ABSTRACT
Background
Accurate accounting of coronavirus disease 2019 (COVID-19) critical care outcomes has important implications for health care delivery.
Research Question
We aimed to determine critical care and organ support outcomes of intensive care unit (ICU) COVID-19 patients and whether they varied depending on the completeness of study follow-up or admission time period.
Study Design and Methods
We conducted a systematic review and meta-analysis of reports describing ICU, mechanical ventilation (MV), renal replacement therapy (RRT), and extracorporeal membrane oxygenation (ECMO) mortality. A search was conducted using PubMed, Embase, and Cochrane databases.
We included English language observational studies of COVID-19 patients, reporting ICU admission, MV, and ICU case fatality, published from December 1, 2019 to December 31, 2020. We excluded reports of less than 5 ICU patients and pediatric populations. Study characteristics, patient demographics, and outcomes were extracted from each article. Subgroup meta-analyses were performed based on the admission end date and the completeness of data.
Results
Of 6,778 generated articles, 145 were retained for inclusion (n = 60,357 patients). Case fatality rates across all studies were 34.0% (95% CI = 30.7%, 37.5%, P < 0.001) for ICU deaths, 47.9% (95% CI = 41.6%, 54.2%, P < 0.001) for MV deaths, 58.7% (95% CI = 50.0%, 67.2%, P < 0.001) for RRT deaths, and 43.3% (95% CI = 31.4%, 55.4%, P < 0.001) for extracorporeal membrane oxygenation deaths. There was no statistically significant difference in ICU and organ support outcomes between studies with complete follow-up versus studies without complete follow-up. Case fatality rates for ICU, MV, and RRT deaths were significantly higher in studies with patients admitted before April 31st 2020.
Interpretation
Coronavirus disease 2019 critical care outcomes have significantly improved since the start of the pandemic. Intensive care unit outcomes should be evaluated contextually (study quality, data completeness, and time) for the most accurate reporting and to effectively guide mortality predictions.
INTRODUCTION
Although overall mortality for coronavirus disease 2019 (COVID-19) approaches 4% or lower, estimated case fatality rates (CFR) for severely ill COVID-19 patients have been much higher, although varying widely, ranging from 10% to 90% over the span of a year. A large observational study in China of 72,314 cases (5% critically ill) reported an intensive care unit (ICU) mortality of 49%.1 In Italy, 16% of total hospitalizations required ICU-level care within the first 2 weeks.2 Of 1,591 critically ill patients in Italy, 72% were mechanically ventilated, 26% died, 58% remained in the ICU, and only 16% were discharged from the ICU.3 In the rush to disseminate information to the medical community and public, it has become apparent that outcomes vary depending on the completeness of data. When initial coverage of the study by Richardson et al. appeared in press, they reported a mortality of 88.1% for patients requiring mechanical ventilation (MV). When the paper was published in April 2020, the mortality for patients requiring MV was reported as 24.5%. This drastically different rate comes from comparing the same numerator (282 deaths) to different denominators (1,151 patients requiring MV total or 320 patients requiring MV that have all died or discharged alive). Neither of these figures gives the reader an accurate portrayal of mortality as 831 (72%) patients remained hospitalized at the time of publication (i.e., final outcome to be determined).4 When a physician counsels a critically ill patient about the need for MV and chances of survival, having an 88% chance of dying could lead some patients to decline a potentially lifesaving intervention that they would accept if there were told that the mortality was closer to 24%. For the lay public, perception that a vital treatment, such as MV, could have a higher mortality than the disease itself is misleading and dangerous. It can impact individual patient decisions, physician decision making, and hospital policy making regarding allocation of scarce resources. The literature quality has been variable as many studies were available for review online prior to peer review.
It is of vital importance that we have accurate and up-to-date information about COVID-19 ICU outcomes for expectation management, facilitating end-of-life discussion, resource allocation, and planning clinical research. Multiple meta-analyses and systematic reviews on COVID-19 ICU outcomes have emerged, but the quality and scope are variable. We conducted a systematic review with meta-analysis to summarize updated outcomes of COVID-19 patients requiring intensive care and MV. The purpose of this study is to determine the overall outcomes associated with ICU and organ support outcomes across the first year of the pandemic.
MATERIALS AND METHODS
This systematic review follows the recommendations established by the Preferred Reporting Items for Systematic Reviews statement and is reported in the Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020180607).5 We included English language peer-reviewed observational studies of confirmed COVID-19 patients, reporting at least 2 of the following parameters: ICU admission or ICU level of care, ICU and MV mortality and published from December 1, 2019 to December 31, 2020. We excluded reports of fewer than 5 patients, articles not representing original data, studies with pediatric populations, and preprints. The search was conducted through April 24, 2020 by a professional medical librarian using PubMed, Embase, and Cochrane databases. The following search terms were used: COVID-19, Novel coronavirus 2019 and MV, intubation, pneumonia, ICU, critical care, critically ill patients, severely ill patients, clinical characteristics, mortality, and outcomes.
The search identified a total of 6,778 records after duplicates were removed. The final results were exported to Covidence (covidence.org), an online systematic review software. After search results were obtained, 2 authors (R.M. and M.L.) independently screened and selected articles by title and abstract and cross-matched selections with the other. Full-text articles were then reviewed by the same method. Two separate authors (S.L. and M.W., J.C and S.A., E.T. and B.F., R.M. and M.L., M.B. and Z.H.) extracted data including study design, country of origin, age, gender, number of COVID-19 patient admissions, number of ICU admissions, number of patients diagnosed with acute respiratory distress syndrome (ARDS), number of ICU patients requiring MV (i.e., invasive MV via endotracheal intubation), renal replacement therapy (RRT) and extracorporeal membrane oxygenation (ECMO), ICU mortality, and mortality associated with organ support (MV, RRT, and ECMO). We defined MV as patients who underwent endotracheal intubation with placement on a mechanical ventilator.
We defined complete datasets or complete follow-up datasets as those with hospitalized populations where all patients had either recovered and been discharged or died. This meant that reported outcomes for endpoints such as mortality did not include patients who remained in the hospital with an unclear disposition at the time of evaluation. Studies with incomplete data were those that included an overall population encompassing those who had recovered and been discharged, those who had died, as well as those who remained in the hospital.
All outcomes were evaluated temporally based on when they occurred during the COVID-19 pandemic (i.e., first 6 months, admission ending on or before April 30, 2020, and after).
The data were recorded on a standardized electronic data collection sheet. Summary tables were constructed, data extraction and resultant figures were reviewed to ensure they were free from discrepancies. Any discrepancies were adjudicated by a fifth author (M.A.A.). The quality of the included studies was assessed independently based on the Newcastle–Ottawa Scale by 2 researchers (J.C. and S.L.).6
All statistical analyses were conducted in Stata statistical software version 15.1. Each fatality outcome variable was dichotomous in nature and was analyzed separately using the meta-analysis of proportions in Stata (metaprop command). In this study, the meta-analysis summary estimate of the proportions represents the case fatality rate of the outcome and is estimated using the meta-analysis random-effects model. This model employs the DerSimonian and Laird method and estimates heterogeneity using the inverse-variance fixed-effect model. In several studies, the proportions were equal to (or close to) 0 or 1. To stabilize their variances, the Freeman–Tukey double arcsine transformation was used in the data. The pooled estimate of the rate was then back transformed and presented, along with their Wald 95% CI estimated using the Score method. Heterogeneity between studies was estimated using the I2 statistic and its P-value. In this study, we used the pooled estimates obtained from the meta-analysis to report the fatality rates, which are often different from the rates obtained by manually combining all the number of deaths and cases from individual studies. Publication bias was assessed by examining visually the funnel plots and performing Egger tests. We also explored subgroup variations based on the date of last admission (admission ending on or before April 30, 2020), or after and completeness of data (with or without follow-up data).
RESULTS
Study Selection and Characteristics
A total of 6,778 articles were retrieved. After screening by abstract and title, 461 articles were selected for full-text assessment (Fig. 1). Three hundred and sixteen studies were excluded due to a lack of information on ICU admissions, MV, and mortality. We retained a total of 145 studies (n = 60,357 patients). The main characteristics of the studies included are shown in Table S1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews flowchart of included and excluded studies.
Demographics of ICU Patients
There were 78 studies that reported exclusively on severely ill patient cohorts (ICU-only studies). A total number of 21,510 ICU patients were included. The majority were male (64.2%). The median ages of these cohorts ranged from 49 to 72 years old. Additionally, 67 other “mixed” cohorts (ICU + Non-ICU hospitalized patients) reported a total of 24,931confirmed COVID-19 positive hospitalized patients with 6,186 (24.8%) ICU patients. An average of 53.8% were males. The median ages of these cohorts ranged from 32 to 72 years old.
ICU Case Fatality Rate
The total combined number of ICU deaths was 9,483 with an overall estimated ICU CFR of 34.0% (95% CI = 30.7%, 37.5%; I2 = 96.2%, P < 0.001). The ICU CFR in studies with complete follow-up (40.1%; 95% CI = 31.0%, 49.5%; I2 = 98.0%, P < 0.001) was worse compared to studies with complete follow-up (31.9%, 95% CI = 28.7%, 35.1%; I2 = 93.4%, P = 0.01), although the difference was not statistically significant (P = 0.091) (Fig. 2A). When stratified based on the date of last admission, the CFRs were statistically different from each other (P = 0.001), with 44.0% (95% CI = 36.4%, 51.8%; I2 = 94.9%, P < 0.001) from studies with last date of admission prior to April 2020 and 29.9% (95% CI = 26.4%, 33.5; I2 = 95.71%, P < 0.001) from studies with last date of admission in/after April 2020 (Fig. 2B; Fig. S1A).
FIGURE 2.
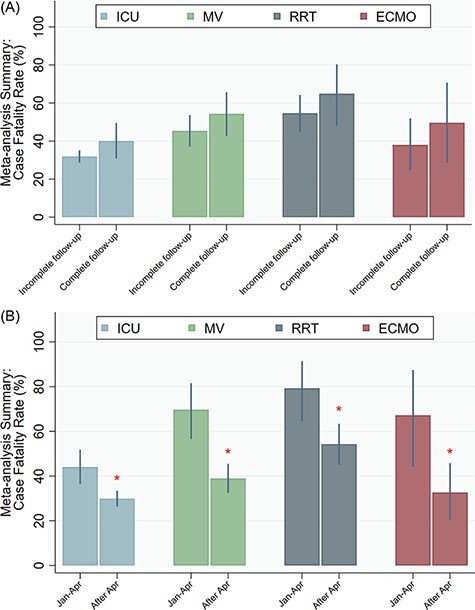
A–B: Case fatality rates. (A) Case fatality rates, complete versus incomplete follow-up. (B) Case fatality rates, hospitalization before and after April 31, 2020. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MV, mechanical ventilation; RRT, renal replacement therapy; *P < 0.05.
MV Case Fatality Rate
A total of 4,821 MV-related deaths (of 10,951 mechanically ventilated patients) were reported in the studies. The overall MV CFR across all studies was 47.9% (95% CI = 41.6%, 54.2%; I2 = 96.9%, P < 0.001) based on a total of 4,446 MV-related deaths. The MV CFR from studies with incomplete follow-up data was 45.3% (95% CI = 37.1%, 53.6%; I2 = 95.7%, P < 0.001), which was not statistically different (P = 0.216) from the CFRs from studies with complete follow-up: 54.3% (95% CI = 42.7%, 65.7%; I2 = 98.5%, P < 0.001) (Fig. 2A). Mechanical ventilation CFRs was significantly different (P < 0.001) based on the date of last admission, with 69.7% (95% CI = 56.6%, 81.6%; I2 = 93.2%, P < 0.001) from studies with last date of admission prior to April 2020 and 38.9% (95% CI = 32.5%, 45.5%; I2 = 96.9%, P < 0.001) from studies with last date of admission in/after April 2020 (Fig. 2B; Fig. S1B).
RRT Case Fatality Rate
There were 867 RRT-related deaths across the studies (of 1,498 patients who received RRT), and the overall RRT CFR was 58.7% (95% CI = 50.0%, 67.2%; I2 = 83.1%, P < 0.001). The mortality CFR from studies with incomplete follow-up data was 54.6% (95% CI = 44.9%, 64.2%; I2 = 77.2%, P < 0.001), which was not significantly different (P = 0.285) from the rate of the studies with complete follow-up data: 64.9% (95% CI = 48.1%, 80.3%; I2 = 86.3%, P < 0.001) (Fig. 2A). The RRT CFRs were significantly different (P = 0.007) based on the date of last admission, with 79.2% (95% CI = 64.6%, 91.5%; I2 = 32.9%, P = 0.155) from studies with last date of admission prior to April 2020 and 54.2%, (95% CI = 45.0%, 63.4%; I2 = 84.6%, P < 0.001) from studies with last date of admission in/after April 2020 (Fig. 2B; Fig. S1C).
ECMO Case Fatality Rate
A total of 269 ECMO-related deaths were reported in the studies (of 1,498 patients who required ECMO). The overall ECMO CFR across these studies was 43.3% (95% CI = 31.4%, 55.4%; I2 = 77.4%, P < 0.001). The ECMO CFRs did not vary statistically (P =0.395) based on whether studies had incomplete follow-up data or complete follow-up data, with rates of 38.0% (95% CI = 24.7%, 51.9%; I2 = 70.3%, P < 0.001) and 49.6% (95% CI = 28.5%, 70.7%; I2 = 80.9%, P < 0.001), respectively (Fig. 2A). The ECMO case fatality for studies that occurred later in the pandemic (32.6%; 95% CI = 20.4%, 45.8%; I2 = 79.3%, P < 0.001) was significantly lower (P = 0.009) compared to studies that occurred later in the pandemic (67.2%; 95% CI = 44.2%, 87.4% I2 = 64.5%, P < 0.001) (Fig. 2B; Fig. S1D).
RISK OF BIAS ASSESSMENT
The funnel plots of the estimates versus the estimate precisions are shown in Figure S2A-D. The Eggers test results showed there is no evidence of “small-study effects” (P > 0.05), suggesting that the estimates from smaller studies did not significantly differ from those from larger studies. However, from visual assessment, the funnel plots of all outcomes, except MV death, showed asymmetry, suggesting that publication bias or other types of biases cannot be excluded.
DISCUSSION
Our study is the largest and most up-to-date systematic review and meta-analysis for COVID-19 critical care outcomes, with more than double the number of studies as the largest meta-analyses to date.7 Although the ICU and organ support outcomes appear to have improved over time (early pandemic to more recent cases), death rates continue to exceed 30% by any measure. Our estimates for ventilator mortality are worse than several other, smaller meta-analyses. Chang et al. estimated ICU mortality at 28.5% and ventilator mortality at 43%.8 A recent meta-analysis by Hasan demonstrated significant heterogeneity by region for mortality in over 10,000 COVID-19 patients with ARDS, averaging 39% overall (15–73%).9 We did find that outcomes appear to be improving later in the pandemic as opposed to earlier, echoing the findings of the recent meta-analysis by Armstrong et al.10 However, the high rates of poor ICU outcomes for those patients that require organ support remain concerning and our updated review would suggest that even with improved outcomes over time, overall ICU and organ support mortality are higher than expected. When a physician counsels a patient or their family member regarding prognosis it is imperative that we have current, high-quality data, and valid comparisons. This has important implications for patient–physician shared decision making, allocation of resources, and the extent of societal response to the pandemic.
The mortality of mechanically ventilated COVID-19 patients appears to be much higher than that of other ICU populations. Sepsis has an in-hospital mortality between 10% and 40% based on a Sequential Organ Failure Assessment score of 2 points or higher.11 Severe community-acquired pneumonia in a prospective cohort of 3,719 patients had a 30-day mortality of 33% in mechanically ventilated patients.12 The high mortality is likely due to a high proportion of patients who develop ARDS. Nearly 43% of patients who required ICU level of care had ARDS. An international multicenter observational study of 459 ICUs across 50 countries by Bellani et al. determined that ARDS represented 10.4% (95% CI, 10.0–10.7%) of ICU admissions with 40% mortality.13 Regardless of the cause, mortality among those requiring MV in COVID-19 is clearly high when compared to historical metrics obtained outside of epidemic or pandemic circumstances.
Prior to COVID-19, there have been 3 recent global viral pneumonia outbreaks: severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, Influenza A H1N1 (2009), and most recently Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in 2012. Between 2002 and 2004, SARS-CoV resulted in over 8,000 admissions (20% developed ARDS) with a CFR exceeding 9%.14,15 Intensive care unit mortality for SARS-CoV was reportedly between 35% and 43%. Among those requiring MV, mortality was reported to be between 52% and64%.14–21 Wide annual vaccinations and existing anti-viral medications have somewhat mitigated the impact of seasonal influenza on morbidity and mortality.22 The 2009 Influenza A H1N1 pandemic led to ICU admission in 9-31% of adults and mortality of 14-27% among the critically ill with rates as high as 42% for patients requiring MV.15,23–28 For MERS-CoV, ICU mortality rates have been reported to be between 58% and 90% and 72% and 75% among those requiring MV.15,29 Certainly, the global mortality implications for COVID-19 are direr given the drastic increase in range. The World Health Organization has documented 854 deaths due to MERS-CoV since 2012. Coronavirus disease 2019 by contrast continues to spread with nearly 6.2 million deaths globally since January 2020. Clinical outcomes in a pandemic are affected by variables beyond pathogenicity, patient risk, and illness severity. Patients treated in the first wave of a pandemic may have worse outcomes due to supply–demand mismatch for intensive care. The current pandemic has exposed limitations for critical care disaster management at large tertiary care centers in first-world nations. Comparing critical care outcomes between illnesses occurring in usual care settings and pandemics or other disasters may fail to account for these factors. Our study adds to the literature by providing updated evidence that COVID-19 ICU outcomes are improving as time progresses, resource shortages improve, and we gain increased experience with this illness.
RRT has a substantial impact on resource utilization and risk to the patient and exposure risk for providers. The need for RRT in patients with respiratory failure portends worsened outcomes with progression to multi-organ failure and death.30 Among critically ill COVID-19 populations, the need for RRT has been documented in 15-58% of patients. The need for RRT is significantly greater among patients that died compared to survivors (53% versus 1%).31,32 One recent study by Eriksson et al. demonstrated a 90-day mortality of 45% and ICU mortality of 39% for a population of critically ill COVID-19 patients that required RRT.33 All the patients in this study were treated with MV. In our series, mortality in patients requiring RRT approached 60%. Although not specified in the studies we evaluated, our assumption from the clinical experience was that RRT rates reflected those patients that were treated with MV and experienced worsening of their critical illness requiring additional support with RRT. The higher mortality for RRT is consistent with that seen for other ARDS populations.
Interventions like ECMO generally require transfer to a specialty center and the impact on resource utilization and provider exposure risk is immense. These patients are often the most severely ill and have proven refractory to other advanced interventions for ARDS. Interestingly, our data found that ECMO mortality was lower than that of patients in the ICU requiring RRT. Data regarding the impact of ECMO on critically ill patients with COVID-19 are also evolving at a rapid pace. Earlier data suggested poor outcomes in the majority of cases, approaching 83% mortality.34–36 More recently, outcomes look better and may mirror outcomes for other patient populations that are placed on ECMO.37 The improved outcomes with ECMO found in our systematic review and meta-analysis may have a number of explanations. Patients that are treated with ECMO are a select population and the criteria for receiving ECMO have been dynamic over the course of the pandemic and vary by local practice and resources. These patients must have proven refractory to other interventions for ARDS, but also be considered good enough candidates physiologically to benefit and survive following ECMO. Several multicenter analyses of ECMO outcomes in COVID-19 have demonstrated mortality rates similar to what we found in our study (40–45%). Although we did not evaluate outcomes in our study populations based on age, some of the literature has demonstrated that as expected, outcomes are improved in younger patients (30%) with worse mortality seen in older patients (50–75%).36,38 A full discussion on the factors influencing ECMO as a treatment in COVID-19 is beyond the scope of this manuscript but worthy of further study.
Our review has several limitations. First, it only analyzed published data as reported in selected manuscripts. Including those studies with patients still in the hospital likely drove the mean mortality rates lower than they might actually have been if all patients were followed through discharge from the hospital. Hence, our outcomes associated with RRT and ECMO were likely underreported. Second, we limited our review to English language studies. While this choice may have limited the scope of the data uncovered during this global pandemic, the articles we reviewed represented a wide geographic scope.
CONCLUSION
Coronavirus disease 2019 critical care outcomes have significantly improved since the start of the pandemic. Ventilator mortality is high, approaching 70% in patients admitted before April 30, 2020, and significantly improved, approaching 40%, in patients admitted after April 30, 2020. Intensive care unit outcomes should be evaluated contextually in terms of study quality, data completeness, and time for the most accurate reporting and to effectively guide mortality predictions. Interventions that avoid, delay, or decrease the duration of organ support may represent a target of opportunity to improve outcomes.
Supplementary Material
Contributor Information
Sahar Leazer, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA; The Metis Foundation, San Antonio, TX 78216, USA.
Jacob Collen, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Karl Alcover, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Erin Tompkins, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Shiva Ambardar, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Rhonda J Allard, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Brian Foster, Walter Reed National Military Medical Center, Bethesda, MD 20814, USA.
Ryan McNutt, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Matthew Leon, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Zachary Haynes, Walter Reed National Military Medical Center, Bethesda, MD 20814, USA.
Makala Bascome, Walter Reed National Military Medical Center, Bethesda, MD 20814, USA.
Matthias Williams, Walter Reed National Military Medical Center, Bethesda, MD 20814, USA.
Jessica Bunin, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Patrick G O’Malley, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Lisa K Moores, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
Kevin K Chung, Department of Medicine, Uniformed Services University of Health Sciences, Bethesda, MD 20814, USA.
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL is available at Military Medicine online.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
The authors have no potential financial or ethical conflicts of interest regarding the contents of this submission.
AUTHOR CONTRIBUTIONS
Dr Leazer and Dr Collen were responsible for guiding article selection, data extraction, data analysis, and writing the manuscript’s first draft and subsequent edits and revisions. Dr Alcover performed data analysis and assisted in writing methods and results sections. He also provided relevant figures and tables. Ms Allard provided expertise from the medical library and set up the COVIDENCE database. Mr McNutt and Mr Leon screened all of the study abstracts and assisted with data abstraction from the full-text articles. Dr Foster, Dr Williams, Dr Bascome, Dr Haynes, Dr Tompkins, and Mr Ambardar all participated in data abstraction and quality assessment. Dr Tompkins assisted with submission to PROSPERO. Mr Shiva Ambardar performed literature reviews and assisted in structuring the manuscript. Dr Bunin, Dr Tompkins, Dr Collen, Dr Moores, and Dr O’Malley all participated in manuscript edits and revisions. Dr Chung directed the research project from inception to completion, including the initial literature search, endpoints, data analysis, manuscript edits, and revision.
REFERENCES
- 1. Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA 2020; 323(13): 1239–42. [DOI] [PubMed] [Google Scholar]
- 2. Grasselli G, Pesenti A, Cecconi M: Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323(16): 1545–6. [DOI] [PubMed] [Google Scholar]
- 3. Grasselli G, Zangrillo A, Zanella A, et al. : Baseline characteristics and outcomes of 1,591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Jama 2020; 323(16): 1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Safiya R, Jamie SH, Mangala N, et al. : Presenting characteristics, comorbidities, and outcomes among 5,700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323(20): 2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tompkins E, Leazer S, Foster B, et al. : Outcomes associated with intensive care and mechanical ventilator support among patients with COVID-19: a systematic review. PROSPERO 2020 CRD42020180607. Available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=180607; accessed May 25, 2021.
- 6. Wells GA, Shea B, O’Connell D, et al. : The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp; accessed May 25, 2021.
- 7. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. : Case fatality rates for COVID-19 patients requiring invasive mechanical ventilation: a meta-analysis. Am J Respir Crit Care Med 2021; 203(1): 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang R, Elhusseiny KM, Yeh YC, et al. : COVID-19 ICU and mechanical ventilation patient characteristics and outcomes. A systematic review and meta-analysis. PLoS One 2021; 16(2): e0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasan SS, Capstick T, Ahmed R, et al. : Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med 2020; 14(11): 1149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstrong RA, Kane AD, Cook TM: Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia 2020; 75(10): 1340–9. [DOI] [PubMed] [Google Scholar]
- 11. Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016; 315(8): 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrer M, Travierso C, Cilloniz C, et al. : Severe community-acquired pneumonia: characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One 2018; 13(1): e0191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellani G, Laffey JG, Pham T, et al. : Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. JAMA 2016; 315(8): 788–800. [DOI] [PubMed] [Google Scholar]
- 14. ECDC : Severe acute respiratory syndrome (SARS). 2016. Available at https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2015-SARS.pdf; accessed April 15, 2021.
- 15. Shah RD, Wunderink RG: Viral pneumonia and acute respiratory distress syndrome. Clin Chest Med 2017; 38(1): 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Booth CM, Stewart TE: Severe acute respiratory syndrome and critical care medicine: the Toronto experience. Crit Care Med 2005; 33(1 Suppl): S53–60. [DOI] [PubMed] [Google Scholar]
- 17. Chen CY, Lee CH, Liu CY, et al. : Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc 2005; 68(1): 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fowler RA, Lapinsky SE, Hallett D, et al. : Critically ill patients with severe acute respiratory syndrome. JAMA 2003; 290(3): 367–73. [DOI] [PubMed] [Google Scholar]
- 19. Hui DS, Memish ZA, Zumla A: Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med 2014; 20(3): 233–41. [DOI] [PubMed] [Google Scholar]
- 20. Lien TC, Sung CS, Lee CH, et al. : Characteristic features and outcomes of severe acute respiratory syndrome found in severe acute respiratory syndrome intensive care unit patients. J Crit Care 2008; 23(4): 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang JT, Sheng WH, Fang CT, et al. : Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 2004; 10(5): 818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jain S, Kamimoto L, Bramley AM, et al. : Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 2009; 361(20): 1935–44. [DOI] [PubMed] [Google Scholar]
- 23. Cantan B, Luyt CE, Martin-Loeches I: Influenza infections and emergent viral infections in intensive care unit. Semin Respir Crit Care Med 2019; 40(4): 488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delaney JW, Fowler RA: 2009 influenza A (H1N1): a clinical review. Hosp Pract (1995) 2010; 38(2): 74–81. [PubMed] [Google Scholar]
- 25. Duggal A, Pinto R, Rubenfeld G, et al. : Global variability in reported mortality for critical illness during the 2009-10 influenza A(H1N1) pandemic: a systematic review and meta-regression to guide reporting of outcomes during disease outbreaks. PLoS One 2016; 11(5): e0155044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dwyer DE, Lynfield R, Losso MH, et al. : Comparison of the outcomes of individuals with medically attended influenza A and B Virus infections enrolled in 2 international cohort studies over a 6-year period: 2009–2015. Open Forum Infect Dis 2017; 4(4): ofx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar A, Zarychanski R, Pinto R, et al. : Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302(17): 1872–9. [DOI] [PubMed] [Google Scholar]
- 28. Sarda C, Palma P, Rello J: Severe influenza: overview in critically ill patients. Curr Opin Crit Care 2019; 25(5): 449–57. [DOI] [PubMed] [Google Scholar]
- 29. ECDC : Risk assessment guidelines for infectious diseases transmitted on aircraft (RAGIDA) Middle East Respiratory Syndrome Coronavirus (MERS-CoV). 2020. Available at https://www.ecdc.europa.eu/en/publications-data/risk-assessment-guidelines-infectious-diseases-transmitted-aircraft-ragida-middle; accessed May 15, 2021.
- 30. Gasparini M, Khan S, Patel JM: Renal impairment and its impact on clinical outcomes in patients who are critically ill with COVID-19: a multicentre observational study. Anaesthesia 2021; 76(3): 320–6.doi: 10.1111/anae.15293 [DOI] [PubMed] [Google Scholar]
- 31. Yang X, Jin Y, Li R, et al. : Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care 2020; 24(1): 356.doi: 10.1186/s13054-020-03065-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wright SE, Bodenham A, Short AIK, et al. : The provision and practice of renal replacement therapy on adult intensive care units in the United Kingdom. Anaesthesia 2003; 58(11): 1063–9.doi: 10.1046/j.1365-2044.2003.03449.x [DOI] [PubMed] [Google Scholar]
- 33. Eriksson KE, Campoccia-Jalde F, Rysz S, et al. : Continuous renal replacement therapy in intensive care patients with COVID-19; survival and renal recovery. J Crit Care 2021; 64: 125–30.doi: 10.1016/j.jcrc.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang X, Yu Y, Xu J, et al. : Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8(5): 475–81.doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henry BM: COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med 2020; 8(4): e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacobs EP, Stammers AH, St Louis J, et al. : Multi-institutional analysis of 100 consecutive patients with COVID-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J 2021; 67(5): 496–502.doi: 10.1097/MAT.0000000000001434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raasveld SJ, Delnoij TSR, Broman LM, et al. : Extracorporeal membrane oxygenation in patients with COVID-19: an international multicenter cohort study. J Intensive Care Med 2021; 36(8): 910–7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen NT, Sullivan B, Sagebin F, et al. : Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at US academic centers. Ann Surg 2021; 274(1): 40–4.doi: 10.1097/SLA.0000000000004870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


