical properties (lipophilicity etc.) of the drug could cause the level of drug administration in animal model (intestinal, blood brain barrier etc.).30 In the present study, the lipophilicity of the selected derivatives 5a, 5c, 10f, and 13o was improved as compared with that of the parent molecule (Table S6†).
Fig. 8. Molecular docking studies of bergenin derivatives. The interaction of bergenin derivative 13o (a–c) with apoptosis inhibitor protein BcL2 (PDB ID: 4LVT).
3. Conclusion
A series of novel bergenin derivatives bearing various arylthiazolyl (5a–e), benzothiazolyl (10a–i), and arylsulfonylpiperazine (13a–o) moieties were synthesized using the Mannich reaction and assessed for anticancer activity. These bergenin derivatives exhibited significant cytotoxic activity against the CAL-27 and SCC09 cancer cell lines with IC50 values ranging from 15.41–92.9 μM and 17.41–91.9 μM, respectively, compared with the parent compound. The compounds 5a, 5c, 10f, and 13o showed cytotoxic activity against tongue and oral cancer cells and the flow cytometry analysis indicated that the selected derivatives inhibited cell cycle arrest at the G0/G1 phase. Consequently, the annexin V/FITC dual staining studies disclosed that the compounds 5a, 5c, 10f, and 13o induced early apoptosis against tongue cancer and necrosis against oral cancer. The compounds 5a, 5c, and 13o induce apoptosis by suppressing the genes responsible for cell migration, invasion and stemness and upregulate the genes responsible for apoptosis. Moreover, the selected compounds have a strong binding affinity with the apoptotic inhibitor protein BcL2. In view of the present data, the compounds 5a, 5c, 10f, and 13o might serve as promising candidates for the development of new potent and efficacious anticancer chemotherapeutics for various solid cancers.
Conflicts of interest
The authors declare that there is no conflict of interest to disclose.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. S. Chandrasekar, Director, IICT, Hyderabad for showing keen interest in this work. BVR acknowledges the CSIR for financial support. The IICT communication number is IICT/Pubs./2021/231.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00116k
References
- Zahavi D. Weiner L. Antibodies. 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbadawi M. M. Eldehna W. M. Wang W. Agama K. K. Pommier Y. Abe M. Eur. J. Med. Chem. 2021;215:113261. doi: 10.1016/j.ejmech.2021.113261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. Tamma R. Annese T. Transl. Oncol. 2020;13(6):100773. doi: 10.1016/j.tranon.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J. Cragg G. M. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Abu Samaan T. M. Samec M. Liskova A. Kubatka P. Büsselberg D. Biomolecules. 2019;9:789. doi: 10.3390/biom9120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amujuri D. Siva B. Poornima B. Sirisha K. Sarma A. V. S. Lakshma Nayak V. Tiwari A. K. Purushotham U. Suresh Babu K. Eur. J. Med. Chem. 2018;149:182–192. doi: 10.1016/j.ejmech.2018.02.066. [DOI] [PubMed] [Google Scholar]
- Dileep Kumar G. Siva B. Ashwini K. Vinod Kumar J. Ramalingam V. Sai Balaji A. Suresh Babu K. Nat. Prod. Res. 2022:1–7. doi: 10.1080/14786419.2021.2024531. doi: 10.1080/14786419.2021.2024531. [DOI] [PubMed] [Google Scholar]
- Gaja S. K. Bandi S. Pavuluri P. K. Sambyal S. Jaina V. K. Sampath Kumar H. M. Andugulapati S. B. Ramalingam V. Suresh Babu K. Nat. Prod. Res. 2022:1–7. doi: 10.1080/14786419.2022.2056889. doi: 10.1080/14786419.2022.2056889. [DOI] [PubMed] [Google Scholar]
- Jain S. K. Singh S. Khajuria A. Guru S. K. Joshi P. Meena S. Nadkarni J. R. Singh A. Bharate S. S. Bhushan S. Bharate S. B. Vishwakarma R. A. J. Med. Chem. 2014;57:7085–7097. doi: 10.1021/jm500901e. [DOI] [PubMed] [Google Scholar]
- Koul B. Kumar A. Yadav D. Jin J.-O. Molecules. 2020;25:5555. doi: 10.3390/molecules25235555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Zhang Y. Yu C. Zhang P. Gu S. Wang G. Xiao H. Li S. Drug Dev. Res. 2020;82:278–286. doi: 10.1002/ddr.21751. [DOI] [PubMed] [Google Scholar]
- Xuan Q. Fang Y. Dan Z. Yuan H. Arzneimittelforschung. 2011;60:198–204. doi: 10.1055/s-0031-1296273. [DOI] [PubMed] [Google Scholar]
- Pavan Kumar P. Siva B. Venkateswara Rao B. Dileep Kumar G. Lakshma Nayak V. Nishant Jain S. Tiwari A. K. Purushotham U. Venkata Rao C. Suresh Babu K. Bioorg. Chem. 2019;91:103161. doi: 10.1016/j.bioorg.2019.103161. [DOI] [PubMed] [Google Scholar]
- Liang C. Pei S. Ju W. Jia M. Tian D. Tang Y. Mao G. Eur. J. Med. Chem. 2017;133:319–328. doi: 10.1016/j.ejmech.2017.03.053. [DOI] [PubMed] [Google Scholar]
- Pawelczyk A. Sowa-Kasprzak K. Olender D. Zaprutko L. Int. J. Mol. Sci. 2018;19:1104. doi: 10.3390/ijms19041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi Manasa K. Thatikonda S. Sigalapalli D. K. Sagar A. Kiranmai G. Kalle A. M. Alvala M. Godugu C. Nagesh N. Nagendra Babu B. Bioorg. Chem. 2020;101:103983. doi: 10.1016/j.bioorg.2020.103983. [DOI] [PubMed] [Google Scholar]
- Wolf L. Quoos N. Mayer J. C. P. de Souza D. Sauer A. C. Meichtry L. Bortolotto V. Prigol M. Rodrigues O. E. D. Dornelles L. Tetrahedron Lett. 2016;57:1031–1034. doi: 10.1016/j.tetlet.2016.01.079. [DOI] [Google Scholar]
- Schiedel M. Rumpf T. Karaman B. Lehotzky A. Oláh J. Gerhardt S. Ovádi J. Sippl W. Einsle O. Jung M. J. Med. Chem. 2016;59:1599–1612. doi: 10.1021/acs.jmedchem.5b01517. [DOI] [PubMed] [Google Scholar]
- Patel R. V. Park S. W. Eur. J. Med. Chem. 2014;71:24–30. doi: 10.1016/j.ejmech.2013.10.059. [DOI] [PubMed] [Google Scholar]
- Patel R. V. Patel P. K. Kumari P. Rajani D. P. Chikhalia K. H. Eur. J. Med. Chem. 2012;53:41–51. doi: 10.1016/j.ejmech.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Raghavendra N. M. Jyothsna A. Venkateswara Rao A. Subrahmanyam C. V. S. Bioorg. Med. Chem. Lett. 2012;22:820–823. doi: 10.1016/j.bmcl.2011.12.062. [DOI] [PubMed] [Google Scholar]
- Akbar A. McNeil N. M. R. Albert M. R. Ta V. Adhikary G. Bourgeois K. Eckert R. L. Keillor J. W. J. Med. Chem. 2017;60:7910–7927. doi: 10.1021/acs.jmedchem.7b01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Chen H. Chen P. Zhang W. Wu C. Sun C. Luo W. Zheng L. Liu Z. Liang G. Eur. J. Med. Chem. 2019;161:22–38. doi: 10.1016/j.ejmech.2018.09.068. [DOI] [PubMed] [Google Scholar]
- Ramalingam V. Rajaram R. Process Biochem. 2021;100:69–81. doi: 10.1016/j.procbio.2020.09.032. [DOI] [Google Scholar]
- Wang S. S. Jiang J. Liang X. H. Tang Y. L. OncoTargets Ther. 2015;8:2973–2980. doi: 10.2147/OTT.S91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi N. Shahgoli V. K. Amini M. Safaei S. Mokhtarzadeh A. Mansoori B. Derakhshani A. Baghbanzadeh A. Baradaran B. Eur. J. Pharmacol. 2021;894:1388–1399. doi: 10.1016/j.ejphar.2021.173871. [DOI] [PubMed] [Google Scholar]
- Na H.-H. Ryu J.-M. Kim K.-C. Biochem. Biophys. Res. Commun. 2021;567:131–137. doi: 10.1016/j.bbrc.2021.06.031. [DOI] [PubMed] [Google Scholar]
- Ramalingam V. Varunkumar K. Ravikumar V. Rajaram R. Chem.-Biol. Interact. 2018;287:1–12. doi: 10.1016/j.cbi.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Khan A. A. Y. F. Ahmed Q. U. Narayanamurthy V. Razali S. Asuhaimi F. A. Saleh M. S. M. Johan M. F. Khatib A. Seeni A. Wahab R. A. Biomed. Pharmacother. 2019;114:108841. doi: 10.1016/j.biopha.2019.108841. [DOI] [PubMed] [Google Scholar]
- Reddy P. R. S. Sambyal S. Mhamane T. B. Sravanthi V. Shafi S. Khan I. A. Sampath Kumar H. M. Bioorg. Med. Chem. 2022;66:116781. doi: 10.1016/j.bmc.2022.116781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.