Abstract
VanD-type resistance to glycopeptides in Enterococcus faecium BM4339 is due to constitutive synthesis of d-alanyl-d-lactate-terminating peptidoglycan precursors (B. Périchon, P. Reynolds, and P. Courvalin, Antimicrob. Agents Chemother. 41:2016–2018, 1997). The sequence of a 5,780-bp fragment was determined and revealed six open reading frames. The 3′ distal part encoded the VanHD dehydrogenase, the VanD ligase, and the VanXD dd-dipeptidase, which were highly similar to the corresponding proteins in VanA and VanB types of resistance. The deduced VanYD protein was homologous to penicillin-binding proteins that display dd-carboxypeptidase activity. The 5′ end coded for the putative VanRD-VanSD two-component regulatory system. Due to a frameshift mutation in the chromosomal ddl gene, BM4339 produced an impaired d-alanine:d-alanine ligase. However, since expression of the resistance genes is constitutive, growth of E. faecium BM4339 was not dependent on the presence of glycopeptides in the culture medium.
In gram-positive bacteria, glycopeptides inhibit the last steps of peptidoglycan synthesis by binding to the C-terminal dipeptide d-alanyl–d-alanine (d-Ala–d-Ala) of peptidoglycan precursors, thus preventing their incorporation into the cell wall (23). Acquired resistance to vancomycin and teicoplanin in enterococci is due to the replacement of d-Ala–d-Ala by the depsipeptide d-alanyl–d-lactate (d-Ala–d-Lac). This substitution leads to the formation of modified peptidoglycan precursors for which glycopeptides exhibit 1,000-fold lower binding affinities (11).
Two types of acquired resistance to glycopeptides have been well characterized (for a recent review, see reference 6). VanA-type enterococci display inducible resistance to high levels of both vancomycin and teicoplanin, whereas VanB-type enterococci display inducible resistance to various levels of vancomycin but remain susceptible to teicoplanin. In addition to the host d-Ala:d-Ala ligase (Ddl), a resistance ligase, VanA or VanB, mediates formation of d-Ala–d-Lac, which competes with d-Ala–d-Ala in cell wall assembly. Two other proteins are required for resistance: VanH (VanHB), a dehydrogenase which converts pyruvate into d-Lac (11), and VanX (VanXB), a dd-dipeptidase which hydrolyzes d-Ala–d-Ala produced by the chromosomal Ddl (24). VanY (VanYB), inessential for resistance, is a dd-carboxypeptidase which acts when significant incorporation of d-Ala–d-Ala occurs in spite of VanX hydrolysis. Under such conditions, VanY hydrolyzes pentapeptides and, to a lesser extent, pentadepsipeptides (1a). Thus, two dd-peptidases, VanX (VanXB) and VanY (VanYB), contribute sequentially to resistance in reducing the pool of pentapeptide precursors, favoring their replacement by pentadepsipeptides in cell wall assembly. Two proteins which belong to the family of two-component regulatory systems control the level of expression of the resistance genes in response to the presence of glycopeptides in the culture medium. The VanS (VanSB) sensor governs phosphorylation of the regulator VanR (VanRB), which acts as a transcriptional activator for the resistance genes. An accessory protein other than VanY, VanZ, has also been identified in VanA-type strains. VanZ confers low-level teicoplanin resistance by an unknown mechanism. The open reading frame (ORF) vanW is present in the vanB operon, but no function has yet been assigned to the corresponding protein (14).
A new type of acquired resistance to glycopeptides, VanD, was recently found in Enterococcus faecium BM4339 (22) and in other E. faecium strains (19). Clinical isolate BM4339 is resistant to intermediate levels of vancomycin (MIC = 64 μg/ml) and to low levels of teicoplanin (MIC = 4 μg/ml) and does not harbor the vanA or vanB operon. Glycopeptide resistance in this strain is constitutively expressed and mediated by synthesis of pentadepsipeptide precursors ending in d-Ala–d-Lac, which represent the main components of cell wall cytoplasmic precursors (22).
In this work, we describe the genetic organization of the vanD gene cluster in E. faecium BM4339. We also show that a frameshift mutation in the chromosomal ddl gene accounts for the lack of precursors terminating in d-Ala–d-Ala in this strain.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are described in Table 1. Unless specified, Escherichia coli JM83 (35) and E. coli TB1 (Focus, Life Technologies Inc., Gaithersburg, Md.) were used as the hosts in cloning experiments. Bacteria were cultured in brain heart infusion broth or agar (Difco Laboratories, Detroit, Mich.) at 37°C. The method of Steers et al. (30) was used to determine the MICs of glycopeptides with 105 CFU per spot on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) after 24 h of incubation.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant propertiesa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM83 | F−ara Δ(lac-proAB) rpsL (Strr)[φ80dlacΔ(lacZ)M15] | 35 |
| TB1 | JM83 hsdR(rK− mK+) | Life Technologies Inc. |
| INVαF′ | F′ endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 relA1 φ80lacZΔM15 Δ(lacZYA-argF)U169λ− | Invitrogen |
| E. faecium | ||
| BM4339 | Vmr Ter (VanD type) | 22 |
| BM4147 | Vmr Ter (VanA type) | 16 |
| BM4409 | BM4339/pAT662 | This work |
| Plasmids | ||
| pUC18 | Apr, lacZα vector | 33 |
| pGB2 | Smr Spr derivative of pSC101 | 12 |
| pCR2.1 | Apr Kmr, oriR from ColE1, lacZα vector | Invitrogen |
| pAT79 | oriR from pAMβ1, oriR from pUC, oriT from RK2; SprlacZα P2 cat | 5 |
| pAT654 | 5.3-kb Sau3AI fragment (vanSD′ vanYDHDDXD) of BM4339 cloned into pUC18 | This work |
| pAT656 | 0.6-kb PCR fragment (vanD′) of BM4339 cloned into pCR2.1 | 22 |
| pAT657 | 1.0-kb PCR fragment (vanRD′SD′) of BM4339 cloned into pCR2.1 | This work |
| pAT658 | 1.8-kb HindIII-ClaI fragment (vanRDSD′) of BM4339 cloned into pUC18 | This work |
| pAT661 | 7.0-kb HindIII fragment (ddl) of BM4339 cloned into pGB2 | This work |
| pAT662 | 1.2-kb SacI-XbaI fragment (ddl) of BM4147 cloned into pAT79 | This work |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Smr, streptomycin resistance; Spr, spectinomycin resistance; Ter, teicoplanin resistance; Vmr, vancomycin resistance; P2, promoter of the aphA-3 gene from enterococcal plasmid pJH1.
Recombinant DNA techniques.
Plasmid DNA isolation, cleavage of DNA with restriction endonucleases (Amersham, Little Chalfont, Buckinghamshire, England; Gibco BRL-Life Technologies Inc.; and Pharmacia, Uppsala, Sweden), purification of restriction fragments from agarose gel, dephosphorylation of vector DNA with calf intestinal phosphatase (Pharmacia), and ligation with T4 DNA ligase (Pharmacia) were performed by standard methods (26).
Plasmid construction.
The plasmids were constructed as explained below (Fig. 1).
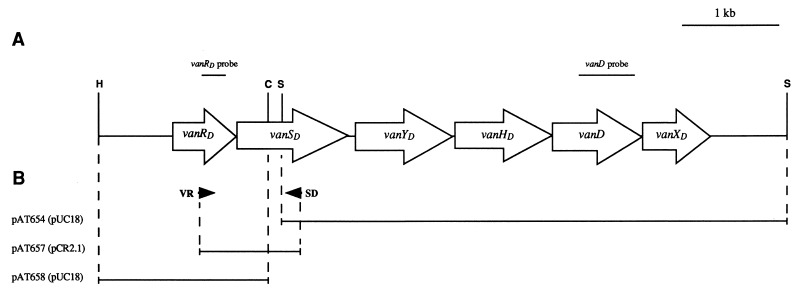
FIG. 1.
Schematic representation of the vanD gene cluster and of recombinant plasmids. (A) Map of the 7.2-kb HindIII-Sau3AI fragment containing the vanRD, vanSD, vanYD, vanHD, vanD, and vanXD genes. Open arrows represent coding sequences. The PCR fragments internal to the vanRD and vanD genes used as probes in hybridization experiments are indicated above the corresponding regions. Abbreviations for restriction sites used for cloning are as follows: C, ClaI; H, HindIII; and S, Sau3AI. (B) Inserts in recombinant plasmids. The inserts are represented by solid lines, and the vectors are indicated in parentheses. Arrowheads indicate binding sites and orientations of the VR and SD oligodeoxynucleotides used for amplification.
(i) Plasmid pAT654.
E. faecium BM4339 total DNA was partially digested with Sau3AI and ligated with pUC18 DNA cleaved by BamHI. To identify recombinant plasmids, clones were screened by colony hybridization (26) with the 605-bp fragment internal to vanD purified from pAT656 (22) as a probe (Fig. 1).
(ii) Plasmid pAT657.
To amplify a fragment internal to the vanRD and the vanSD genes, the degenerate oligodeoxynucleotide VR (1) was used in combination with the specific primer SD (5′ GTTCTTCCAGACGCTCA), complementary to the 5′ end of the insert in pAT654. Oligodeoxynucleotide VR [5′ GGIGCIGA(T/C)GA(T/C)TA(T/C)ITIIIIAA(A/G)CCITT, where I is deoxyinosine] was deduced from the sequences of conserved motifs located in the C termini of the effector domains of VanR, OmpR, and PhoB response regulators (5). The PCR product obtained with BM4339 total DNA as a template and Taq DNA polymerase (U.S. Biochemical-Amersham) was cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.) and introduced into E. coli INVαF′ (Invitrogen) by transformation.
(iii) Plasmid pAT658.
To complete the sequence of the vanRD gene, total DNA from E. faecium BM4339 was digested with EcoRI and ClaI, HindIII and ClaI, EcoRI and SspI, and HindIII and SspI, and the sizes of the fragments hybridizing with a 271-bp probe corresponding to the 5′ end of the pAT657 insert (Fig. 1) were estimated (26). Cloning was performed with restriction endonucleases generating fragments of more than 1 kb in length. The recombinant plasmids were screened by hybridization with the same probe, and plasmid pAT658, selected for further studies, contained a 1.8-kb HindIII-ClaI insert.
(iv) Plasmid pAT661.
A strategy similar to that used with plasmid pAT658 was followed to clone the chromosomal ddl gene from BM4339. The 600-bp fragment internal to the E. faecium BM4147 ddl gene (13) was used as a probe. Plasmid pAT661 consisted of a 7-kb HindIII chromosomal fragment of BM4339 cloned into the low-copy-number vector pGB2.
(v) Plasmid pAT662.
The ddl gene from E. faecium BM4147 with its ribosome binding site (RBS) was amplified by PCR with total DNA as a template and oligodeoxynucleotides 4147-1 and 4147-2 as primers. Primer 4147-1 (5′ ccgctgcagagctcTTAGAATACAGGAGGAC) contained a SacI site (underlined) and 17 bases complementary to the sequence upstream from the BM4147 ddl gene (in uppercase letters) including the RBS (italicized). Primer 4147-2 (5′atttgggatctagaTACGCAATCACTCCAGC) contained an XbaI site (underlined) and 17 bases complementary to the sequence downstream from the BM4147 ddl gene (in uppercase letters). The PCR product was digested with SacI and XbaI and placed under the control of the constitutive promoter P2 of the expression vector pAT79 (chloramphenicol resistant [Cmr]), leading to plasmid pAT662.
Strain construction.
E. faecium BM4409 was obtained by introduction of plasmid pAT662 (Cmr Ωddl BM4147) into E. faecium BM4339 by electrotransformation and selection on SR medium (28) containing chloramphenicol (10 μg/ml). The presence of pAT662 in BM4409 was confirmed by plasmid DNA extraction (26).
Nucleotide sequencing.
DNA sequencing was performed by the dideoxynucleotide chain termination method (27) with [α-35S]dATP (Amersham) and the T7 Sequenase version 2.0 DNA sequencing kit (Amersham). The plasmid DNA used as the template was extracted with the commercial Wizard Plus Minipreps DNA Purification System (Promega, Madison, Wis.).
Computer analysis of sequence data.
Sequence data were analyzed with the Sequence Analysis Software Package (version 7; Genetics Computer Group, Madison, Wis.).
Nucleotide sequence accession numbers.
The 5,781-bp fragment containing the vanD gene cluster was submitted to GenBank and assigned accession no. AF130997. The nucleotide sequence of the 1,240-bp chromosomal region containing the BM4339 ddl gene was allotted accession no. AF130998.
RESULTS AND DISCUSSION
Identification of the van genes and protein sequence analysis.
Partial digests of E. faecium BM4339 total DNA were cloned into E. coli, and transformants were screened by hybridization with a vanD internal probe (Fig. 1). Plasmid pAT654 (vanSD′ vanYDHDDXD) carried an insert of 5.3 kb that was sequenced with specific primers. Analysis of the sequence revealed five ORFs with the same orientation, the 5′ one being truncated (Fig. 1). The deduced amino acid sequences were compared to those of the proteins encoded by the vanA and vanB operons (Table 2). Based on homology, four ORFs could be assigned to the 3′ end of the vanSD gene, to vanHD, to vanD, and to vanXD. The identities between the VanHD, VanH, and VanHB dehydrogenases; the VanD, VanA, and VanB ligases; and the VanXD, VanX, and VanXB dd-dipeptidases were high (from 59 to 70%) (Table 2). The three conserved residues, Arg, Glu, and His, predicted to participate in substrate binding and catalysis of d-Lac dehydrogenases (31) were present in VanHD at positions 232, 260, and 292, respectively (data not shown). VanHD also contained the GXGXXG(17X)D sequence (positions 154 to 177) characteristic of nucleotide-binding domains in NAD+ cofactor-dependent dehydrogenases (31). The PEKG motif specifically found in the ω loop of VanA and VanB d-Ala:d-Lac ligases (13) was also present in VanD between positions 249 and 252 (data not shown). The presence of VanHD, which was homologous to dehydrogenases producing d-Lac, and of VanD, which was related to d-Ala:d-Lac ligases, is consistent with the fact that the vanD gene cluster (22), like the vanA and vanB operons (6, 8), confers glycopeptide resistance by production of d-Ala–d-Lac-ending peptidoglycan precursors. The deduced product of the fifth ORF was homologous to penicillin-binding proteins (PBPs) displaying dd-carboxypeptidase activity (Fig. 1), and the gene was designated vanYD. VanYD displayed 26% identity with a PBP from Streptomyces sp. strain K15 (20), with the putative DacF dd-carboxypeptidase involved in the sporulation of Bacillus subtilis MB24 (34), and with PBP 6 from E. coli (9). VanYD contained in the right order the motifs predicted to define the active sites of these PBPs (20): SXXK, which includes the catalytic serine, the SG(C/N) triad, and the KTG motif (Fig. 2). Consistent with these observations, the dd-carboxypeptidase activity detected in BM4339 was sensitive to penicillin G (22), unlike the activities of VanY (4) and VanYB (14).
TABLE 2.
Levels of identity between the deduced sequences of the proteins encoded by the vanA, vanB, and vanD gene clusters
| Sequence compared | % Identity
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VanR | VanS | VanH | VanA | VanX | VanY | VanRB | VanSB | VanHB | VanB | VanXB | VanYB | |
| VanRD | 58 | 34 | ||||||||||
| VanSD | 42 | 19 | ||||||||||
| VanHD | 59 | 63 | ||||||||||
| VanD | 69 | 69 | ||||||||||
| VanXD | 68 | 70 | ||||||||||
| VanYD | 13 | 15 | ||||||||||
| VanR | 35 | |||||||||||
| VanS | 17 | |||||||||||
| VanH | 68 | |||||||||||
| VanA | 76 | |||||||||||
| VanX | 75 | |||||||||||
| VanY | 30 | |||||||||||
FIG. 2.
Partial alignment of the deduced amino acid sequences of VanYD from E. faecium BM4339, PBP from Streptomyces sp. strain K15 (20), the putative DacF dd-carboxypeptidase from B. subtilis MB24 (34), and PBP 6 from E. coli (9). Conserved motifs involved in the scaffolding of the active site are indicated in boldface. The numbers of amino acids between the NH2 terminus and motif I, motifs I and II, motifs II and III, and motif III and the COOH terminus are indicated.
A PCR strategy was used to complete the sequence of vanSD. Since the gene organization in BM4339 was similar to those of the vanA and vanB clusters (6), an ORF related to vanR and vanRB was expected to be located upstream from vanSD. In two-component regulatory systems, response regulators display well-conserved N-terminal domains (15). Based on the alignment of VanR, OmpR, and PhoB (5), the degenerate oligodeoxynucleotide VR was designed and used in combination with primer SD, specific for vanSD (Fig. 1). A product with the 1-kb expected size was obtained and cloned. Recombinant plasmid pAT657 (vanRD′SD′) contained the 5′ missing portion of vanSD and the 3′ half of the vanRD gene (Fig. 1). To recover the 5′ extremity of vanRD, total DNA from BM4339 was digested and cloned into E. coli and transformants were screened by hybridization with a probe internal to vanRD (Fig. 1). Recombinant plasmid pAT658 (vanRDSD′) carried a 1.8-kb insert which contained the entire vanRD gene (Fig. 1). A higher degree of identity was observed between VanRD and VanR and between VanSD and VanS than with the corresponding proteins encoded by the vanB gene cluster (Table 2). VanRD and VanSD were respectively as related to VanRB and VanSB as are VanR and VanS (Table 2) (14). No genes homologous to vanZ and vanW from the vanA and vanB operons, respectively, were found.
Cloning and sequence analysis of the E. faecium BM4339 ddl gene.
The insert in recombinant plasmid pAT661 (ddl BM4339) (Table 1) was sequenced on 1,300 consecutive base pairs with divergent primers complementary to the termini of the 600-bp fragment internal to the BM4147 ddl gene (13) and specific oligodeoxynucleotides. In turn, the sequence of the BM4339 ddl region allowed the cloning by PCR of the entire BM4147 ddl gene (14a). Comparison of the two ddl sequences revealed the presence of a 5-bp insertion near the 5′ end in the BM4339 gene (Fig. 3). The insertion was responsible for a frameshift leading to the synthesis of a 26-amino-acid peptide instead of the putative 358-amino-acid Ddl. Production of a truncated protein accounts for the lack of d-Ala–d-Ala-containing peptidoglycan precursors in BM4339 (22). VanA-type (25, 29) and VanB-type (7, 32) mutants of Enterococcus impaired in Ddl activity grow only in the presence of glycopeptides. These antibiotics are required to induce production of the resistance ligase and dehydrogenase and, therefore, to synthesize peptidoglycan from d-Ala–d-Lac- instead of d-Ala–d-Ala-containing precursors. In E. faecium BM4339, constitutive expression of glycopeptide resistance (22) accounts for the fact that this strain is not glycopeptide dependent.
FIG. 3.
Partial alignment of the nucleotide sequence of the ddl genes from E. faecium BM4339 and E. faecium BM4147 (14a). Numbers at the left refer to the position of the first nucleotide in the corresponding line. Identical bases are indicated by dashes in the BM4147 sequence. The putative RBS is indicated in boldface lettering. The 5-bp insertion in the BM4339 sequence is underlined and corresponds to a gap represented by dots in the BM4147 sequence. The deduced amino acid sequence of the E. faecium BM4339 ddl gene is indicated below the alignment. Numbers at the right refer to the position of the last amino acid of the corresponding line. The putative translation stop codon is indicated by an asterisk.
trans complementation of the insertional mutation in the BM4339 ddl gene.
The ddl gene from E. faecium BM4147 was cloned under the control of the heterologous enterococcal P2 promoter in the gram-positive expression vector pAT79 (5), leading to pAT662 (ddl BM4147) (Table 1). The recombinant plasmid was introduced by electrotransformation into E. faecium BM4339, and transformants, such as BM4409 (Table 1), were susceptible to vancomycin and teicoplanin (MICs = 0.5 μg/ml). The decrease in glycopeptide resistance was most likely due to expression of the heterologous Ddl since no VanX dd-dipeptidase activity is present in cytoplasmic extracts from E. faecium BM4339 and only low levels of VanY dd-carboxypeptidase activity are found in membrane preparations (22).
Peculiarities of VanD-type glycopeptide resistance in E. faecium BM4339.
Inducible expression of the resistance genes in VanA- and VanB-type strains is regulated by the two-component systems VanRS and VanRBSB, respectively. VanB-type constitutive variants harbor mutations in the vanSB sensor gene (7) that are thought to impair dephosphorylation of the VanRB regulator (2, 7). The sequences of VanRD and VanSD were analyzed for the presence of the amino acids involved in protein phosphorylation and of the motifs conserved in response regulators and protein kinases (21). The three amino acids Asp10, Asp53 (which corresponds to the putative site of phosphorylation), and Lys101, highly conserved in the effector domains of response regulators (21), were present in VanRD (data not shown). The five motifs characteristic of protein kinases (21), namely, H (positions 164 to 172), N (273 to 284), G1 (309 to 317), F (324 to 328), and G2 (340 to 346), including the histidine at position 166, which is the putative site of autophosphorylation (data not shown), were found in VanSD. The constitutive phenotype of BM4339 may be due to mutations located near the putative autophosphorylation site and known to alter the phosphatase activity of VanSB (7). Alternatively, the signal recognition properties of VanSD may be impaired, leading to phosphorylation of VanRD even in the absence of glycopeptides. Another possibility is that alternate phosphorylation of VanRD by acetyl phosphate or by a heterologous protein kinase (2) maintains high concentrations of VanRD-phosphate in spite of VanSD phosphatase activity.
As already mentioned, insertional inactivation of the BM4339 chromosomal ddl gene accounts for the absence of d-Ala–d-Ala-containing peptidoglycan precursors in this strain (22). Lack of a substrate for dd-dipeptidase hydrolysis makes VanXD superfluous in achieving glycopeptide resistance in BM4339. As a matter of fact, although VanXD does not exhibit mutations in the conserved residues involved in zinc binding and catalysis (17) (data not shown), BM4339 does not produce dd-dipeptidase activity (22). It has been shown that a mutation in the host ddl gene can compensate for inactivation of vanX in VanA-type strains (1a). Conversely, loss of production of VanXD dd-dipeptidase activity in BM4339 may be secondary to the impairment of the host ligase.
The VanYD dd-carboxypeptidase exhibited the same hydrophobicity profile as VanY (4) and VanYB (14), with a cluster of hydrophobic residues near the N terminus of the protein (data not shown). VanYD may thus be a membrane-anchored protein that acts like VanY and VanYB. The dd-carboxypeptidase contributes to resistance by hydrolyzing precursors containing the d-Ala–d-Ala target of glycopeptides (1a, 3). This activity in BM4339 may explain the presence of tetrapeptide peptidoglycan precursors (17% of the precursors synthesized [22]). In this strain, the substrates for the dd-carboxypeptidase may be the pentapeptides (which represent only 2% of all precursors [22]) and, to a minor extent, the pentadepsipeptides. The pentapeptide precursors may conceivably originate from a very low rate of production of d-Ala–d-Ala by VanD. Like VanA (10) and VanB (18), the related VanD ligase may display broad substrate specificity, leading to synthesis of d-Ala–d-Ala at a level lower than that of d-Ala–d-Lac but in sufficient amount to require a weak contribution of VanYD dd-carboxypeptidase activity.
In conclusion, E. faecium BM4339 harbors the vanD gene cluster responsible for glycopeptide resistance. The d-Ala:d-Ala ligase in this strain is not functional following a mutation in the chromosomal ddl gene. However, replacement of the host metabolic pathway for synthesis of d-Ala-ending peptidoglycan precursors by the constitutively expressed resistance pathway leading to production of d-Lac-terminating precursors allows glycopeptide-independent growth of BM4339.
ACKNOWLEDGMENTS
We thank M. Arthur for helpful discussions and help with the writing of the manuscript and P. Reynolds for critical reading of the manuscript. B.C. is grateful to B. Périchon for constant technical advice and for construction of pAT656.
This work was supported in part by a Bristol-Myers Squibb unrestricted biomedical research grant in infectious diseases. B.C. was the recipient of a grant from the Centre National de la Recherche Scientifique.
REFERENCES
- 1.Arthur, M., et al. Unpublished data.
- 1a.Arthur M, Depardieu F, Cabanié L, Reynolds P, Courvalin P. Requirement of the VanY and VanX d,d-peptidases for glycopeptide resistance in enterococci. Mol Microbiol. 1998;31:819–830. doi: 10.1046/j.1365-2958.1998.01114.x. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Depardieu F, Gerbaud G, Galimand M, Leclercq R, Courvalin P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546and related elements in the absence of induction. J Bacteriol. 1997;179:97–106. doi: 10.1128/jb.179.1.97-106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Depardieu F, Snaith H A, Reynolds P E, Courvalin P. Contribution of VanY dd-carboxypeptidase to glycopeptide resistance in Enterococcus faecalisby hydrolysis of peptidoglycan precursors. Antimicrob Agents Chemother. 1994;38:1899–1903. doi: 10.1128/aac.38.9.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur M, Molinas C, Courvalin P. Sequence of the vanY gene required for production of a vancomycin-inducible d,d-carboxypeptidase in Enterococcus faeciumBM4147. Gene. 1992;120:111–114. doi: 10.1016/0378-1119(92)90017-j. [DOI] [PubMed] [Google Scholar]
- 5.Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faeciumBM4147. J Bacteriol. 1992;174:2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 7.Baptista M, Depardieu F, Reynolds P, Courvalin P, Arthur M. Mutations leading to increased levels of resistance to glycopeptide antibiotics in VanB-type enterococci. Mol Microbiol. 1997;25:93–105. doi: 10.1046/j.1365-2958.1997.4401812.x. [DOI] [PubMed] [Google Scholar]
- 8.Billot-Klein D, Gutmann L, Sablé S, Guittet E, van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994;176:2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broome-Smith J K, Ioannidis I, Edelman A, Spratt B G. Nucleotide sequences of the penicillin-binding protein 5 and 6 genes of Escherichia coli. Nucleic Acids Res. 1988;16:1617. doi: 10.1093/nar/16.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugg T D H, Dutka-Malen S, Arthur M, Courvalin P, Walsh C T. Identification of vancomycin resistance protein VanA as a d-alanine:d-alanine ligase of altered substrate specificity. Biochemistry. 1991;30:2017–2021. doi: 10.1021/bi00222a002. [DOI] [PubMed] [Google Scholar]
- 11.Bugg T D H, Wright G D, Dutka-Malen S, Arthur M, Courvalin P, Walsh C T. Molecular basis for vancomycin resistance in Enterococcus faeciumBM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991;30:10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- 12.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 13.Evers S, Casadewall B, Charles M, Dutka-Malen S, Galimand M, Courvalin P. Evolution of structure and substrate specificity in d-alanine:d-alanine ligases and related enzymes. J Mol Evol. 1996;42:706–712. doi: 10.1007/BF02338803. [DOI] [PubMed] [Google Scholar]
- 14.Evers S, Courvalin P. Regulation of VanB-type vancomycin resistance gene expression by the VanSB-VanRB two-component regulatory system in Enterococcus faecalisV583. J Bacteriol. 1996;178:1302–1309. doi: 10.1128/jb.178.5.1302-1309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Gholizadeh, Y., et al. Unpublished data.
- 15.Goudreau P N, Stock A M. Signal transduction in bacteria: molecular mechanisms of stimulus-response coupling. Curr Opin Microbiol. 1998;1:160–169. doi: 10.1016/s1369-5274(98)80006-4. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty D G, Lessard I A, Walsh C T. Mutational analysis of potential zinc-binding residues in the active site of the enterococcal d-Ala–d-Ala dipeptidase VanX. Biochemistry. 1997;36:10498–10505. doi: 10.1021/bi970543u. [DOI] [PubMed] [Google Scholar]
- 18.Meziane-Cherif D, Badet-Denisot M A, Evers S, Courvalin P, Badet B. Purification and characterization of the VanB ligase associated with type B vancomycin resistance in Enterococcus faecalisV583. FEBS Lett. 1994;354:140–142. doi: 10.1016/0014-5793(94)01096-x. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowsky B, Clark N, Eliopoulos C T, Venkataraman L, Samore M, Tenover F, Eliopoulos G M, Moellering R C, Jr, Gold H S. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. A cluster of VanD glycopeptide-resistant (GR) Enterococcus faecium (Efcm): molecular characterization and clinical epidemiology, abstr. C-96; p. 96. [Google Scholar]
- 20.Palomeque-Messia P, Englebert S, Leyh-Bouille M, Nguyen-Distèche M, Duez C, Houba S, Dideberg O, Van Beeumen J, Ghuysen J M. Amino acid sequence of the penicillin-binding protein/d,d-peptidase of StreptomycesK15. Biochem J. 1991;279:223–230. doi: 10.1042/bj2790223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkinson J, Kofoid E. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 22.Périchon B, Reynolds P, Courvalin P. VanD-type glycopeptide-resistant Enterococcus faeciumBM4339. Antimicrob Agents Chemother. 1997;41:2016–2018. doi: 10.1128/aac.41.9.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds P E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds P E, Depardieu F, Dutka-Malen S, Arthur M, Courvalin P. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of d-alanyl–d-alanine. Mol Microbiol. 1994;13:1065–1070. doi: 10.1111/j.1365-2958.1994.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosato A, Pierre J, Billot-Klein D, Buu-Hoi A, Gutmann L. Inducible and constitutive expression of resistance to glycopeptides and vancomycin dependence in glycopeptide-resistant Enterococcus avium. Antimicrob Agents Chemother. 1995;39:830–833. doi: 10.1128/aac.39.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepard B D, Gilmore M S. Electroporation and efficient transformation of Enterococcus faecalisgrown in high concentrations of glycine. Methods Mol Biol. 1995;47:217–226. doi: 10.1385/0-89603-310-4:217. [DOI] [PubMed] [Google Scholar]
- 29.Sifaoui F, Gutmann L. Vancomycin dependence in a VanA-producing Enterococcus avium strain with a nonsense mutation in the natural d-Ala–d-Ala ligase gene. Antimicrob Agents Chemother. 1997;41:1409. doi: 10.1128/aac.41.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steers E, Foltz E L, Graves B S, Rindel J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother (Basel) 1959;9:307–311. [PubMed] [Google Scholar]
- 31.Stoll V S, Kimber M S, Pai E F. Insights into substrate binding by d-2-ketoacid dehydrogenases from the structure of Lactobacillus pentosusd-lactate dehydrogenase. Structure. 1996;4:437–447. doi: 10.1016/s0969-2126(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 32.Van Bambeke F, Chauvel M, Reynolds P E, Fraimow H S, Courvalin P. Vancomycin-dependent Enterococcus faecalisclinical isolates and revertant mutants. Antimicrob Agents Chemother. 1999;43:41–47. doi: 10.1128/aac.43.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira J, Messing J. The pUC plasmids and M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu J-J, Schuch R, Piggot P J. Characterization of a Bacillus subtilis sporulation operon that includes genes for an RNA polymerase ς factor and for a putative dd-carboxypeptidase. J Bacteriol. 1992;174:4885–4892. doi: 10.1128/jb.174.15.4885-4892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]