ABSTRACT
Background
In the general population, the seroconversion rate after primary vaccination with two doses of an anti-severe acute respiratory syndrome coronavirus 2 messenger RNA (mRNA) vaccine reaches nearly 100%, with significantly higher antibody titers after mRNA-1273 vaccination compared to BNT162b2 vaccination. Here we performed a systematic review and meta-analysis to compare the antibody response after two-dose mRNA-1273 versus BNT162b2 vaccination in solid organ transplant (SOT) recipients.
Methods
A systematic literature review was performed using PubMed, Web of Science and the Cochrane Library and original research papers were included for a meta-analysis to calculate vaccine-specific seroconversion rates for each of the mRNA vaccines. Next, the pooled relative seroconversion rate was estimated.
Results
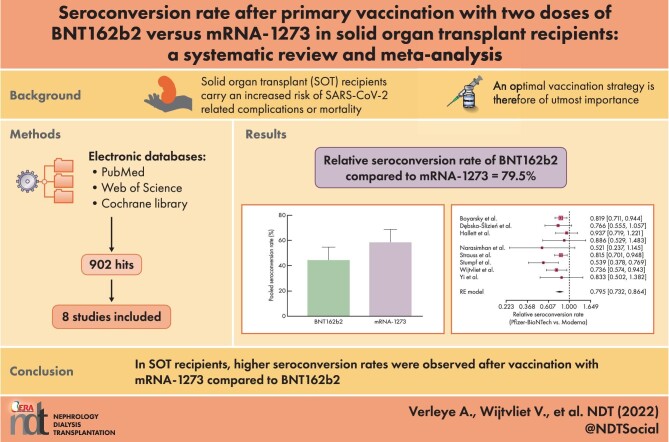
Eight studies that described the development of antibodies against receptor-binding domain (RBD) and/or spike protein were eligible for meta-analysis. Two of these studies also reported antibody titers. The meta-analysis revealed lower seroconversion rates in SOT recipients vaccinated with two doses of BNT162b2 {44.3% [95% confidence interval (CI) 34.1–54.7]} as compared with patients vaccinated with two doses of mRNA-1273 [58.4% (95% CI 47.2–69.2)]. The relative seroconversion rate was 0.795 (95% CI 0.732–0.864).
Conclusions
This systematic review and meta-analysis indicates that in SOT recipients, higher seroconversion rates were observed after vaccination with mRNA-1273 compared with BNT162b2.
Keywords: antibody response, meta-analysis, mRNA vaccines, SARS-CoV-2/COVID-19, solid organ transplant recipients
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Solid organ transplant (SOT) patients carry an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related complications or mortality. An efficient vaccination strategy is critical in this population.
As patients on immunosuppressive drugs were excluded from phase 3 trials, little is known about the efficacy of anti-SARS-CoV-2 messenger RNA (mRNA) vaccines in SOT recipients.
In the general population, several studies have demonstrated significantly higher antibody titers after mRNA-1273 vaccination (Moderna) compared with BNT162b2 vaccination (Pfizer).
What this study adds?
In this study, the published literature concerning antibody responses after a two-dose anti-SARS-CoV-2 mRNA vaccination in SOT patients is united in a systematic review and meta-analysis.
The results of the meta-analysis show that in SOT recipients, higher seroconversion rates were observed after vaccination with mRNA-1273 compared with BNT162b2.
What impact this may have on practice or policy?
This study provides a platform for the design of novel studies aimed at investigating the preferred vaccine strategy in SOT recipients.
This study will aid in the discussion of whether mRNA-1273 should be the preferred vaccine in SOT recipients.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has raged for >2 years now. It is estimated that >300 million people have been infected and that ∼5.5 million individuals have died [1]. Solid organ transplant (SOT) patients carry a greater risk of complications and mortality attributable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [2]. Therefore an efficient vaccination strategy is critical for this population.
Both BNT162b2 and mRNA-1273 vaccines have each shown >90% efficacy in preventing COVID-19 illness in the general population [3, 4]. As patients on immunosuppressive drugs were excluded from phase 3 trials, little is known about the efficacy of these vaccines in SOT recipients. Multiple reports show that in this latter group, only ∼50% of patients develop anti-SARS-CoV-2 antibodies after a primary vaccination with two injections of BNT162b2 (Pfizer-BioNTech, New York, NY, USA) or mRNA-1273 (Moderna, Cambridge, MA, USA) [5, 6].
Although both vaccines induce a nearly 100% seroconversion rate in the general population, several studies have demonstrated significantly higher antibody titers after mRNA-1273 vaccination compared with BNT162b2 vaccination [7–10]. In this systematic review and meta-analysis, we aimed to investigate the proportion of SOT patients developing a humoral response to both vaccines as well as the corresponding anti-SARS-CoV-2 spike antibody levels by performing a systematic review and meta-analysis of the existing literature. Higher seroconversion rates and/or antibody titers following either mRNA vaccine could potentially affect vaccination strategies targeting this vulnerable group.
MATERIALS AND METHODS
A clinical research question was formulated according to the following Population, Intervention, Comparison and Outcome (PICO) question [11]: in SOT patients (P), do two doses of mRNA-1273 vaccination (I), compared with two doses of BNT162b2 vaccination (C), result in a higher seroconversion rate (O1) and/or higher anti-SARS-CoV-2 antibody titers (O2)?
Studies in which results are reported on both the antibody response after two doses of BNT162b2 and mRNA-1273 in SOT recipients were considered eligible. Randomized controlled trials (RCTs), cohort studies, case–control studies and cross-sectional studies were included. The search period ranged from 2020 to 2022. No age restriction was applied. Literature reviews, case reports and commentaries were excluded.
A systematic search of three databases was conducted (PubMed, Web of Science and Cochrane Library) using the following search terms: transplant* AND {[(mRNA OR Moderna OR Pfizer BioNTech) AND vaccin*] OR mRNA-1273 OR BNT162b2 OR Comirnaty OR Spikevax}. The last search date was 8 January 2022.
To minimize selection bias, studies were screened independently by two reviewers (A.V. and R.B.). First, duplicates were removed, after which articles were screened by title and abstract. Remaining reports were subsequently assessed for eligibility through full-text screening. Finally, the methodological quality of the included studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) [12]. Indeed, we could not retrieve any RCTs on this topic. Disagreements were resolved by consensus.
A.V. extracted the following data from the included studies: cohort size, transplant type, seroconversion rate, antibody titer, immunological assay and time of measurement. A second author (K.J.L.) checked the data for correctness.
The meta-analysis was performed using the packages metafor and meta in the statistical software package R, version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) [13]. More specifically, a single-group random-effects (RE) meta-analysis approach was considered to pool the seroconversion rates for each of the mRNA vaccines (mRNA-1273 and BNT162b2) obtained from the eligible studies. A Freeman–Tukey double arcsine transformation of the study-specific seroconversion rates and corresponding standard errors were used in the pooling procedure. The inverse-variance (IV) method was used to weigh the study-specific transformed seroconversion rates (with the inverse of the within-study variance as study-specific weights). The between-study variability was estimated using the DerSimonian–Laird estimator. Heterogeneity across the studies was quantified by means of the inconsistency index or I2 statistic. After the single-group meta-analysis models for each of the mRNA vaccines we performed an RE meta-analysis of the relative seroconversion rates for the two two-dose mRNA vaccination schemes in SOT patients. Again, the IV method and DerSimonian–Laird estimator were used.
RESULTS
Our search yielded a total of 902 results (PubMed n = 371, Web of Science n = 273 and Cochrane Library n = 258). After removal of duplicates and screening by title/abstract, 62 articles were found to be eligible for full-text reading. Of these, 54 articles were excluded, mostly because no comparison was made between the mRNA vaccines. Alternatively, no data on seroconversion rates for both vaccines were available. A total of eight studies were included in this meta-analysis. The full study selection process is shown in Fig. 1.
Figure 1:
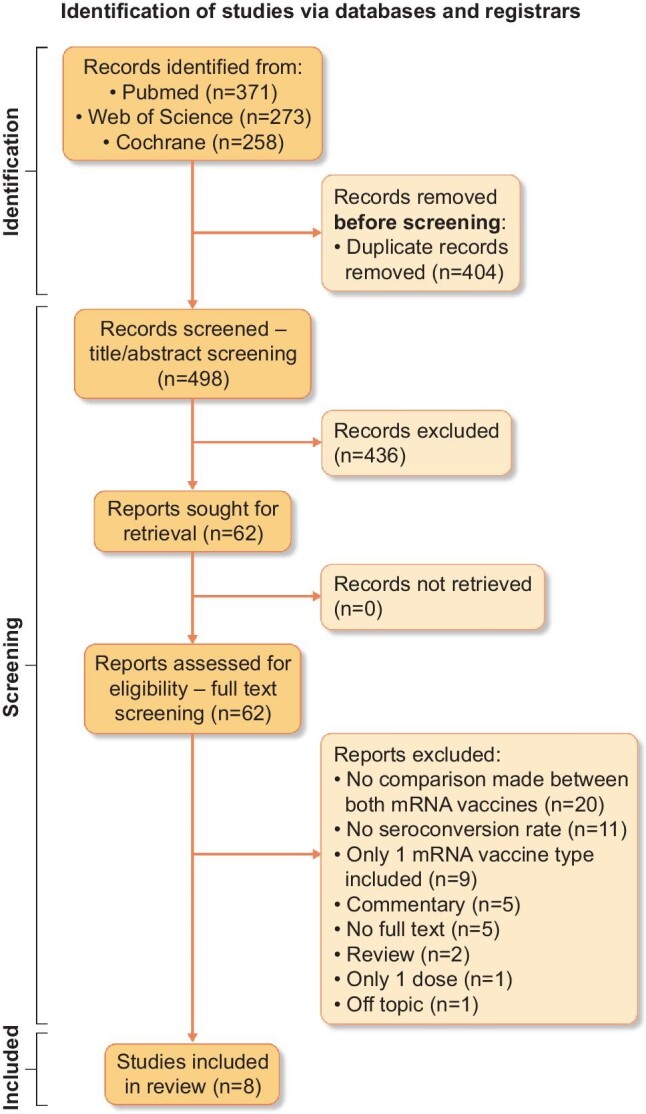
Flow diagram of study selection. Source: Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71.
Study characteristics
Patient characteristics are summarized in Table 1. One study was retrospectively designed [14], the other seven studies were prospective in design [15–21]. Furthermore, an overview of the variables related to seroresponses described in the separate studies is depicted in Table 2. There was considerable heterogeneity in transplant type as well as in the immunological assay that was used to measure antibodies. Among the 1877 SOT patients studied, 1070 were transplanted with a kidney, 290 with a liver, 247 with a lung, 231 with a heart, 5 with a pancreas and 26 were multiorgan recipients. Data were not available regarding transplant type for eight SOT recipients. The vaccine-specific seroconversion rate was available for 1833 patients. A total of 956 patients were vaccinated with BNT162b2. Of those, 877 patients received the mRNA-1273 vaccine. The anti-SARS-CoV-2 antibodies against receptor-binding domain (RBD) and/or spike protein were detected with the Elecsys (anti-RBD; Roche, Basel, Switzerland) or the Euroimmun (Lübeck, Germany) test (anti-S1) in three studies [15, 17, 19], with only the Euroimmun test in one study [20], with the Alimiti (anti-RBD; Abbott, Abbott Park, IL, USA) in one study [18], with the DiaSorin (Saluggia, Italy) anti-trimeric S-protein test [16] or Ortho Clinical Diagnostics (Raritan, NJ, USA) anti-SARS-CoV-2 Spike Ig assay [14] and with an in-house-designed Luminex bead assay (anti-RBD; Austin, TX, USA) [21]. The median time between the second vaccination and measuring the antibody response varied between 17 and 94 days after the second vaccine administration.
Table 1.
Patient characteristics per study
| Reference | Patients (n) | Male (%) | Age (years)a | BMI (kg/m²)a | Time after transplantation (years)a | >2 immunosuppressants (%) | Subjects on MMF/MPA (%) | Serum creatinine, median (IQR) | Lymphocyte count |
|---|---|---|---|---|---|---|---|---|---|
| Boyarsky et al. [15] | 658 | 41 | 18–39: 17% 40–59: 31% ≥60: 51% |
NA | <3: 28% 3–6: 21% 7–11: 22% ≥12: 29% |
NA | 66a | NA | NA |
| Dębska-Ślizień et al. [16] | 142 | 58 | Median 54 (43–63) | 25 (22.55–28.37) | Median 8 (IQR 3.5–15) | 73 | 79 | 1.35 (1.12–1.7) | NA |
| Hallett et al. [17] | 237 | 46 | 62 (46–69) | 25.6 (21.9–29.7) | 5.1 (2.5–11.0) | 34 | 62 | NA | NA |
| Narasimhan et al. [18] | 73 | 64 | 65 (53.5–69.5) | NA | 3.3 (1.6–5.3) | NA | 99 b | NA | NA |
| Strauss et al. [19] | 161 | 43 | 64 (48–69) | 26.0 (23.0–30.5) | 6.9 (2.9–15.0) | NA | 35 | NA | NA |
| Stumpf et al. [20] | 368 | 65 | 57.3 ± 13.7 | 26.4 ± 4.8 | 9.9 ± 6.8 | 35 | 76 | NA | NA |
| Wijtvliet et al. [21] | 133 | 59 | NA | NA | NA | NA | 53 | NA | NA |
| Yi et al. [14] | 105 | 62 | 57.0 (46.0–64.0) | NA | 1.0 (0.0–3.0) | NA | 81 | NA | NA |
NA, not available.
Frequencies were calculated of those with available data.
aValues presented as percentage, median (IQR) or mean ± standard deviation.
bAlso included azathioprine.
Table 2.
Variables related to seroresponse
| Reference | SOT recipients | Transplant type (n) | Seroresponse after first vaccination, % | Seroresponse after second vaccination, % | BNT162b2 vaccinated, n | BNT162b2 seroconverted, n | mRNA-1273 vaccinated, n | mRNA-1273 seroconverted, n | Immunological assay | Cutoff seronversion | Median time between second vaccination and measuring the antibody response |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boyarsky et al. [15] | 658a | Kidney (322) Liver (129) Heart (97) Lung (71) Pancreas (5) Multiorgan (26) Missing data (8) |
15 | 54 | 342 | 167 | 307 | 183 | Roche Elecsys (anti-RBD total Ab) OR Euroimmun (anti-S1 IgG) | ≥0.8 U/mL (Roche Elecsys) ≥1:1 AUs (Euroimmun) |
29 days |
| Dębska-Ślizień et al. [16] | 142 | Kidney (142) | NA | 51 | 105 | 50 | 37 | 23 | DiaSorin (anti-trimeric S-protein test) | ≥12 AU/mL | 14–21 days |
| Hallett et al. [17] | 237 | Heart (134) | 14 | 48 | 70 | 42 | 64 | 41 | Roche Elecsys (anti-RBD total Ab) OR Euroimmun (anti-S1 IgG) | ≥0.8 U/mL (Roche Elecsys) ≥1:1 AUs (Euroimmun) |
29 days |
| Lung (103) | 9 | 27 | 56 | 19 | 47 | 18 | |||||
| Narasimhan et al. [18] | 73 | Lung (73) | NA | 25 | 48 | 9 | 25 | 9 | Abbott Alinity i (anti-RBD IgG) | ≥50 AU/mL | 17.5 days (BNT162b) 19 days (mRNA-1273) |
| Strauss et al. [19] | 161 | Liver (161) | 34 | 81 | 85 | 62 | 76 | 68 | Roche Elecsys (anti-RBD total Ab) or Euroimmun (anti-S1 IgG) | ≥0.8 U/mL (Roche Elecsys) ≥1:1 AUs (Euroimmun) |
30 days |
| Stumpf et al. [20] | 368a | Kidney (368) | 8 | 42 | 99 | 26 | 234 | 114 | Euroimmun (anti-S1 IgG or anti-S1 IgA) | A positive serologic response was defined as de novo antibody development (seroconversion) | 4–5 weeks |
| Wijtvliet et al. [21] | 133 | Kidney (133) | 14 | 62 | 91 | 51 | 42 | 32 | Luminex (anti-RBD IgG) | Signal:noise ratio > 1 | 28 days |
| Yi et al. [14] | 105 | Kidney (105) | NA | 36 | 60 | 20 | 45 | 18 | Ortho Clinical Diagnostics anti-SARS-CoV-2 Spike Ig assay | Antibody response was defined as the presence of either anti-SARS-CoV-2 IgG or total antibody or anti-SARS-CoV-2 Spike total Ig ≥ 1:50 | 91 days |
Ab, antibodies; AU, arbitrary units; Ig, immunoglobulin; S-protein, spike protein.
aVaccine-specific seroconversion rate not available from all included patients.
Risk of bias within studies
The MINORS criteria revealed a mean score of 74%. Three of eight studies were considered high quality [17, 20, 21], the others were scored as moderate quality [14–16, 18, 19], as shown in Table 3. The risk of bias was thus acceptable.
Table 3.
Individual MINORS score
| Boyarsky et al.[15] | Dębska-Ślizień et al. [16] | Hallett et al. [17] | Narasimhan et al. [18] | Strauss et al. [19] | Stumpf et al. [20] | Wijtvliet et al. [21] | Yi et al. [14] | |
|---|---|---|---|---|---|---|---|---|
| Clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 |
| Prospective data collection | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 |
| Endpoints appropriate to study aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of study endpoint | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Follow-up period appropriate to study aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| <5% loss to follow-up | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 |
| Prospective calculation of study size | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Adequate control group | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Contemporary groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalence of groups | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total score | 16/24 (67%) | 17/24 (71%) | 19/24 (79%) | 17/24 (71%) | 17/24 (71%) | 19/24 (79%) | 20/24 (83%) | 17/24 (71%) |
The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The global ideal score is 24 for comparative studies. The corresponding scores are 0–6, very low quality; 7–12, low quality; 13–18, moderate quality and 19–24, high quality.
Synthesis of results
No patients had a prior polymerase chain reaction-confirmed diagnosis of COVID-19. Four studies screened for anti-nucleocapsid protein immunoglobulin G (IgG) prior to vaccination. While Narasimhan et al. [18] included one patient with a past SARS-CoV-2 infection, those patients were excluded by Yi et al. [14], Dębska-Ślizień et al. [16] and Stumpf et al. [20].
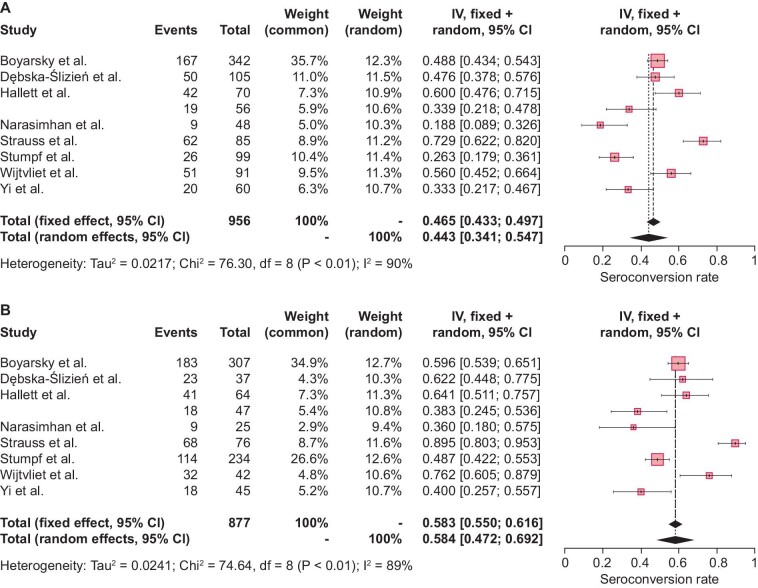
The single-group meta-analysis models indicated considerable heterogeneity across different studies, with high I2 values {89.3% [95% confidence interval (CI) 81.9–93.7] and 89.5, [95% CI 82.3–93.8] for mRNA-1273 and BNT162b2, respectively}. The pooled seroconversion rate was estimated to be higher for mRNA-1273 [58.4% (95% CI 47.2–69.2); Fig. 2A] as compared with BNT162b2 [44.3% (95% CI 34.1–54.7); Fig. 2A]. As presented in Fig. 3, the relative seroconversion rate was estimated to be 0.795 (95% CI 0.732–0.864) for BNT162b2 versus mRNA-1273 vaccination based on an RE meta-analysis model as described above. Consequently, a lower seroconversion rate was observed after two-dose vaccination with BNT162b2 as compared with mRNA-1273.
Figure 2:
Study-specific and pooled estimates for the seroconversion rate after two-dose mRNA vaccination with (A) BNT162b2 or (B) mRNA-1273 based on RE meta-analysis models and relying on the IV method. Box sizes in the forest plots are proportional to the weight assigned to each study. Limits of the displayed intervals are defined as 95% CIs. Eight studies calculated the seroconversion rates in SOT recipients after two-dose BNT162b2 vaccination (n = 956), (A) resulting in a pooled seroconversion rate of 44.3% (95% CI 34.1–54.7). The same eight studies also described seroconversion rates in SOT recipients after two-dose mRNA-1273 vaccination (n = 877), (B) resulting in a pooled seroconversion rate of 58.4% (95% CI 47.2–69.2). df, degrees of freedom; I2, inconsistency index.
Figure 3:
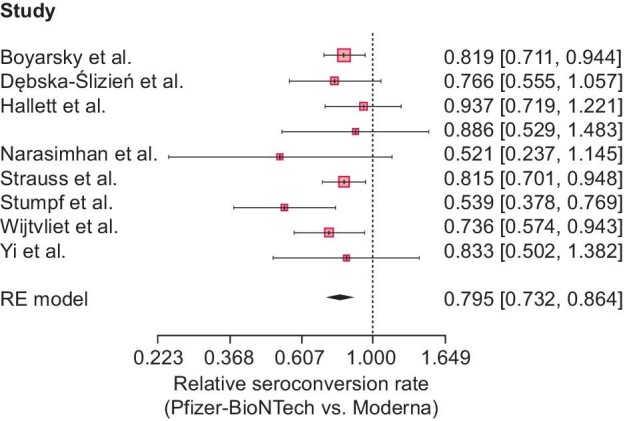
Meta-analytic result for the relative seroconversion rate (BNT162b2 versus mRNA-1273) based on an RE meta-analysis model and relying on the IV method. Seroconversion rates appeared to be significantly lower in patients vaccinated with two doses of BNT162b2 than in patients vaccinated with two doses of mRNA-1273 [79.5% (95% CI 73.2–86.4)].
Subsequently a subgroup meta-analysis was performed regarding seroconversion rates after two-dose BNT162b2 versus mRNA-1273 vaccination in kidney transplant recipients. Four of the included studies provided individual seroconversion data for both vaccines in kidney transplant recipients and were therefore incorporated [14, 16, 20, 21]. Again, the single-group meta-analysis showed considerable heterogeneity across the studies, with high I2 values [80.7% (95% CI 49.3–92.7) and 85.7 (95% CI 65.0–94.2) for mRNA-1273 and BNT162b2, respectively]. mRNA-1273 induced higher pooled seroconversion rates [56.4% (95% CI 42.0–70.2); Supplementary data, Figure 1B] compared with BNT162b2 [40.7% (95% CI 27.4–54.6); Supplementary data, Figure 1A]. The relative seroconversion rate was estimated to be 0.705 (95% CI 0.599–0.830) for BNT162b2 versus mRNA-1273 vaccination (Supplementary data, Figure 2).
Only two studies directly compared anti-SARS-CoV-2 antibody titers after vaccination with either mRNA vaccine [18, 21], thus precluding a meta-analysis on this issue. While Narasimhan et al. [18] did not find a significant difference in antibody titers between the BNT162b2 vaccine [median 0.9 AU/mL (95% CI 0.0–4.1)] and the mRNA-1273 formulation [median 20.6 AU/mL (95% CI 0.8–80.2)] among lung transplant patients (P = .96), Wijtvliet et al. [21] showed significantly higher antibody titers after two doses of mRNA-1273 compared with BNT162b2 in kidney transplant recipients [mean log-transformed antibody levels were 0.289 units higher for mRNA-1273 versus BNT162b2 vaccination in multiple linear mixed models (P = .005)].
Two of eight studies reported on T-cell anti-SARS-CoV-2 responses [18, 20]. Interestingly, Stumpf et al. [20] showed a numerically higher cellular immune response after vaccination with mRNA-1273 as compared with BNT162b2. Narasimhan et al. [18] did not compare T-cell responses between both mRNA vaccines, but they studied the humoral response in relation to T-cell activity.
DISCUSSION
This systematic review and meta-analysis reveal that in SOT patients, vaccination with mRNA-1273 leads to a significantly higher seroconversion rate than BNT162b2 vaccination [58.4% versus 44.3%, respectively, with a relative seroconversion rate of 0.795 (95% CI 0.732–0.864)]. A subanalysis with data regarding kidney transplant recipients revealed similar outcomes and resulted in a relative seroconversion rate of 0.705 (95% CI 0.599–0.830). In similar research in patients with hematologic malignancies, the seroconversion rate was 56% with mRNA-1273 versus 33% with BNT162b2 (P = .013) [22]. This contrasts with dialysis patients, where the seroconversion rate is much higher with both the BNT162b2 (∼73–88%) [21, 23, 24] and the mRNA-1273 vaccine (∼94.4–100%) [21, 23, 24]. In the article by Lacson et al. [23], no difference in seroconversion rate was observed between both vaccines in dialysis patients (P = 0.42), while in the study by Wijtvliet et al. [21] and Yau et al. [24], mRNA-1273 led to a higher seroconversion rate. Moreover, a recent study by Van Praet et al. [25] reported higher geometric mean antibody titers in hemodialysis patients vaccinated with mRNA-1273 versus BNT162b2, and a larger proportion achieved the threshold of 4160 AU/mL with higher neutralizing antibody titers in vitro (53.6% versus 31.8% at 8 or 9 weeks; P < .0001). Yau et al. [24] described similar results, where 35 of 70 (50%) dialysis patients vaccinated with BNT162b2 reached the convalescent level for anti-RBD compared with 69 of 87 (79%) who received mRNA-1273 (P < .001). Garcia et al. [26] confirmed these findings and reported that a greater number of dialysis patients vaccinated with BNT162b2 had no detectable or diminished IgG response compared with patients vaccinated with mRNA-1273.
Among the studies included in this systematic review and meta-analysis, only two articles directly compared anti-SARS-CoV-2 antibody titers after vaccination with either mRNA vaccine [18, 21], thus precluding a meta-analysis on this issue. While Narasimhan et al. [18] did not find a significant difference in antibody titers between the BNT162b2 vaccine and the mRNA-1273 formulation among lung transplant patients (P = .95), in our own study [21], significantly higher antibody titers after two doses of mRNA-1273 compared with BNT162b2 in kidney transplant recipients were observed after multivariate analysis. In the general population, whereas the seroconversion rates are similar between the two vaccines, there is now clear evidence that higher titers of anti-SARS-CoV-2 antibodies are present after vaccination with mRNA-1273 as compared with BNT162b2 [7–10]. This has already resulted in serious clinical consequences in the general population. Indeed, although the incidence of severe or critical COVID-19 illness remains low in the fully vaccinated general population, a higher number of breakthrough SARS-CoV-2 infections have been observed after two-dose BNT162b2 versus mRNA-1273 vaccination [27–29]. First, Wang et al. [29] noted 2.8 versus 1.6 breakthrough cases per 1000 person-days in November 2021, respectively, a finding that was confirmed in the studies by Abu-Raddad et al. [28] and Dickerman et al. [27]. Moreover, the severity of breakthrough SARS-CoV-2 infections appeared to be higher in patients who received the BNT162b2 vaccine compared with those who received mRNA-1273 [27, 30, 31]. Dickerman et al. [27] showed higher 24-week risk ratios after BNT162b2 vaccination for both COVID-19 and admission to the intensive care unit. Furthermore, in a case–control study, beyond 120 days after vaccination, a higher estimated effectiveness to prevent COVID-19 hospitalizations was observed after vaccination with mRNA-1273 versus BNT162b2 (85% versus 64%) [31].
The difference in immunogenicity between the two mRNA vaccines could relate to the amount of mRNA used in the respective vaccines. Indeed, the mRNA-1273 vaccine contains 100 µg of mRNA while the BNT162b2 formulation only contains 30 µg. Another possible explanation is the longer interval between priming and boosting for mRNA-1273 (4 weeks as compared with 3 weeks for BNT162b2). A longer interval between the first and second dose has recently been shown to increase antibody levels [32]. Furthermore, there are differences in the lipid composition of the nanoparticles used for packaging the mRNA. BNT162b2 has a lipid nanoparticle composed of ALC-0315, ALC-0159, DSPC and cholesterol, whereas the lipid nanoparticle of mRNA-1273 is composed of SM-102, PEG-DMG, DSPC and cholesterol [33].
This meta-analysis makes clear that the current research on immunity after SARS-CoV-2 vaccination in vulnerable patients has several limitations. First, given the fact that the response after vaccination against SARS-CoV-2 has only been investigated for 1 year, only eight studies reporting on 1833 patients could be included in this systematic review and meta-analysis. However, even with this restricted number of articles, the results were consistent across the studies, making this meta-analysis scientifically sound. Second, the included studies were all observational in nature; none of them was an RCT. Third, the number of studies reporting on vaccine-specific antibody titers was too small to allow for a meta-analysis and only two studies reported on T-cell responses. Fourth, a considerable level of heterogeneity across the different studies was observed, which could be at least partly explained by different transplant types analysed in different studies. However, heterogeneity disappeared when looking at relative differences in seroresponse following two-dose BNT162b2 versus mRNA-1273 vaccination across patient groups (cf. I2 value: 1% of total variability due to between-study variability). Moreover, a subgroup meta-analysis with regard to seroconversion rates after two-dose BNT162b2 versus mRNA-1273 vaccination in kidney transplant recipients only showed similar results as compared with the meta-analytic results in all SOT recipients. Furthermore, a subgroup meta-analysis was not possible for lung, liver or heart transplant recipients separately, as no data were available for more than two studies. Finally, a comparison of factors associated with lower seroconversion rates in patients receiving either two-dose BNT162b2 or mRNA-1273 vaccination across studies would be of interest. However, such an assessment requires sufficient information regarding such factors across all eligible studies. In the absence thereof, only a qualitative assessment was possible with regard to the relationship between the use of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), which has been described as one of the strongest predictors of impaired immune response after vaccination [34], and seroresponse following vaccination. Although no formal statistical assessment has been performed, results from the studies included in this meta-analysis do suggest that the use of MMF/MPA is inversely related to seroconversion rates [14, 18–20]. Additional data on time after transplantation, use of MMF, estimated glomerular filtration rate (eGFR) levels and lymphocyte counts would be useful to better understand observed differences in seroconversion rates between both mRNA vaccines and for a specific vaccine across studies. Unfortunately, detailed data on patient characteristics were often not reported in the eight studies included in this meta-analysis. New, bigger studies with stratification by age, gender, time since transplantation, eGFR levels, lymphocyte counts, transplant type and immunosuppressive drugs are needed to overcome this problem.
In conclusion, the seroconversion rate appeared to be higher after mRNA-1273 vaccination versus BNT162b2 vaccination in SOT recipients. Future studies are needed to assess whether these differences are confirmed after third-dose vaccination and whether they also associated with a better protection against severe disease, hospitalization and/or mortality. This will help to determine whether mRNA-1273 should be the preferred vaccine in SOT recipients. In addition, all efforts should be made to vaccinate kidney transplant candidates before transplantation, as the overall efficacy of SARS-CoV-2 vaccines is better during dialysis than after kidney transplantation [35].
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Erik Snelders for his outstanding secretarial help.
Contributor Information
Arno Verleye, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium.
Veerle Wijtvliet, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Steven Abrams, Global Health Institute, Family Medicine and Population Health, University of Antwerp, Wilrijk, Belgium; Data Science Institute, Interuniversity Institute for Biostatistics and Statistical Bioinformatics, Hasselt University, Hasselt, Belgium.
Rachel Hellemans, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Rania Bougrea, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium.
Annick Massart, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Lissa Pipeleers, Department of Nephrology, Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium.
Karl Martin Wissing, Department of Nephrology, Universitair Ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium.
Kevin K Ariën, Virology Unit, Department of Biomedical Sciences, Institute of Tropical Medicine, Antwerp, Belgium; Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
Benedicte Y De Winter, Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Pierre Van Damme, Centre for the Evaluation of Vaccination, Vaccine and Infectious Disease Institute, University of Antwerp, Antwerp, Belgium.
Daniel Abramowicz, Department of Nephrology and Hypertension, Antwerp University Hospital, Edegem, Belgium; Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
Kristien J Ledeganck, Laboratory of Experimental Medicine and Pediatrics and Member of the Infla-Med Centre of Excellence, University of Antwerp, Antwerp, Belgium.
FUNDING
No funding was received to assist with the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONS
A.V. and R.B. performed the literature search independently and assessed the methodological quality. A.V. extracted data from the articles that met the inclusion criteria. K.J.L. checked the data for correctness. S.A. performed the meta-analysis. A.V., R.H., V.W., K.J.L. and D.A. drafted the manuscript. A.V., V.W., S.A., R.H., A.M., L.P., K.M.W., K.K.A., B.D.W., P.V.D., D.A. and K.J.L. reviewed and approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (30 May 2022, date last accessed) [Google Scholar]
- 2. Azzi Y, Bartash R, Scalea Jet al. COVID-19 and solid organ transplantation: a review article. Transplantation 2021; 105: 37–55 [DOI] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin Net al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383: 2603–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink Bet al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384: 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marinaki S, Adamopoulos S, Degiannis Det al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant 2021; 21: 2913–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marion O, Del Bello A, Abravanel Fet al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med 2021; 174: 1336–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steensels D, Pierlet N, Penders Jet al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021; 326: 1533–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Markewitz R, Pauli D, Dargvainiene Jet al. The temporal course of T- and B-cell-responses to vaccination with BNT162b2 and mRNA-1273. Clin Microbiol Infect 2022; 28: 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richards NE, Keshavarz B, Workman LJet al. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open 2021; 4: e2124331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Self WH, Tenforde MW, Rhoads JPet al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson WS, Wilson MC, Nishikawa Jet al. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995; 123: A12–A13 [PubMed] [Google Scholar]
- 12. Slim K, Nini E, Forestier Det al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716 [DOI] [PubMed] [Google Scholar]
- 13. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2018 [Google Scholar]
- 14. Yi SG, Moore LW, Eagar Tet al. Risk factors associated with an impaired antibody response in kidney transplant recipients following 2 doses of the SARS-CoV-2 mRNA vaccine. Transplant Direct 2022; 8: e1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyarsky BJ, Werbel WA, Avery RKet al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325: 2204–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dębska-Ślizień A, Muchlado M, Ślizień Zet al. Significant humoral response to mRNA COVID-19 vaccine in kidney transplant recipients with prior exposure to SARS-CoV-2. The COViNEPH Project. Pol Arch Intern Med 2021; 132: 16142. [DOI] [PubMed] [Google Scholar]
- 17. Hallett AM, Greenberg RS, Boyarsky BJet al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant 2021; 40: 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narasimhan M, Mahimainathan L, Clark AEet al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines (Basel) 2021; 9: 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strauss AT, Hallett AM, Boyarsky BJet al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl 2021; 27: 1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stumpf J, Siepmann T, Lindner Tet al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 2021; 9: 100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wijtvliet VPWM, Ariën KK, Abrams Set al. mRNA-1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? Nephrol Dial Transplant 2022; 37: 799–803 [DOI] [PubMed] [Google Scholar]
- 22. Ollila TA, Lu S, Masel Ret al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol 2021; 7: 1714–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lacson E, Argyropoulos CP, Manley HJet al. Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol 2021; 32: 2735–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yau K, Chan CT, Abe KTet al. Differences in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. CMAJ 2022; 194: E297–E305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Praet J, Reynders M, De Bacquer Det al. Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: a multicenter observational study. J Am Soc Nephrol 2021; 32: 3208–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia P, Anand S, Han Jet al. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol 2022; 33: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dickerman BA, Gerlovin H, Madenci ALet al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med 2022; 386: 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu-Raddad LJ, Chemaitelly H, Bertollini Ret al. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med 2022; 386: 799–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Davis PB, Kaelber DCet al. Comparison of mRNA-1273 and BNT162b2 vaccines on breakthrough SARS-CoV-2 infections, hospitalizations, and death during the delta-predominant period. JAMA 2022; 327: 678–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Juthani PV, Gupta A, Borges KAet al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis 2021; 21: 1485–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tenforde MW, Self WH, Adams Ket al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021; 326: 2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Payne RP, Longet S, Austin JAet al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021; 184: 5699–5714.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoenmaker L, Witzigmann D, Kulkarni JAet al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm 2021; 601: 120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kantauskaite M, Müller L, Kolb Tet al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant 2022; 22: 634–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quiroga B, Soler MJ, Ortiz Aet al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant 2021; doi: 10.1093/ndt/gfab313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.