Abstract
Aims
Multisystem inflammatory syndrome in children (MIS-C) with cardiovascular manifestations are frequent. However, there is lacking evidence regarding cardiological follow-up of this cohort of patients. The aim of our study was to describe the early and mid-term cardiac abnormalities assessed by standard and speckle-tracking echocardiography (STE), and cardiac MRI (CMR).
Methods and results
We enrolled 32 patients (21 male, 11 female), mean age 8.25 ± 4years, with diagnosis of MIS-C. During admission, all children underwent TTE, STE with analysis of left ventricle global longitudinal strain (GLS) and CMR. Patients underwent cardiological evaluation at 2 (T1) and 6 months (T2) after discharge. Cardiac MRI was repeated at 6 months after discharge. Mean left ventricular ejection fraction (LVEF) at baseline was 58.8 ± 10% with 10 patients (31%) below 55%. Speckle-tracking echocardiography showed reduced mean LV GLS (−17.4 ± 4%). On CMR, late gadolinium enhancement (LGE) with non-ischaemic pattern was evident in 8 of 23 patients (35%). Follow-up data showed rapid improvement of LVEF at T1 (62.5 ± 7.5 vs. 58.8 ± 10.6%, P-value 0.044) with only three patients (10%) below ≤ 55% at T1. Left ventricular (LV) GLS remained impaired at T1 (−17.2 ± 2.7 vs.−17.4 ± 4, P-value 0.71) and significantly improved at T2 (−19 ± 2.6% vs. −17.4 ± 4%, P-value 0.009). LV GLS was impaired (>−18%) in 53% of patients at baseline and T1, whereas only 13% showed persistent LV GLS reduction at T2. Follow-up CMR showed LGE persistence in 33.4% of cases.
Conclusion
Early cardiac involvement significantly improves during follow-up of MIS-C patients. However, subclinical myocardial dysfunction seems to be still detectable after 6 months of follow-up in a not negligible proportion of them.
Keywords: multisystem inflammatory syndrome in children, COVID-19, speckle-tracking echocardiography, longitudinal strain, cardiac magnetic resonance, myocardial injury
Introduction
During the early phase of the COVID-19 pandemic, children were considered relatively spared from severe manifestations of SARS-CoV-2 infection compared with adults.1–3 However, starting from mid-April 2020, multisystem inflammatory syndrome in children (MIS-C) has emerged as a major cause of morbidity in the paediatric population, previously exposed to SARS-CoV2 2–6 weeks earlier.4,5
The characteristic features of the disease are fever, gastrointestinal symptoms, muco-cutaneous inflammation signs, myocardial and coronary artery involvement. Interestingly, this multisystemic illness shares features with other paediatric inflammatory conditions, such as Kawasaki disease (KD), bacterial sepsis, toxic shock syndrome and macrophage activation syndrome.6 However, haemodynamic shock, myocardial dysfunction, and gastrointestinal involvement are peculiar findings of this unique syndrome.7,8
Cardiovascular manifestations in MIS-C are common, occurring in 34–82% of cases.9 They include myocardial dysfunction, coronary artery dilatation or aneurysms, conduction abnormalities, arrhythmias, pericarditis, and valvulitis. Severe cases can present with distributive or cardiogenic shock requiring fluid resuscitation and inotropic support.10,11 Among the others, left ventricular (LV) systolic dysfunction is the most frequent cardiac abnormal finding. Recent evidence support a rapid improvement of myocardial dysfunction after the acute event and that it may not be detected on cardiac MRI few weeks after symptoms onset. These data suggest an immune-mediated pathogenesis of MIS-C.12,13
Multimodality and specific cardiac imaging techniques have highlighted subclinical myocardial dysfunction in a significant proportion of MIS-C patients.14,15 However, there is lacking evidence regarding mid-term cardiological follow-up of this cohort of patients. This issue is pivotal to establish the reversibility of such alterations and even more in view of providing advice for sports participation after recovery.
Therefore, the aims of our study were as follows: (i) to evaluate early cardiac involvement in a cohort of patients in the acute phase of MIS-C with two advanced cardiovascular imaging techniques, speckle-tracking echocardiography (STE), and cardiac MRI. (ii) To assess the evolution of cardiac function and myocardial disfunction at 3 and 6 months after diagnosis.
Methods
Study design and population
This is a retrospective, cohort and single-center study performed at the Department of Women's and Children's Health of Padua University Hospital, Italy.
We enrolled patients with MIS-C diagnosis from 26 April 2020 to 2 October 2021. The diagnosis of MIS-C was made according to WHO criteria.4 On admission, clinical, laboratory, and microbiological data were measured. All subjects were tested for SARS-CoV-2 infection by reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal swab on admission. Serological assay included IgG detection, targeting a recombinant nucleocapsid (N)-spike (S) protein of SARS-CoV-2 (IgG cut-off 1.1 AU/mL).
All patients underwent routinely blood tests including blood cell counts, biochemical profile, and markers of systemic inflammation (C-reactive protein—CRP, erythrocyte sedimentation rate—ESR, procalcitonin, ferritin, D-dimer, fibrinogen, LDH). Troponin I (TnI, normal value <34 ng/L) and brain natriuretic peptide (BNP, normal value <100 ng/L) were measured on admission and serially during hospital stay.
Admission to the paediatric intensive care unit was assessed according to the patient's haemodynamic status at the time of presentation in the emergency department. Pharmacologic treatment was established following the American College of Rheumatology guidelines for paediatric patients with MIS-C.16 All patients received a course of intravenous immunoglobulin (IVIG), intravenous corticosteroids (methylprednisolone), and antiplatelet therapy (aspirin). The use of biologic agents, such as Anakinra, an interleukin-1 receptor inhibitor, was reserved for patients with severe/critical disease refractory to standard therapy. One patient, with giant coronary aneurysms, coronary thrombosis, and myocardial ischaemia, also required thrombolytic therapy with Alteplase (t-PA), anticoagulant therapy, and dual antiplatelet therapy.
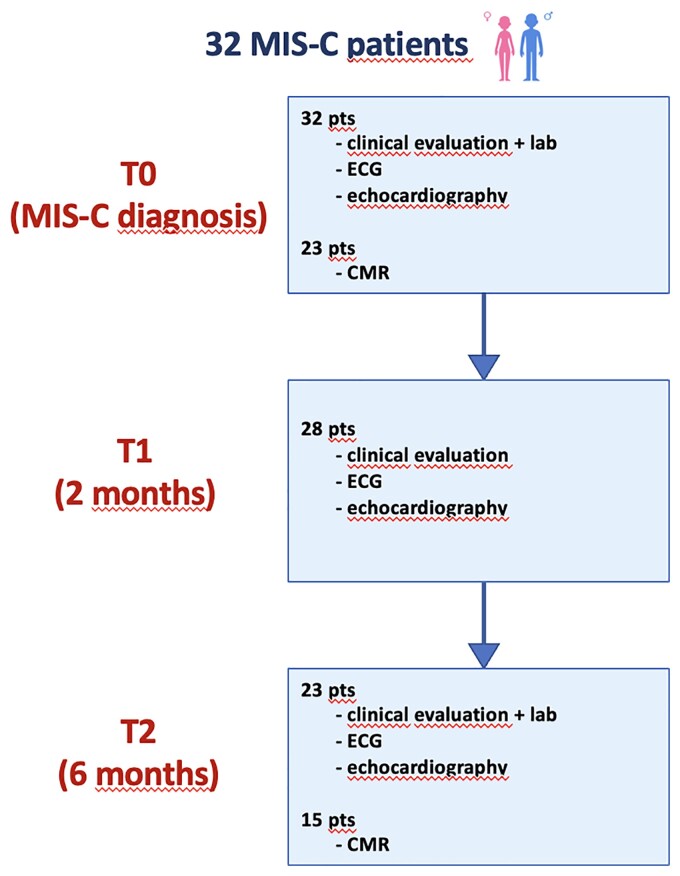
All patients underwent regular follow-up at 2 and 6 months after discharge in the paediatric cardiology outpatient clinic department, Padua University Hospital, Italy. During the follow-up evaluation, patients underwent repeated clinical evaluation, electrocardiogram (ECG), standard and STE. Cardiac MRI (CMR) was performed only at 6-month time (Figure 1). Moreover, depending on patient's clinical status and blood tests, immunologic and antiplatelet therapy was modified accordingly and the need for further investigations was assessed.
Figure 1.
Study timeline.
Echocardiography
During the first day of hospitalization, all children underwent full cardiac evaluation, including ECG and transthoracic echocardiogram (TTE). During follow-up, cardiological evaluation with ECG and transthoracic echocardiography was performed 2 and 6 months after MIS-C diagnosis.
Standard 2D echocardiography was made by experienced paediatric echocardiographers using GE Vivid E9 (GE Healthcare, Chicago—IL, USA) and EpiQ 5 (Philips Medical Systems, Bothell—WA, USA) devices, following the recommendations for cardiovascular imaging during pandemic COVID- 19.17,18 Standard echocardiographic parameters analyzed included: left ventricular ejection fraction (LVEF) by Simpson biplane method, early and late peak mitral inflow velocity by Power Doppler (E/A), right ventricular function estimation by Fractional Area Change (RV FAC), tricuspid annular plane systolic excursion (TAPSE) by M-mode, and early mitral annular lateral and septal diastolic peak velocity by TDI (E/e’ lateral and medial). Coronary arteries were also evaluated following the American Heart Association (AHA) guidelines for KD.19
Longitudinal strain (LS) analysis of the left ventricle, by STE, was performed offline using GE EchoPac Software (GE Healthcare, USA).20 Specifically, the best apical four-chamber, two-chamber, and three-chamber projections were selected for visualization of all segments of the left ventricle. Subsequently, by identifying 3 points (2 annular and 1 apical), the software is allowed to semi-automatically plot the myocardial profile during the cardiac cycle. The region of interest is adjusted by careful inspection of the endocardial boundary, and manual correction was performed when necessary. Finally, the automatic algorithm allows calculation of global longitudinal strain (GLS). Left ventricular longitudinal strain, calculated by speckle-tracking, is defined as the mean peak-negative value on the strain curve during systole (end of T wave on the ECG) of all segments studied.21,22 The peak-negative value of systolic strain for each regional segment of the left ventricle was also analyzed. Normal mean LV GLS values were considered as more negative than -18% according to recent meta-analysis by Levy et al.23 on paediatric population. This is in agreement with our experience on normal healthy children. Analysis of the standard TTE and STE was performed by two experienced sonographers blinded to the clinical data.
Cardiac MRI
Twenty-three patients underwent cardiac magnetic resonance imaging (CMR) during the acute phase of the disease. During the follow-up, 15 (65%) of these patients were referred for repeated CMR at ∼6 months after MIS-C diagnosis.
The scan was performed with a 1.5-Tesla scanner (Philips Achieva, Philips Healthcare, Best, The Netherlands). Sedation with intravenous midazolam or ketamine was required for patients younger than 6 years old. cardiac MRI included cine SSFP and T2-weighted images with fat suppression. First-pass perfusion with intravenous administration of contrast agent (0,1 mmol/kg body weight gadoterate meglumine) was realized in all patients. Late gadolinium enhancement (LGE) sequences, in short axis and four-chambers planes, were acquired 10 min after contrast administration using a standard 2-dimensional breath-hold phase-sensitive inversion recovery sequence with the inversion time selected to null the myocardial signal. In all patients, LV and RV end-diastolic volume, end-systolic volume, and ejection fraction were measured. The CMR images were analyzed for detecting the presence of myocardial oedema and fibrosis using standard LV 17-segment model and specifying the pattern (transmural, subendocardial, subepicardial). To rule out coronary dilatation or aneurysm, 3D SSFP isovolumetric MRA ECG and navigator gating was performed. Other findings, such as pericardial or pleural effusion and the presence of coronary thrombosis, were reported. All the CMR studied were performed by the same experienced physician (E.R.).
Statistical analysis
Categorical variables were presented as percentage (%), and continuous variables as mean ± standard deviation. Shapiro–Wilk test and histogram were used to test normality for each variable. Student’s t-test was performed for normally distributed continuous variables and Mann–Whitney U test for non-parametric continuous variables. χ2 test or Fisher’s exact test were performed for categorical variables to examine if there were significant differences between the groups. Wilcoxon's test was used to compare continuous variables of two non-independent samples from the same population. Linear mixed model analysis with random effects was used to evaluate changes in variables measured multiple times over time. Statistical analysis was performed using SPSS 21.0 (IBM, NY, USA).
Results
Thirty-two patients (11 females and 21 males) with confirmed MIS-C diagnosis were enrolled (Table 1). Mean age at diagnosis was 8.25 ± 4 years, mean BSA 1.08 ± 0.37m2. The majority was of Caucasian origin (75%), the remaining had African (18.8%) or Asian (6.2%) ethnicity. Twenty-seven patients (84.4%) had serologic confirmation of previous SARS-CoV-2 infection, and most had no documented underlying disease. Thirteen children (40.6%) shared KD-like symptoms.
Table 1.
Clinical characteristics of the patients
| MIS-C n = 32 | |
|---|---|
| Age at onset (years) | 8.25 ± 4 |
| Male | 21 (65.6) |
| Comorbidity | 3 (9.4) |
| Associated CHD | 1 bicuspid aortic valve |
| positive NF SARS-CoV-2 swab | 4 (12.5) |
| positive SARS-CoV-2 IgG title | 27 (84.4) |
| Clinical manifestations | |
| Fever | 32 (100) |
| GI | 27 (84.4) |
| Muco-cutaneous | 22 (68.8) |
| Neurological | 7 (22) |
| Hypotension | 9 (28.1) |
| Sinus Bradicardia | 9 (28.1) |
| KD-like | 13 (40.6) |
| ICU admission | 5 (15.6) |
| Laboratory | |
| TnI ≤ 34 ng/L TnI >34 ng/L |
12 (37.5) 20 (62.5) |
| BNP (pg/mL) | 612 ± 859 |
| CRP (mg/L) | 162 ± 91.4 |
| PCT (ng/mL) | 12.3 ± 86 |
| ESR (mm/h) | 54.5 ± 36.5 |
| Ferritin (mcg/L) | 922 ± 837 |
| D-dimer (mcg/mL) | 2069.4 ± 2484 |
| LDH (U/L) | 385 ± 255 |
| Treatment | |
| IV steroids | 32 (100) |
| IGIV | 32 (100) |
| Aspirin | 31 (97) |
| Anakinra | 3 (9,4) |
| Other | 4 (12,5) |
Values are numbers (%) or mean ± standard deviation.
CHD, congenital heart disease, BNP, brain natriuretic peptide; CRP, C-reactive protein; ERS, erythrocyte sedimentation rate); GI, gastrointestinal symptoms; ICU, intensive care unit; IV, intravenous; IVIG, intravenous immunoglobulins; KD, Kawasaki disease; LDH, lactic dehydrogenase; PCT, procalcitonin; NP swab, naso-pharingeal swab; TnI, troponin I; WBC, white blood count.
All patients (100%) presented with fever. The most involved organs were the gastrointestinal system (84.4%) and the muco-cutaneous system (68.8%). Multisystem involvement, treatment, and laboratory findings are summarized in Table 1. In five cases (15.6%), ICU admission was needed, and four of them required inotropic support. Regarding cardiovascular symptoms, nine patients (28.1%) presented hypotension and nine (28.1%) sinus bradycardia. In-hospital survival was 100%. All patients showed a hyperinflammatory state characterized by elevation of PCR, ESR, and D-Dimer. Troponin I was abnormal (>34 ng/L) in 20 children (62.5%). Brain natriuretic peptide was significantly elevated in 28 (87.5%) patients (mean value 612 ± 859 pg/mL).
ECG abnormalities were reported in 50% of cases, of which 10 patients with abnormalities of the repolarization phase showing negative T waves in eight patients, mostly in the lateral and inferior leads, and ST segment depression in two patients in the lateral leads. Nine patients (28.1%) presented sinus bradycardia, whereas three (9.4%) showed atrioventricular block (first-degree block in two cases and second degree Mobitz 1 in the other).
All patients underwent echocardiographic evaluation with a median time of 7 days since fever onset and 1 day since hospital admission. Mean left ventricular ejection fraction (LVEF) was 58.8 ± 10.6%, and 10 patients (31%) presented an LVEF below 55%. Mean right ventricular FAC and TAPSE were 43 ± 7% and 19 ± 4.8 mm, respectively. Coronary dilatation (Z-score >2) was detected in nine patients (28.1%), of which eight had mild-to-moderate dilatation (2< Z-score <4). One 4-year-old child showed severe left descending artery dilation (z-score +20) with giant aneurysm and coronary thrombosis leading to acute anterior and lateral myocardial infarction and acute heart failure necessitating thrombolysis with t-PA, anticoagulant therapy, and adjunctive antiplatelet drug. Pericardial effusion was described in nine patients (28.1%).
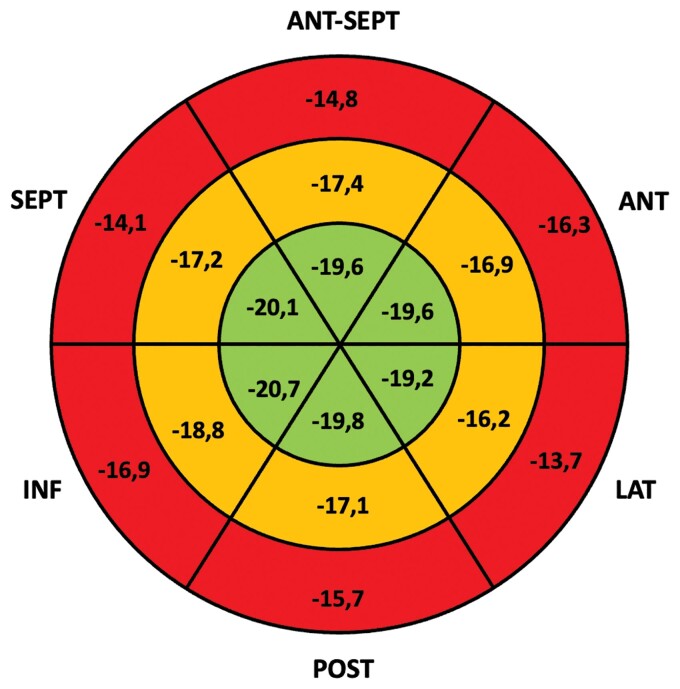
Speckle-tracking echocardiography analysis showed impaired LV GLS higher than −18% in 53% of patients with a mean LV GLS in all the cohort of −17.4 ± 4%. Basal and mid LV segments were the most affected (Figure 2).
Figure 2.
LV GLS bull’s eye. The bull's eye shows mean values of longitudinal strain (%) for each segment of the left ventricle. Basal segments (outer circle in red) presented more impaired deformation compared with mid (mid circle in orange) and apical segments (inner circle in green).
Median time to CMR was 18 days from fever onset and 11 days from hospital admission. Left ventricular ejection fraction on CMR was 60 ± 9.8% and four patients showed LVEF ≤ 55%. Late gadolinium enhancement with non-ischaemic pattern was detected in 8 of 23 (35%), and all of them had the imaging performed within 19 days from the symptom’s onset. Pericardial effusion was observed in five patients (21.7%). Myocardial oedema was detected in three patients (13%): mild oedema in two cases, who underwent examination in the first 13 days of disease, whereas in the patient with acute myocardial infarction secondary to coronary thrombosis, the oedema involved the middle anterior wall, septum, and apex. The acute cardiac involvement data in MIS-C are summarized in Table 2.
Table 2.
Early abnormal cardiac finding in MIS-C
| MIS-C (n = 32) | |
|---|---|
| ECG | |
| ECG abnormalities | 16 (50) |
| Ripolarization abnormalities | 10 (31.2) |
| Sinus bradycardia | 9 (28.1) |
| AV block | 3 (9.4) |
| Echocardiography | |
| LVEF (%) | 58.8 ± 10.6 |
| GLS (%) | −17.4 ± 4 |
| E/A ratio | 1.9 ± 0.6 |
| Deceleration time (msec) | 151 ± 39 |
| Average E/e’ ratio | 7.8 ± 1.9 |
| TAPSE (mm) | 19 ± 4.8 |
| RV FAC (%) | 43 ± 7 |
| coronary dilatation | 9 (28.1) |
| coronary thrombosis | 1 (3) |
| pericardial effusion | 9 (28.1) |
| Cardiac MRI (n = 23/32) | |
| LVEF | 60 ± 9.8 |
| LV oedema | 3 (13) |
| LV LGE | 8 (35) |
| RVEF | 62 ± 6 |
Values are numbers (%) or mean ± standard deviation.
AV block, atrioventricular block; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; LV LGE, left ventricular late gadolinium enhancement; MIS-C, multisystem inflammatory syndrome in children; RV FAC, right ventricular fractional area change; RVEF, right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
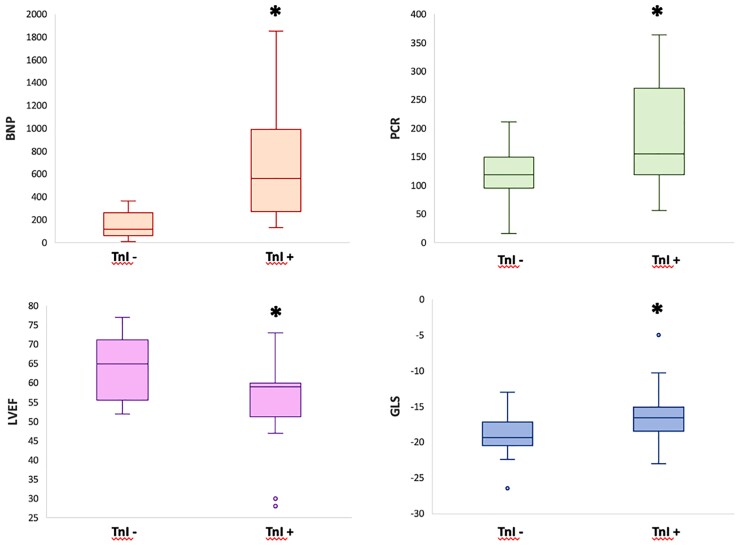
Patients with elevated TnI levels (>34 ng/L) showed significantly higher levels of circulating BNP compared with patients with normal TnI levels (891 ± 988 vs. 146 ± 120, P-value 0.03). Patients with higher TnI did not show significant differences in SARS-CoV-2 IgG titles, nor in inflammatory markers (ESR, D-Dimer, Ferritin), except for CRP (188 ± 100 vs. 118 ± 53, P = 0.016) and white blood cell count (10332 ± 4079 vs. 7471 ± 2503, P-value 0.02). On echocardiography, patients with higher levels of TnI showed significantly lower LVEF (55 ± 10 vs. 64 ± 8%, P-value 0.032) and impaired LV GLS (−16.3 ± 4.2 vs. −19.3 ± 3.2%, P-value 0.02), whereas there was no difference in right ventricular systolic functional parameters (TAPSE: 19.5 ± 5.3 vs. 18.1 ± 4 mm, P-value 0.44; RV FAC: 44.8 ± 7.7 vs. 40.3 ± 6%, P-value 0.15), or the presence of coronary dilatation (X2 = 0.09; P = 0.76). In addition, the two groups showed comparable LGE on CMR (X2 = 0.82; P = 0.36) (Table 3, Figure 3).
Table 3.
Cardiac findings in patient with or without myocardial injury
| TnI+(n = 20) | TnI-(n = 12) | P-value | |
|---|---|---|---|
| BNP (pc/mL) | 891 ± 988 | 146 ± 120 | 0.03* |
| PCR (mg/L) | 188 ± 100 | 118 ± 53 | 0.016* |
| PCT (ng/mL) | 48.8 ± 103 | 19.8 ± 36 | 0.22 |
| VES (mm/h) | 56 ± 35 | 51 ± 40 | 0.73 |
| D-dimer (mcg/mL) | 1827 ± 2717 | 2473 ± 2085 | 0.19 |
| SARS-CoV-2 Ig title (kAU/L) | 11.5 ± 28.1 | 10.3 ± 8.6 | 0.12 |
| LVEF echo (%) | 55 ± 10 | 64 ± 8 | 0.032* |
| GLS (%) | −16.3 ± 4.2 | −19.3 ± 3.2 | 0.02* |
| E/A | 2 ± 0.66 | 1.7 ± 0.57 | 0.14 |
| E/e’ average | 8.1 ± 1.7 | 7.5 ± 2.2 | 0.42 |
| TAPSE (mm) | 19.5 ± 5.3 | 18.1 ± 4 | 0.44 |
| RV FAC (%) | 44.8 ± 7.7 | 40.3 ± 6 | 0.15 |
| Coronary dilatation | 6 (30) | 3 (25) | 0.76 (X2 0.09, p 1 FET) |
| Pericardial effusion | 4 (20) | 5 (42) | 0.18 (X2 1.7) |
| CMR (n = 23) | TnI+(n = 17) | TnI-(n = 6) | P-value |
| CMR LVEF (%) | 59.4 ± 11.4 | 64 ± 1.3 | 0.38 |
| CMR LV LGE | 5 (30) | 3 (50) | 0.36 (X2 0.82, p 0.62 FET) |
Values are numbers (%) or mean ± standard deviation.
BNP, brain natriuretic peptide; CRP, C-reactive protein; ERS, erythrocyte sedimentation rate); GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; LV LGE, left ventricular late gadolinium enhancement; MIS-C, multisystem inflammatory syndrome in children; PCT, procalcitonin; RV FAC, right ventricular fractional area change; RVEF, right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; TnI, troponin I. * = P < 0.05.
Figure 3.
MIS-C and myocardial involvement. Patients with elevated TnI levels (>34 ng/L) showed higher levels of BNP and CRP, reduced LVEF, and higher LV GLS compared with patients with normal values of TnI. * = P < 0.05.
Follow-up study
Twenty-eight patients underwent T1 follow-up 2 months after diagnosis with median time of 48.5 ± 17 days. Two patients (7%) were still taking cardiovascular therapy, antiarrhythmic (beta-blocker) in one case, and heart failure therapy (ACE inhibitor, beta-blocker, spironolactone) in the other. Electrocardiogram abnormalities were reported in five patients (18%); only one patient (3.5%) presented first-degree atrioventricular block. Standard echocardiography showed mean LVEF of 62.5 ± 7.5% with three patients (10%) below 55%. TAPSE was 19.7 ± 3.2 mm and mean right ventricular FAC 42 ± 5.5%. Coronary dilatation persisted in only 2 of the 28 patients (7.2%), in one case of mild degree (Z-score +2.2). None presented pericardial effusion.
Overall, on T1 assessment our cohort of patients showed mean left ventricular GLS values of −17.2 ± 2.7%, persistently reduced compared with basal assessment. Fifty-three percent of patients presented an abnormal LV GLS value higher than −18%. Consistently with baseline evaluation, basal segments of left ventricle were more affected by GLS impairment.
Subsequent cardiological follow-up (T2) was performed in 23 patients with a median time of 207 days from MIS-C diagnosis. Five patients (21.7%) had ECG abnormalities: three cases showed abnormal ventricular repolarization, two cases showed interventricular conduction delays, and in one case the first-grade atrioventricular block was confirmed. Standard echocardiography showed a mean LVEF of 65.7 ± 8.2%, with one patient (4%) below 55%, and a mean TAPSE of 21.1 ± 3.6 mm. No patient presented pericardial effusion, whereas coronary dilatation persisted in two patients. The analysis of STE images displayed normalization of mean GLS of the left ventricle in most of the cohort (87%). The mean LV GLS value of our cohort at 6 months was in the normal range, −19 ± 2.6%. Follow-up echocardiographic data are summarized in Table 4.
Table 4.
Cardiac findings in MIS-C follow-up
| T1 (n = 28) | |
|---|---|
| Median time (days) | 48.5 |
| ECG T1 | |
| ECG abnormalities | 5 (18) |
| Ripolarization abnormalities | 3 (10.7) |
| AV block | 1 (3.5) |
| Echocardiography T1 | |
| VEF (%) | 62.5 ± 7.5 |
| GLS (%) | −17.2 ± 2.7 |
| E/A ratio | 1.8 ± 0.4 |
| TAPSE (mm) | 19.7 ± 3.2 |
| RV FAC (%) | 42 ± 5.5 |
| coronary dilatation | 2 (7.2) |
| pericardial effusion | 0 (0) |
| T2 (n = 23) | |
| Median time (days) | 207 |
| ECG T2 | |
| ECG abnormalities | 5 (21,7) |
| Ripolarization abnormalities | 3 (13) |
| AV block | 1 (4.3) |
| Echocardiography T2 | |
| LVEF (%) | 65.7 ± 8.2 |
| GLS (%) | −19 ± 2.6 |
| TAPSE (mm) | 1.9 ± 0.5 |
| RV FAC (%) | 21.1 ± 3.6 |
| Coronary dilatation | 2 (8,7) |
| Pericardial effusion | 0 (0) |
Values are numbers (%) or mean ± standard deviation.
AV block, atrioventricular block; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; MIS-C, multisystem inflammatory syndrome in children; RV FAC, right ventricular fractional area change; RVEF, right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
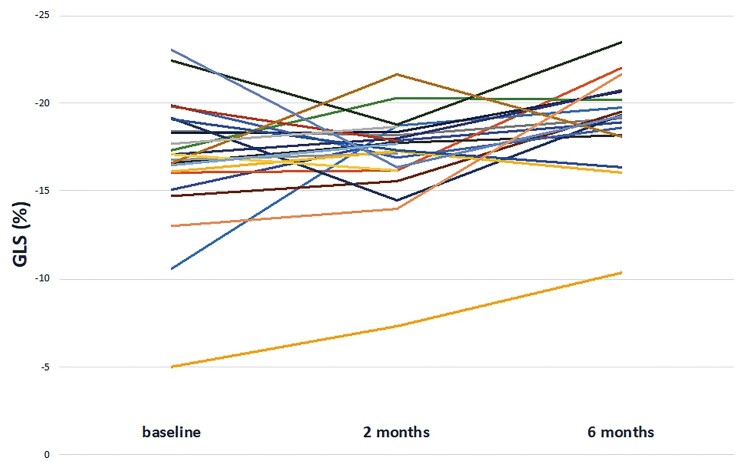
T2 follow-up data showed a statistically significant improvement in left ventricular systolic function parameters compared to disease onset (LVEF 65.7 ± 8.2 vs. 58.8 ± 10.6%, P-value < 0.001 and LV GLS −19 ± 2.6 vs. −17.4 ± 4%, P-value 0.009) (Figure 4). Furthermore, on mixed effect analysis LVEF and LV GLS improved significantly during follow-up (P < 0.001 and P = 0.048, respectively) with an estimated increase at each evaluation of 3.39% for EF and an estimated decrease of -0.81% regarding GLS. There was no correlation between the absolute improvement of LV systolic function and the basal levels of TnI or BNP. In particular, LVEF improved during the first 2 months of follow-up (58.8 ± 10.6 vs. 62.5 ± 7.5%, P-value 0.044), whereas LV GLS showed a significant improvement between 2 and 6 months after MIS-C onset (−17.2 ± 2.7 vs. −19 ± 2.6%, P-value 0.007). Particularly, at baseline and T1, 53% of patients (17/32 and 15/28, respectively) showed reduced LV GLS, whereas only 3 of 23 patients (13%) had impaired LV GLS at T2. There was no significant difference in right ventricular systolic functional parameters over time (Table 5).
Figure 4.
Distribution of GLS values during follow-up.
Table 5.
Distribution of ventricular functional parameters at baseline and during follow-up
| Baseline | 2 months | 6 months | P* | P † | P ‡ | |
|---|---|---|---|---|---|---|
| LVEF (%) | 58.8 ± 10.6 | 62.5 ± 7.5 | 65.7 ± 8.2 | 0.044 | 0.72 | <0.001 |
| LV GLS (%) | −17.4 ± 4 | −17.2 ± 2.7 | −19 ± 2.6 | 0.71 | 0.007 | 0.009 |
| TAPSE (mm) | 19 ± 4.8 | 19.7 ± 3.2 | 21.1 ± 3.6 | 0.37 | 0.09 | 0.24 |
Values are numbers (%) or mean ± standard deviation.
GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion. P* value between baseline and 2 months; P† value between 2 and 6 months; P‡ value between baseline and 6 months.
Follow-up CMR was performed in 15 patients at 6 months from MIS-C diagnosis. LVEF was 58.8 ± 8.8% comparable with baseline, and RVEF was 60.8 ± 5%. Of the eight patients presenting LGE on baseline CMR, six underwent a follow-up scan and five of them showed persistence of LGE with non-ischaemic pattern. However, LGE extension was reduced at quantitative analysis compared with the acute phase. Oedema was detected only in the patient with previous myocardial infarction secondary to anterior descending thrombosis. Small pericardial effusion was detected in four patients (26.7%).
Discussion
To the best of our knowledge, this is the first study to analyze and correlate echocardiographic and CMR combined data during the initial acute phase of MIS-C and later during follow-up.
We demonstrated that myocardial abnormalities revert in a large proportion of the studied patients with a different time course over follow-up. However, LGE detected by CMR at baseline persists in a significant proportion of patients after 6 months from MIS-C diagnosis.
Early phase
This study confirms our preliminary data in 23 patients regarding early phase cardiac involvement in MIS-C patients.14 Myocardial injury based on troponin elevation is hereby confirmed to affect about two-thirds (65%) of our cohort, and subclinical left ventricle systolic dysfunction estimated by a decrease in mean GLS values was persistently evident in a substantial proportion (53%) of our MIS-C population. Moreover, CMR performed early after diagnosis showed non-ischemic pattern of LGE in a third (34,7%) of evaluated patients, in agreement to our previous data.
Follow-up
Follow-up data from our population show an excellent clinical response to treatment with an overall survival of 100% and almost all patients asymptomatic at two and 6 months after MIS-C diagnosis. Only two children were on cardiovascular therapy at T1. Electrocardiogram abnormalities, present in 50% of cases in the acute phase, showed a gradual improvement, accounting for 20% of cases at 2 and 6 months. Noteworthy, our data show almost complete resolution of bradyarrhythmias during follow-up.
Echocardiographic monitoring of our cohort of MIS-C patients showed a statistically significant improvement in left ventricular systolic function parameters, LVEF and LV GLS, 6 months after disease diagnosis. Particularly, LVEF increased significantly during the first 2 months of follow-up, in contrast to LV GLS, which improvement was mainly evident in the second part of our follow-up. In support of this finding, considering an abnormal value of mean LV GLS as higher than −18%, we identified 53% patients with impaired deformation at T0 and T1 and only 13% at T2.23
The stable reduction in LV GLS values at T1 reflects the persistence of subclinical myocardial dysfunction that can only be detected by STE as demonstrated in different studies in children.24,25 On the other side, LVEF is less sensitive to subclinical myocardial injury.26 Furthermore, LVEF is recognized to be more load dependent compared to GLS, possibly explaining the faster recovery after the resolution of the acute phase. The later improvement and disappearance of an apex-base gradient of GLS alteration support the normalization of left ventricular regional/segmental contractility at 6 months after disease diagnosis.
No statistically significant differences were detected regarding RV systolic function parameters (TAPSE and FAC), which were always within normal limits, highlighting left ventricle higher sensitivity to myocardial involvement in MIS-C. Furthermore, echocardiographic assessment has demonstrated a significant improvement of coronary arteries dilation during follow-up with a prevalence of 8.6% at T2 compared with 28% in the acute phase.
Conversely, CMR performed at 6 months after MIS-C diagnosis showed the persistence of LGE with non-ischaemic pattern in almost all patients with LGE detection during the acute phase. However, quantitative analysis showed a reduction of the LGE extension among the involved myocardial segments compared to the early phase. Overall LGE was detected in 5 of 15 patients (33.4%). The high percentage of persisting LGE abnormalities may also reflect a selection bias for CMR study. In fact, it is more likely that parents of patients with LGE at the baseline CMR study were more incline to accept a second CMR study, whereas some of the MIS-C patients (and parents) with normal CMR baseline study and a more benign clinical course were more reluctant to perform a follow-up CMR.
This finding demonstrated that CMR is a useful tool for the detection of subclinical myocardial damage in MIS-C. However, our findings support the hypothesis that to document the complete resolution of such injury, CMR should be procrastinated beyond 6 months.
Limitations
The single-center nature of our study may constitute a limitation. However, this increases the reproducibility and decrease the inter-observer variability of our measurements. Another possible limitation may lie in the speckle-tracking analysis, which was limited to the assessment of the longitudinal deformation of the left ventricle. On this purpose, multiple studies demonstrated the strongprognostic value of longitudinal deformation, and, on the other side, the clinical value of circumferential and radial strain has to be proven yet.27–29
Conclusions
Patients with MIS-C show an excellent clinical outcome in the short term. Standard and STE are useful tools in the diagnosis and follow-up. GLS improves significantly at 6 months after diagnosis, and its normalization might clear the patient from further follow-up.
Our data suggest to regularly follow-up MIS-C patients by performing echocardiography and speckle-tracking analysis, whereas CMR, including LGE, could be limited to patients with a more severe acute clinical picture or in patients who do not normalize LV GLS within 6 months after the onset of the disease.
Funding
This research received no external funding.
Conflict of interest: None declared.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
D Sirico, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
A Basso, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
J Sabatino, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
E Reffo, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
A Cavaliere, Institute of Radiology, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
R Biffanti, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
A Cerutti, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
B Castaldi, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
F Zulian, Pediatric Rheumatology Unit, Department for Women's and Children's Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
L Da Dalt, Pediatric Emergency Unit, Department for Women's and Children's Health, University Hospital of Padova, Padua, Italy.
G Di Salvo, Pediatric and Congenital Cardiology Unit, Department for Women's and Children’s Health, University Hospital of Padova, Via Nicolò Giustiniani 2, 35128 Padua, Italy.
References
- 1. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and canadian pediatric intensive care Units. JAMA Pediatr 2020;174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, et al. . Screening and severity of coronavirus disease 2019 (COVID-19) in children in madrid, spain. JAMA Pediatr 2020:e201346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parri N, Lenge M, Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med 2020;383:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pawar SM. Multi system inflammatory syndrome in children and adolescents temporally related to COVID-19. GFNPSS-Int J Multidiscip Res 2020;1:97. [Google Scholar]
- 5. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hameed S, Elbaaly H, Reid CEL, Santos RMF, Shivamurthy V, Wong J, et al. . Spectrum of imaging findings at chest radiography, US, CT, and MRI in multisystem inflammatory syndrome in children associated with COVID-19. Radiology 2021;298:E1–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. . Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020;183:982–995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. . Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 2021;143:21–32. [DOI] [PubMed] [Google Scholar]
- 9. Kwak JH, Lee SY, Choi JW. Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Clin Exp Pediatr 2021;64:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021;180:307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. . Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021;143:78–88. [DOI] [PubMed] [Google Scholar]
- 12. Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. . Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 2020;76:1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bermejo IA, Bautista-Rodriguez C, Fraisse A, Voges I, Gatehouse P, Kang H, et al. . Short-Term sequelae of multisystem inflammatory syndrome in children assessed by CMR. JACC Cardiovasc Imaging 2021;14:1666–7. [DOI] [PubMed] [Google Scholar]
- 14. Sirico D, Basso A, Reffo E, Cavaliere A, Castaldi B, Sabatino J, et al. . Early echocardiographic and cardiac mri findings in multisystem inflammatory syndrome in children. J Clin Med 2021;10:3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, et al. . Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging 2021;22:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. . American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol 2021;73:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zoghbi WA, DiCarli MF, Blankstein R, Choi AD, Dilsizian V, Flachskampf FA, et al. . Multimodality cardiovascular imaging in the midst of the COVID-19 pandemic: ramping up safely to a new ormal. JACC Cardiovasc Imaging 2020;13:1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sirico D, Castaldi B, Ciliberti P, Sabatino J, Cazzoli I, Secinaro A, et al. . Cardiac imaging in congenital heart disease during the coronavirus disease-2019 pandemic: recommendations from the Working Group on Congenital Heart Disease of the Italian Society of Cardiology. J Cardiovasc Med (Hagerstown) 2020;21:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. . Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135:e927–e999. [DOI] [PubMed] [Google Scholar]
- 20. Prota C, Di Salvo G, Sabatino J, Josen M, Paredes J, Sirico D, et al. . Prognostic value of echocardiographic parameters in pediatric patients with Ebstein’s anomaly. Int J Cardiol 2019;278:76–83. [DOI] [PubMed] [Google Scholar]
- 21. Voigt J-U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. . Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Hear journal Cardiovasc Imaging 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
- 22. Di Salvo G, Al Bulbul Z, Issa Z, Fadel B, Al-Sehly A, Pergola V, et al. . Left ventricular mechanics after arterial switch operation: a speckle-tracking echocardiography study. J Cardiovasc Med (Hagerstown) 2016;17:217–224. [DOI] [PubMed] [Google Scholar]
- 23. Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, et al. . Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 2016;29:209–225.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sirico D, Di Chiara C, Costenaro P, Bonfante F, Cozzani S, Plebani M, et al. . Left ventricular longitudinal strain alterations in asymptomatic or mildly symptomatic paediatric patients with SARS-CoV-2 infection. Eur Heart J Cardiovasc Imaging 2021;22(Suppl. 1). 10.1093/ehjci/jeaa356.167 [DOI] [PubMed] [Google Scholar]
- 25. Di Salvo G, Siblini G, Issa Z, Mohammed H, Abu Hazeem A, Pergola V, et al. . Left ventricular mechanics in patients with abnormal origin of the left main coronary artery from the pulmonary trunk late after successful repair. Cardiology 2017;136:71–76. [DOI] [PubMed] [Google Scholar]
- 26. Croft LB, Krishnamoorthy P, Ro R, Anastasius M, Zhao W, Buckley S, et al. . Abnormal left ventricular global longitudinal strain by speckle tracking echocardiography in COVID-19 patients. Future Cardiol 2021;17:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC. Cardiovasc. Imaging 2018;11:260–274. [DOI] [PubMed] [Google Scholar]
- 28. Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. . Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol 2019;4:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castaldi B, Di Salvo G. LV-GLS in congenital heart disease, time to go beyond ejection fraction. Echocardiography 2021;38:384–385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.