Abstract
A temperature-sensitive lethal mutant of Staphylococcus aureus was found to harbor a mutation in the uncharacterized two-component histidine kinase (HK)-response regulator (RR) pair encoded by yycFG; orthologues of yycFG could be identified in the genomes of Bacillus subtilis and other gram-positive bacteria. Sequence analysis of the mutant revealed a point mutation resulting in a nonconservative change (Glu to Lys) in the regulator domain of the RR at position 63. To confirm that this signal transduction system was essential, a disrupted copy of either the RR (yycF) or the HK (yycG) was constructed with a set of suicide vectors and used to generate tandem duplications in the chromosome. Resolution of the duplications, leaving an insertion in either the yycF or the yycG coding region, was achieved only in the presence of an additional wild-type copy of the two open reading frames. Phenotypic characterization of the conditional lethal mutant showed that at permissive growth conditions, the mutant was hypersusceptible to macrolide and lincosamide antibiotics, even in the presence of the ermB resistance determinant. Other mutant phenotypes, including hypersensitivity to unsaturated long-chain fatty acids and suppression of the conditional lethal phenotype by high sucrose and NaCl concentrations, suggest that the role of the two-component system includes the proper regulation of bacterial cell wall or membrane composition. The effects of this point mutation are strongly bactericidal at the nonpermissive temperature, indicating that this pathway provides an excellent target for the identification of novel antibiotics.
The mounting problem of antibiotic resistance among pathogenic bacteria, including Staphylococcus aureus, has prompted renewed efforts toward the discovery of novel antimicrobial agents. Useful targets for novel antimicrobial agents will be found among genes that are essential for bacterial survival. In an effort to identify genes essential for the growth of S. aureus, a collection of temperature-sensitive (ts) mutants has been generated. One of the mutant strains, NT372, was found to be complemented by genes encoding a novel two-component signal transduction system.
Two-component signal transduction systems are widely distributed among prokaryotes (6, 38) and mediate diverse adaptive responses (52), including sporulation (18), elaboration of virulence factors (34), and chemotaxis (31). Examples of structural homologues from eukaryotes have also been reported (25). A prokaryotic two-component system usually consists of a histidine kinase (HK) and a response regulator (RR). Commonly, an environmental signal is sensed by the HK, resulting in a change in the phosphorylation state of a conserved histidine residue in the cytoplasmic kinase domain of the protein. This phosphohistidyl residue serves as a source for phosphotransfer to a conserved aspartate residue of the cognate RR, resulting in modulation of the activity of the RR. The activated RR can initiate subsequent actions, such as transcriptional regulation or phosphorylation of other proteins, ultimately resulting in transduction of the primary signal.
Several essential two-component systems have now been described for bacteria. An essential multicomponent pathway (60) in the dimorphic bacterium Caulobacter crescentus requires the RR gene ctrA (44) to restrict DNA replication to the stalked cell type, as well as to control the transcription of a number of genes (43). The resDE system is required for the proper expression of genes involved in respiration in Bacillus subtilis (55), although strains bearing insertional inactivations of either the HK or the RR are viable in the presence of glucose or fructose. A recent report has offered a preliminary characterization of the yycFG genes of B. subtilis (13); these genes are essential for the in vitro growth of B. subtilis and are orthologues of the genes described in this report.
The two-component system identified in this work is essential for the in vitro viability of S. aureus. Several characteristics of the genes and mutant phenotypes suggest that they will be uniquely suitable for serving as targets for novel antibacterial agents.
MATERIALS AND METHODS
Media and chemicals.
Antibiotics and medium supplements were obtained from Sigma (St. Louis, Mo.). Commercially available media for routine strain cultivation, including Trypticase soy broth (TSB), Mueller-Hinton broth, and Luria-Bertani broth, were obtained from Difco Laboratories (Detroit, Mich.). Chemically defined medium and dropout media were prepared as described by Pattee and Neveln (41). Oxyrase reagents and plates were purchased from Oxyrase, Inc. (Mansfield, Ohio) and used to generate anoxic growth conditions in combination with a GasPak chamber from BBL (Cockeysville, Md.).
Selection for the drug resistance markers ermC and tetK was performed on Trypticase soy agar (TSA) plates (TSA with erythromycin [TSA-Em] or TSA with tetracycline [TSA-Tc], respectively) with 1 μg of antibiotic per ml for initial selection and scoring on 10 μg/ml. To select for the presence of the rescue plasmid, chloramphenicol (10 μg/ml) was added when required. Unless otherwise noted, strains carrying the yycF1 mutation were grown at 30°C as a permissive temperature; growth curves were acquired in a microtiter format with a SpectraMAXplus plate reader from Molecular Devices (Sunnyvale, Calif.). MICs were determined by twofold broth dilution on microtiter plates; end points were determined after overnight growth (35°C, 20 h).
Bacterial strains and plasmids.
The strains and vectors used in this work are listed in Table 1; all S. aureus strains used in this study were derivatives of 8325-4 (SAM23). The standard molecular biological procedures used are described by Sambrook et al. (45). All enzymes were purchased from New England Biolabs (Beverly, Mass.); deoxynucleotides were obtained from Pharmacia (Piscataway, N.J.). Recombinant constructs were introduced into bacteria either by electroporation with a Gene Pulser (BioRad, Inc.) or by transduction (47).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Phenotype or relevant marker | Source or comment |
|---|---|---|---|
| Strains | |||
| Staphylococcus aureus | |||
| SAM13 | RN4220 (r− m+) | S. Arvidson (28) | |
| SAM23 | 8325-4 (r+ m+) | S. Arvidson (37) | |
| NT372 | SAM23 yycF1 | TS | UV-induced mutant |
| SAM970 | NT372 purA571::Tn917lac yycF+ | Emr Cmr | This work |
| SAM1010 | SAM23 purA571::Tn917lac yycF1 | Emr Cmr TS | This work |
| SAM1011 | SAM23 purA571::Tn917lac yycF+ | Emr Cmr | This work |
| SAM1061 | SAM13/pMP782 | Cmr | This work |
| SAM1156 | SAM13 DUP[yycFG, tetK, yycF::ermC] | Emr Tcr | pMP626 integrant |
| SAM1157 | SAM13 DUP[yycFG, tetK, yycG::ermC] | Emr Tcr | pMP705 integrant |
| SAM1158 | SAM13 yycF::ermC/pMP782 | Emr Cmr | This work |
| SAM1159 | SAM13 yycG::ermC/pMP782 | Emr Cmr | This work |
| SAM1443 | SAM13 DUP[yycFG, tetK, yycFG] | Tcr | pMP1008 integrant |
| Escherichia coli DH5α | F−endA1 hsdR17(rK− mK+) recA1 gyrA96 relA1 | Life Technologies, Inc./pUC host strain | |
| Plasmids | |||
| pGEM3Zf(+) | Cloning vector | Apr | Promega |
| pLTV1 | Source of Tn917lac | Emr Cmr | P. Youngman (8) |
| pT181 | Source of tetK | Tcr | S. Arvidson (35) |
| pMIN164 | pE194 (ermC) ligated into pBR325 | Emr | G. A. Bohach (20) |
| pC194 | S. aureus plasmid (cat) | Cmr | Bacillus Genetic Stock Center (19) |
| pMP16 | S. aureus-E. coli shuttle vector | Emr | This work |
| pMP373 | NT372-complementing clone | Emr | This work |
| pMP621 | pGEM3Zf(+) containing yycFG | Apr | This work |
| pMP626 | pMP621 (yycF::ermC, yycG, tetK) | Apr Emr Tcr | This work |
| pMP705 | pMP621 (yycF, yycG::ermC, tetK) | Apr Emr Tcr | This work |
| pMP749 | Clone recovered from SAM970 | Apr | This work |
| pMP782 | Rescue plasmid | Apr Cmr | This work |
| pMP1008 | pMP621 (tetK) | Apr Tcr | This work |
Mutant isolation and complementation.
Heat-sensitive mutant NT372 was obtained by UV mutagenesis of S. aureus SAM23; mutagenized cultures were diluted, plated onto TSA, and incubated overnight (30°C, 18 h). The growing colonies were replica printed in duplicate and incubated under either permissive (30°C) or nonpermissive (43°C) growth conditions. Colonies growing at 30°C but not at 43°C were isolated, and their temperature-sensitive (TS) phenotypes were reconfirmed in a second round of plating. Genomic DNA for library construction and for PCR was prepared from S. aureus by the method of Dyer and Iandolo (12). Genomic DNA from SAM23 was partially digested (Sau3AI), and fragments (2 to 8 kb) were isolated by sucrose gradient centrifugation; the fragments were ligated into the pMP16 shuttle vector (BamHI), and the mixture was electroporated into Escherichia coli DH5α. The shuttle library plasmids were introduced into S. aureus SAM13 by electroporation; the pooled erythromycin-resistant (Emr) transformants were then infected with bacteriophage φ11 at a multiplicity of infection of 0.01. The resulting lysates were used to transduce the ts mutant NT372 to temperature resistance for the selection of complementing clones. Transducing lysates of each of the colonies surviving incubation at 43°C were used to retransduce the original NT372 mutant to confirm the complementation.
DNA sequence and analysis.
Complementing plasmids and PCR amplicons were sequenced with a PRISM dye terminator kit from Applied Biosystems, Inc. (Foster City, Calif.) and an ABI 373A automated DNA sequencer. Oligonucleotides for DNA sequencing were produced on an ABI 392 synthesizer. All sequencing reactions were performed in multiple passes from both directions. Multiple genomic PCR amplicons from the NT372 mutant and the SAM23 parent were sequenced in parallel and compared to differentiate genomic mutations from possible PCR-induced artifacts. Version 8.0.1 of the Wisconsin Genetics Computer Group programs (11) was used for sequence analysis. Potential open reading frames (ORFs) were identified with Genemark (5) by use of an S. aureus matrix; similarity searches were performed with the BLASTX (1) program against the GenBank database (release 110).
Strain construction.
To generate a transposon insertion near the ts mutation in NT372, a Tn917lac transposon insertion library from SAM23 was generated with pLTV1 (42). A transducing lysate prepared from this insertion library was used to transduce the NT372 mutant to a temperature-resistant phenotype (26). In a modified protocol (see Results), the transduction mixture was plated on TSA-Em plates supplemented with sodium citrate (500 μg/ml) and Oxyrase (1:10 [vol/vol]). The plates were incubated under anoxic conditions until colonies began to appear (30°C, 48 h); colonies were reselected on TSA-Em plates at 43°C. A transducing lysate from one of the temperature-resistant derivatives, SAM970, was used to establish a genetic linkage between the transposon and the ts lesion (46). Genomic DNA flanking the transposon insertion was cloned in E. coli as described previously (62). Chromosomal digests (SmaI), used to map Tn917lac insertions and plasmid integrants (see below), were analyzed on a contour-clamped homogeneous electric field (CHEF) gel system (Bio-Rad) according to the manufacturer’s directions. The Tn917lac insertion identified in SAM970 was used to create the isogenic strains SAM1010 (yycF1) and SAM1011 (yycF+) by backcrossing into SAM23 (wild type).
Disruption and rescue constructs.
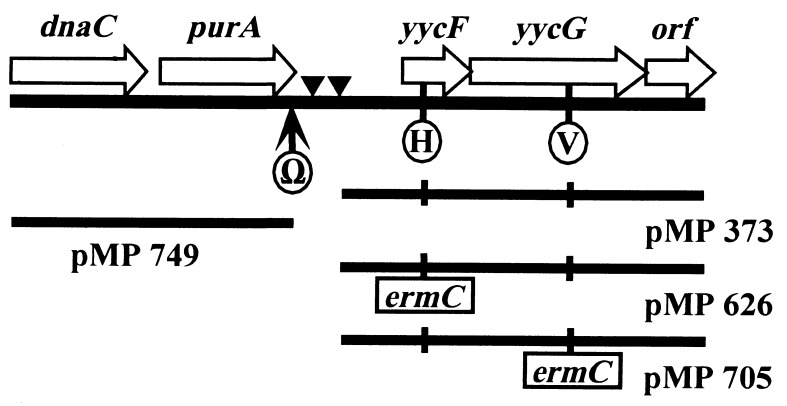
To generate integration constructs, the 3.7-kb yycFG genomic insert from the complementing plasmid (pMP373) was subcloned (EcoRI/HindIII) into pGEM3Zf(+), maintaining a rare SmaI site that could be used to map the integrants by CHEF gel analysis. A 2.1-kb fragment (HindIII) containing the tetK gene from plasmid pT181 was then introduced into the polylinker (pMP1008). A 1.2-kb fragment (MspI/ClaI) containing the ermC gene from plasmid pMIN164 was blunt ended (T4 DNA polymerase) and then introduced into one of two sites (Fig. 1): insertion at the EcoRV site created the yycF disruption (pMP626), and insertion at the HpaI site created the yycG disruption (pMP705).
FIG. 1.
Partial chromosomal map detailing the local organization around the yycFG locus. The location in the C-terminal portion of purA of the Tn917lac insertion used in the construction of the isogenic strains SAM1010 and SAM1011 is shown (Ω), along with the HpaI (H) and EcoRV (V) restriction sites used to create ermC insertions in either yycF or yycG. Predicted tRNA sequences are denoted by an inverted triangle. The relative locations of the original complementing clone (pMP373), the clone rescued from SAM970 (pMP749), and the two inactivation constructs are shown below the sequence. The ORFs that have clear orthologues in B. subtilis are labeled; the last ORF exhibits only limited sequence similarity to yycH from B. subtilis (data not shown).
Each plasmid was introduced into S. aureus SAM13 by electroporation and selected on TSA-Tc plates. Chromosomal integrants were isolated and scored for tetracycline resistance; isolates bearing the ermC marker were also scored for erythromycin resistance. Site-specific integration of each construct was confirmed by CHEF gel analysis of a SmaI genomic digest; single-copy integration was confirmed by genomic PCR. Resolution of the tandem duplication, leaving a single disrupted copy of either ORF, was attempted by overnight growth in the absence of antibiotic selection. Single colonies were inoculated into TSB and grown to the postexponential phase (35°C, 24 h); the cultures were then diluted, plated on TSA, and allowed to grow overnight (35°C, 24 h). The resulting colonies were replica printed onto a selective medium (TSA-Tc); any colonies that were identified as tetracycline sensitive (Tcs) were scored for retention of the ermC insertion.
The rescue plasmid (pMP782) was constructed by linearizing pC194 (HindIII) and ligating it into plasmid pMP621 (HindIII). The rescue plasmid was introduced by electroporation into strains bearing a tandem duplication of the yycFG locus (containing either a yycF::ermC or a yycG::ermC insertion in one copy) and selected on TSA containing chloramphenicol. Strains obtained in this manner were grown overnight (35°C, 18 h) in TSB supplemented with erythromycin (1 μg/ml) and then scored on TSA-Em plates. Colonies were replica plated onto TSA-Tc plates and examined for Tcs. The loss of the integration construct from the chromosome, indicated by the loss of the tetK marker and the loss of the additional SmaI site from the integration construct, was confirmed by CHEF gel analysis and genomic PCR. Transducing lysates were made from strains bearing either chromosomal disruption (yycF::ermC or yycG::ermC) and used to attempt to transduce SAM13, either with or without the rescue plasmid, to erythromycin resistance.
Nucleotide sequence accession number.
The DNA sequence corresponding to the complementing clone from pMP373 has been deposited with GenBank under accession no. AF136709.
RESULTS
Mutant isolation and complementation.
The S. aureus ts mutant NT372 was initially identified by its inability to form colonies on TSA plates at 43°C; subsequent experiments showed that it could not grow on any standard rich medium (solid or liquid) or in liquid or on solid defined medium (chemically defined medium) at temperatures above 40°C. A Tn917lac insertion linked to the TS phenotype in NT372 was identified (see Materials and Methods) and used to move the ts mutation into unmutagenized strain backgrounds. The isogenic strains SAM1010 (yycF1) and SAM1011 (yycF+) have the TS and temperature-insensitive growth phenotypes, respectively. SAM1010 failed to form colonies on rich and defined media at 43°C, demonstrating that a single locus was solely responsible for the inability of NT372 to grow at a high temperature.
CHEF gel analysis of a SmaI chromosomal digest followed by Southern hybridization with a probe specific for Tn917lac localized the transposon insertion to the SmaI-G fragment (data not shown). Sequence analysis of a clone containing the chromosomal DNA flanking the Tn917lac insertion localized the insertion to the extreme C-terminal end of the purA locus of S. aureus, which has been shown to reside in the SmaI-G fragment (40). These results position the ts mutation site near the purA locus of S. aureus.
Three independently isolated overlapping clones were selected from a genomic library by complementation of the ts defect of the NT372 mutant. The shortest complementing clone, pMP373, contained an insert of 3,731 nucleotides; sequence analysis identified two complete ORFs, predicted to encode proteins of 233 and 608 amino acids. No ORFs were predicted within the first 650 bp of this clone, and two tRNA consensus motifs were identified in the genomic DNA proximal to the yycFG locus (Fig. 1). The first ORF was preceded by a Shine-Dalgarno sequence (AGAGG) by 9 bp, and a predicted promoter region (positions −35 and −10) showing agreement with the consensus sequences recognized by ςA from B. subtilis could be identified 215 bp upstream (TTGTCA and ACTAAT, respectively).
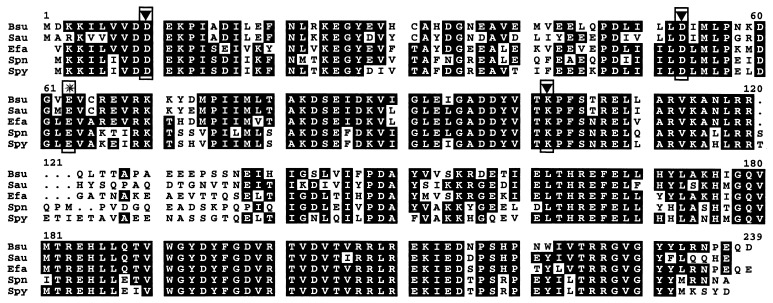
Searches against the GenBank database with the first putative ORF revealed a high degree of similarity to a number of RR genes. The highest degree of similarity observed was to yycF from the B. subtilis genomic sequence database (29); we found 89% amino acid similarity and 74% amino acid identity. Extensive similarity to PhoP (48) from B. subtilis was also noted, placing the product of this ORF in the OmpR-PhoB subfamily of RRs. Structural alignments with orthologues of YycF from other gram-positive organisms highlighted conserved features characteristic of RRs (Fig. 2). In B. subtilis (29) and in S. aureus (Fig. 1), the yycFG locus is located immediately downstream of the purA locus, in agreement with the chromosomal mapping data.
FIG. 2.
Structural alignment of RRs with a high degree of similarity to YycF. The conserved residues necessary for proper phosphotransfer (56) in the regulatory domain are boxed and highlighted with an inverted triangle. The location of the amino acid substitution (E63K) in the variant form of the RR is highlighted by a star. Abbreviations: Bsu, B. subtilis; Sau, S. aureus; Efa, E. faecalis; Spn, S. pneumoniae; Spy, Streptococcus pyogenes. The S. pneumoniae (type 4) unpublished genomic sequence data used to identify the YycF orthologue were provided by The Institute for Genomic Research (25a). The E. faecalis sequence (strain V583) was obtained by designing PCR primers based on the unpublished genomic sequence data provided by The Institute for Genomic Research (25a) and then resolving frameshifts by sequencing PCR-derived amplicons from strain ATCC 29212. The S. pyogenes sequence (strain M1 GAS) was provided by the University of Oklahoma (44a).
Searches with the second putative ORF revealed significant similarity to a number of HK genes. The highest degree of similarity observed was to an ORF designated yycG from B. subtilis (29); we found overall 69% similarity and 46% identity. Hydrophobicity analysis of the translated yycG ORF predicted two strongly hydrophobic stretches in the N-terminal third of the polypeptide, each consisting of a stretch of approximately 25 amino acids and bounding a hydrophilic sequence of 145 amino acids. In the absence of structural studies, these predictions suggested two membrane-spanning regions (TM1 and TM2) with an intervening cell surface loop that could define a sensory domain (6); the remaining hydrophilic C-terminal portion of the polypeptide, which contained sequence motifs characteristic of the kinase domains of HKs (39), could define a cytoplasmic signaling domain.
Identification of the ts mutation in NT372.
To determine the identity of yycF1, the genomic mutation causing the TS phenotype of NT372, genomic fragments containing yycFG were amplified by PCR from the chromosomal DNAs of wild-type strain SAM23 and ts mutant strain NT372. Sequence analysis of the PCR amplicons confirmed that a point mutation existed in the regulatory domain of the RR at nucleotide 187 (G187A), resulting in a predicted E63K variant polypeptide. The mutated residue (Lys) represents a nonconservative change from the acidic residues (Asp and Glu) usually occurring at this position (56). By comparison of the translated regulatory domain of yycF with the structurally characterized RR Spo0F from B. subtilis (14), the mutation is predicted to occur in the third α helix of the RR. This residue is a solvent-exposed side chain and may identify one point of interaction between this RR and its cognate HK. No other mutations in the NT372 genomic sequence were identified within the 3.7-kb region containing the yycFG locus.
Phenotypic effects of the yycF1 mutation.
Experiments designed to test the ability of NT372 cells to recover from a nonpermissive temperature incubation showed that the loss of YycF function had a bactericidal effect. Transient incubation of NT372 at a nonpermissive growth temperature (43°C, 2 h), followed by extended incubation at 30°C, resulted in less than 15% survival; longer incubation times at the higher temperature (43°C, 4 h) reduced the number of viable counts recovered at 30°C (<0.1% survival). Microscopic examination of the yycF1 mutants did not reveal gross changes in cell morphology or altered rates of autolysis when liquid cell cultures were shifted to the nonpermissive temperature.
The yycF1 mutation caused novel nutritional requirements in chemically defined medium at permissive growth temperatures. An apparent Ilv− (isoleucine, leucine, and valine) auxotrophy was observed in both yycF1-containing strains (NT372 and SAM1010) but not in the corresponding wild-type strains (SAM23 and SAM1011). Single additions of isoleucine, leucine, or valine or of metabolic intermediates required for the biosynthesis of these amino acids (51) failed to correlate this apparent auxotrophy with a specific enzymatic defect. However, the combination of valine and pyruvate restored growth to the yycF1-containing strains, suggesting that the defect was related to branched-chain fatty acid biosynthesis, perhaps through improper regulation of 2-ketoisovalerate levels, rather than to an amino acid auxotrophy (15, 33). Additionally, this apparent auxotrophy could be corrected by supplementation with pantothenate, which is also required for branched-chain fatty acid biosynthesis as a precursor for coenzyme A (22).
The possible connection between the apparent Ilv− phenotype of the yycF1 mutants and membrane integrity was investigated further. Certain long-chain cis unsaturated fatty acids (UFAs) are known to inhibit the growth of S. aureus (7), presumably by affecting the permeability of the membrane (17). A strain carrying the yycF1 mutation, SAM1010, was more susceptible to the bactericidal action of the long-chain cis UFAs linoleic acid and oleic acid than the isogenic wild-type strain, SAM1011 (Table 2). The in vitro interference of UFAs specifically in HK autophosphorylation has also been reported for KinA from B. subtilis (53); whether the observed hypersusceptibility was due to direct inhibition of YycG autophosphorylation or was an indication of altered membrane integrity is not currently known. Other phenotypic characteristics of S. aureus that might depend upon the integrity of the phospholipid bilayer, including the production of cell surface and secreted proteins (e.g., coagulase, lipase, and alpha-toxin), appeared to be unaffected in strains carrying the yycF1 mutation (data not shown).
TABLE 2.
Differential susceptibilities to UFAs
| Fatty acid | MIC (μg/ml) for strain:
|
|
|---|---|---|
| SAM1010 | SAM1011 | |
| cis-Vaccenic acid (C18:1) | >1,024 | >1,024 |
| trans-Vaccenic acid (C18:1) | >1,024 | >1,024 |
| Oleic acid (C18:1) | 64 | 1,024 |
| Linoleic acid (C18:2) | 16 | 64 |
| Linolenic acid (C18:3) | 64 | 64 |
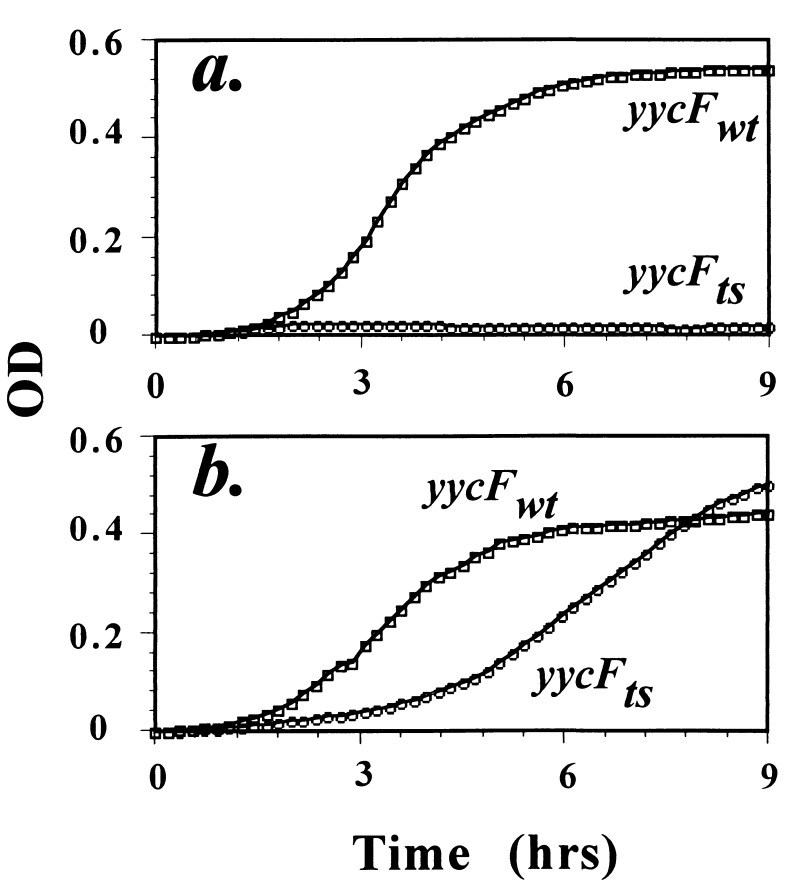
The yycF1 mutation completely prevented growth in a variety of media at temperatures above 40°C; the rate of growth of the mutant was comparable to that of the wild type below 39°C. It was found that strain SAM1010 could grow under anoxic conditions in liquid media (Fig. 3) at 41°C but not 43°C. The TS phenotype of SAM1010 could be suppressed by growth on solid media supplemented with NaCl (500 mM) or sucrose (1 M) at 43°C; however, the addition of NaCl (from 500 mM to 2 M) to liquid media did not restore growth at 43°C but did allow growth at 41°C. Although the alteration of some environmental conditions was found to permit the growth of the ts mutant only on solid media at 43°C, no conditions that allowed the cells to survive when either ORF, yycF or yycG, was insertionally inactivated were found (see below). It is well established that increased salt concentrations can suppress the growth conditional phenotypes of many ts mutants, as well as mutants with defects in cell wall and membrane integrity (27). In addition, these high-salt conditions are known to influence the fatty acid composition of the membrane of S. aureus (23).
FIG. 3.
Comparison of the growth of isogenic strains SAM1010 and SAM1011 at 41°C. (a) The SAM1010 mutant could not survive above 40°C in the presence of oxygen in a variety of liquid media, including TSB. (b) The rate of growth of the yycF1 mutant was only partially restored under anoxic conditions at a nonpermissive temperature in the same liquid media. OD, optical density; ts, temperature sensitive; wt, wild type.
The yycF1 mutation affects resistance to MLSB antibiotics.
The yycF1 mutation was also found to increase the susceptibility of S. aureus to macrolides and lincosamides, although not to other classes of antibiotics, including β-lactams, quinolones, aminoglycosides, and glycopeptides (Table 3). In addition, the Tn917lac insertions present in the isogenic strains SAM1010 and SAM1011, which bear the ermB resistance determinant (50), conferred different levels of Emr (Table 3). The NT372 mutant strain was fourfold more susceptible to erythromycin than was the unmutagenized parent, SAM23 (data not shown), suggesting that the difference might be indicative of a specific defect in the yycF1 strains. The mechanism of erm-encoded resistance to macrolide-lincosamide-streptogramin B (MLSB) antibiotics is well studied (58) and involves control by translational attenuation (2); accordingly, no difference in ermB mRNA levels was observed in samples from SAM1010 and SAM1011 (data not shown).
TABLE 3.
Susceptibilities to known antibiotics
| Antibiotic | MIC (μg/ml) for strain:
|
|
|---|---|---|
| SAM1010 | SAM1011 | |
| Chloramphenicol | 16 | 16 |
| Clindamycin phosphate | 1,024 | >1,024 |
| Clindamycin HCl | 0.25 | >1,024 |
| Erythromycin | 16 | >1,024 |
| Ethidium bromide | 4 | 8 |
| Gentamicin | 1 | 1 |
| Lincomycin HCl | 0.25 | >1,024 |
| Methicillin | 1 | 1 |
| Norfloxacin | 0.5 | 0.5 |
| Vancomycin | 1 | 1 |
The differences in the susceptibilities of the SAM1010 mutant to the lincosamide antibiotics lincomycin hydrochloride, clindamycin hydrochloride, and clindamycin phosphate were large; the SAM1010 mutant was highly susceptible to lincomycin and clindamycin hydrochloride but not to clindamycin phosphate (Table 3). Since the YycF protein exhibits a high degree of similarity to other RRs of the PhoB subfamily, the effect of added Pi on the MICs of erythromycin and lincomycin was examined. A simple addition of Pi (100 μM) to the assay medium substantially increased the MIC of lincomycin from 0.25 μg/ml to >1,024 μg/ml for SAM1010; similar differences were noted for the two commercial preparations of clindamycin. No change in susceptibility to erythromycin was noted; since the lincosamides are more hydrophilic, factors governing susceptibility to the more hydrophobic erythromycin may be different (e.g., partitioning into the membrane [10]). Indeed, the addition of low levels of the UFA linoleic acid (2 ng/ml) to Mueller-Hinton medium significantly affected the MIC of erythromycin for SAM1010 (lowered it from 16 μg/ml to 0.25 μg/ml) but not for SAM1011 (>1,024 μg/ml under both conditions).
These differences in macrolide and lincosamide susceptibility were influenced by the same conditions that influenced the growth of the yycF1 mutant SAM1010 at the semipermissive temperature; for example, the MIC of erythromycin for SAM1010 increased from 16 μg/ml to 256 μg/ml when the strain was grown under anoxic conditions at a neutral pH. It is interesting to note that this correction was only partial and that growth under anoxic conditions allowed the ts mutant to grow at 41°C but not at 43°C. Changes in the fatty acid compositions of membrane lipid pools have been observed to occur in S. aureus transitioning between aerobic growth and anaerobic growth (59); it is possible that an altered balance of fatty acids in this example led to the measured increase in resistance to erythromycin under anoxic growth conditions.
Attempted insertional inactivation of yycFG.
Two chromosomal integration constructs were created to achieve insertional disruption of either the RR (yycF) or the HK (yycG) ORF. By using the suicide plasmid pMP1008, the ermC gene was inserted either at the EcoRV site to disrupt the RR at amino acid 53, creating pMP626, or at the HpaI site to disrupt the HK at amino acid 344, creating pMP705 (Fig. 1). Both of these constructs were integrated separately into the chromosome of SAM13, creating a tandem duplication consisting of a wild-type copy of the yycFG locus, an intervening tetK gene, and an additional copy of the locus with a disruption of either yycF (SAM1156) or yycG (SAM1157). Successful integration of these constructs added a rare SmaI site; CHEF gel analysis of the SmaI genomic digests of both of the integrants (data not shown) confirmed the addition of a SmaI site in the “G” fragment. Both tandem-duplication constructs were stable, although the strain bearing the insertion in the second copy of the RR grew more slowly under antibiotic selection than the corresponding strain bearing the insertion in the second copy of the HK.
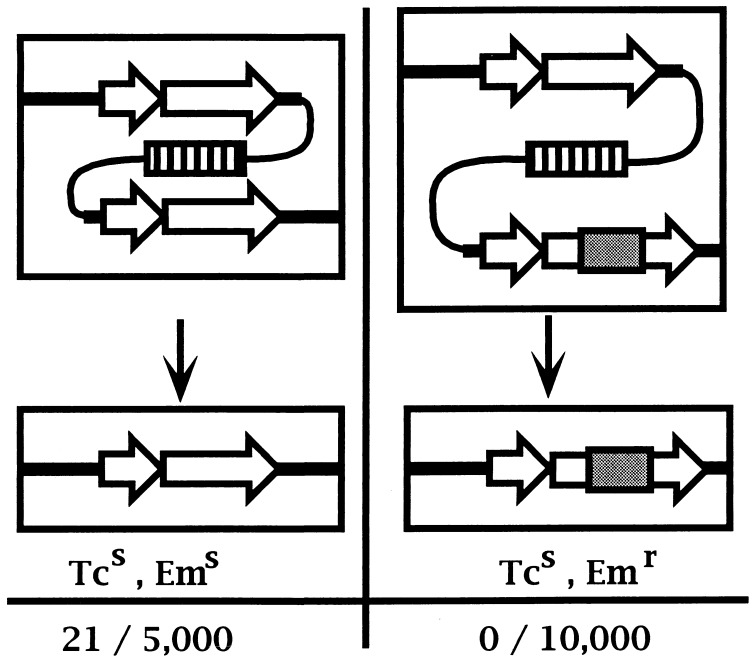
For comparison, the original suicide plasmid (pMP1008) was also integrated into the chromosome of SAM13 at the SmaI-G fragment, resulting in two complete copies of the yycFG locus with an intervening tetK marker. The resulting merodiploid strain, SAM1443, did not exhibit growth-related phenotypes distinct from those of the parental strain. In the absence of selection during overnight growth, resolution of the yycFG duplication was first identified by replica plating for the Tcs phenotype and then confirmed by genomic PCR and CHEF gel analysis. When this procedure was used, Tcs segregants arose from SAM1443 at 21 in 5,000 CFU. This result is in contrast to that for either of the tandem-duplication constructs. Attempts to resolve the tandem duplication and retain the chromosomal insertion in yycF or yycG were not successful in the absence of a wild-type copy of the gene; this finding held true for either disruption construct (SAM1156 and SAM1157). For both strains, resolution of the chromosomal tandem duplications was initially identified by the loss of the tetK marker and then scored for retention of the ermC insertion. In the absence of the rescue plasmid, Tcs segregants arose at 18 in 10,000 CFU; none of these resulting colonies (0 of 18) was Emr, indicating that the duplications had resolved without retaining the ermC insertion (Fig. 4).
FIG. 4.
Resolution frequencies for chromosomal duplications. The ability to resolve a tandem duplication of the yycFG locus with an intervening tetK marker (hatched box) was compared for strains either bearing a complete duplication (left) or bearing an ermC insertion (shaded box) within the second copy of yycG (right). Not shown is the parallel insertion in yycF.
To demonstrate further the essential nature of the yycFG locus, attempts were made to transduce the chromosomal disruption of either ORF to a new set of strains either lacking or bearing a separate copy of the wild-type yycFG locus. To do this, a rescue plasmid (pMP782) constructed with the complementing clone from pMP373 (Fig. 1) was used to aid in the generation of derivatives of SAM1156 or SAM1157 that had resolved the tandem duplication and retained the ermC insertion in the chromosome. In the presence of the rescue plasmid, retention of the ermC insertion in either SAM1156 or SAM1157 was selected by overnight growth in liquid cultures in the presence of erythromycin (1 μg/ml); resulting colonies that were Tcs and Emr could be identified at 6 in 10,000 CFU. Successful resolution of the tandem duplication in either case, resulting in one genomic copy of the disrupted locus, was confirmed by genomic PCR and CHEF gel analysis (data not shown). Transducing lysates were prepared from the resulting strains, SAM1158 (yycF::ermC/pMP782) and SAM1159 (yycG::ermC/pMP782). The recipient strain was either SAM13 or SAM1061 (SAM13 with rescue plasmid pMP782). In the presence of the rescue plasmid, Emr transductants were isolated successfully (on average, 200 CFU per 109 PFU of transducing lysate); genomic PCR and CHEF gel analysis confirmed the presence of a single disrupted chromosomal copy of either of the ORFs in the transductants. In the absence of the rescue plasmid, no Emr transductants (0 CFU) were observed; no altered growth conditions, including selection with high salt, high sucrose, or low oxygen levels, that could be used to select Emr transductants of SAM13 were found.
DISCUSSION
This work identifies a ts mutation in a two-component system from S. aureus that is essential for survival in vitro. An isogenic pair of strains, SAM1010 and SAM1011, was constructed from the original mutant (NT372) for further characterization by use of a closely linked Tn917lac chromosomal insertion. Strains carrying the yycF1 ts mutation exhibited attenuated virulence in two different in vivo models (4), implying that this system could be defined as essential for in vivo survival as well. Given the in vitro and in vivo effects of a ts mutation in the yycFG locus on the proliferation of S. aureus, this two-component system is an attractive target for antimicrobial compound screening.
The yycF1 mutation causes hypersensitivity to macrolides.
The yycF1 strain SAM1010 was affected in the phenotypic expression of MLSB resistance; this partial resistance could be influenced by the same environmental factors that influenced the TS phenotype. The observed differences in susceptibility to macrolides between SAM1010 and SAM1011 could be an indication either of increased permeability or of decreased efflux in the mutant (36). For example, macrolide susceptibility in S. aureus has been observed to increase markedly for strains bearing an insertional inactivation in either femAB (30) or lysC (3), both of which contribute to the structural integrity of the cell envelope. Conditional lethal alleles of genes involved in phospholipid biosynthesis in S. aureus, including pgsA (phosphatidylglycerol phosphate synthase; EC 2.7.8.5) and cdsA (phosphatidate cytidylyl transferase; EC 2.7.7.41), have also been found to confer hypersusceptibility to macrolides (32). Alternatively, disruption of the norA efflux pump in S. aureus results in hypersusceptibility to a number of antimicrobial compounds (21). Since the SAM1010 mutant is not broadly hypersusceptible to antibiotics, and those UFAs that are incorporated into membrane lipid pools at subinhibitory concentrations (16) also affect the erythromycin susceptibility of this mutant, it is proposed that the observed differences in susceptibility are due to a defect in the permeability barrier of the mutant.
The yycFG locus encodes an essential HK-RR pair.
Sequence analysis of the genomic DNA of the mutant confirmed that a point mutation existed in the genomic copy of the RR (encoded by yycF). The Glu-63 residue that occurs in the regulatory domain of the wild-type RR may contribute to the proper interaction with its cognate HK, whereas the Lys-63 variation in the mutant may destabilize the interaction of this HK-RR pair at a high temperature. Thus, a temperature shift to a nonpermissive temperature (43°C) may result in premature dissociation of the variant RR from the HK before an essential phosphotransfer event can occur. If the RR requires phosphorylation for activity (i.e., transcriptional activation), the conditional lethal phenotype would be recessive, as we have reported here, and thus suppressible in trans by a complete wild-type copy of the HK-RR pair.
An attempt was made to disrupt the genomic copy of the yycFG locus to demonstrate its essential nature in vitro; toward this end, insertional inactivation constructs of either ORF were first established as tandem duplications in the chromosome. The inability to resolve the duplication while retaining the ermC insertion was used as an initial measure of the essential nature of this locus. Attempts to disrupt either the HK or the RR in the chromosome failed in the absence of a wild-type copy of the locus. In the presence of a plasmid-borne copy of the wild-type locus, it was possible to identify strains with a disrupted copy of either ORF in the chromosome. Once the resolution of the duplications was achieved, the inactivated loci could be transduced only to a recipient strain bearing a separate, functional copy of the locus. Importantly, environmental conditions that influenced the temperature sensitivity of mutant strains carrying yycF1, such as 0.5 M NaCl or exclusion of oxygen, did not allow the viability of strains with an insertional inactivation of yycF or yycG. Thus we believe that these conditions allow partial suppression of the effects of the variant YycF1 protein but do not bypass the essential function of the protein.
The yycFG system regulates an essential process.
Based upon unusually high peptide-level similarities (∼89%), there are clear orthologues of this HK-RR pair in the genomes of other low-G+C-content gram-positive bacteria, including B. subtilis, Enterococcus faecalis, and Streptococcus pneumoniae. Similarly, these genomes also appear to contain distinct orthologues of genes encoding other members of the OmpR-PhoB subfamily of HK-RR pairs, including phoPR (48, 49) and resDE (55). Since phoPR and resDE have been demonstrated to interact in B. subtilis (54) and the modulation of at least one related environmental condition (i.e., anoxic growth conditions) significantly influences the growth of the ts mutant described here, it is possible that the yycFG system also plays a role in this regulatory network. The sensing of limiting phosphate levels by phoPR and how it might relate to the regulatory role of yycFG in S. aureus remain unclear; the response of S. aureus to phosphate limitation has only recently received extensive attention (57), and genetic studies characterizing the PHO regulon in this organism have not been reported. Studies characterizing the in vitro and in vivo pathway(s) regulated by yycFG and possible relationships to other members of the PHO regulon in S. aureus are currently under way.
Several features of these genes make them uniquely suitable as targets for new antibiotic agents. A point mutation in the genomic copy of the RR confers a strongly bactericidal phenotype at a nonpermissive temperature, suggesting that inhibitors of this pathway would also have bactericidal effects. The mutant is also hypersusceptible to the bactericidal action of the UFAs linoleic acid and oleic acid, which are found at the sites of S. aureus infections (9, 61); it is possible that an inhibitor of this specific two-component system may render the bacteria more vulnerable to killing by the host. The essential nature of this system in the soil bacterium B. subtilis has been reported (13, 24); in addition to the findings reported here with S. aureus, this system may also play an essential role in other pathogens (e.g., E. faecalis) (Fig. 2), providing the opportunity for the development of an antibacterial compound active against a range of pathogenic gram-positive bacteria. The existence of multiple HK-RR proteins in prokaryotes provides the possibility that a single agent might inhibit more than one signal transduction pathway. Furthermore, inhibition of the activity of the YycF protein causes hypersensitivity to macrolides, suggesting that YycF inhibitors would show synergy with macrolide antibiotics.
ACKNOWLEDGMENTS
This work was supported in collaboration with the Robert Wood Johnson Pharmaceutical Research Institute.
We acknowledge helpful discussions with Laura McDowell and Jerry Buysse and the technical assistance of Kelly Winterberg, Henry Fang, and Skip Bond.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bechhofer D H, Zen K H. Mechanism of erythromycin-induced ermC mRNA stability in Bacillus subtilis. J Bacteriol. 1989;171:5803–5811. doi: 10.1128/jb.171.11.5803-5811.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton, B. Personal communication.
- 4.Bond, S. Personal communication.
- 5.Borodovsky M, Rudd K E, Koonin E V. Intrinsic and extrinsic approaches for detecting genes in a bacterial genome. Nucleic Acids Res. 1994;22:4756–4767. doi: 10.1093/nar/22.22.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourret R B, Borkovich K A, Simon M I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- 7.Butcher G W, King G, Dyke K G. Sensitivity of Staphylococcus aureusto unsaturated fatty acids. J Gen Microbiol. 1976;94:290–296. doi: 10.1099/00221287-94-2-290. [DOI] [PubMed] [Google Scholar]
- 8.Camilli A, Portnoy A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell I M, Crozier D N, Caton R B. Abnormal fatty acid composition and impaired oxygen supply in cystic fibrosis patients. Pediatrics. 1976;57:480–486. [PubMed] [Google Scholar]
- 10.Dette G A, Knothe H, Kaula S. Modifying effects of pH and temperature on (14C)erythromycin uptake into Staphylococcus aureus—relation to antimicrobial activity. Zentbl Bakteriol Mikrobiol Hyg A. 1987;265:393–403. [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer D W, Iandolo J J. Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol. 1983;46:283–285. doi: 10.1128/aem.46.1.283-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabret C, Hoch J A. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol. 1998;180:6375–6383. doi: 10.1128/jb.180.23.6375-6383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feher V A, Zapf J W, Hoch J A, Whiteley J M, McIntosh L P, Rance M, Skelton N J, Dahlquist F W, Cavanagh J. High-resolution NMR structure and backbone dynamics of the Bacillus subtilisresponse regulator, Spo0F: implications for phosphorylation and molecular recognition. Biochemistry. 1997;36:10015–10025. doi: 10.1021/bi970816l. [DOI] [PubMed] [Google Scholar]
- 15.Gollop N, Damri B, Chipman D M, Barak Z. Physiological implications of the substrate specificities of acetohydroxy acid synthases from varied organisms. J Bacteriol. 1990;172:3444–3449. doi: 10.1128/jb.172.6.3444-3449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenway D L, Dyke K G. Isolation and properties of a linoleic acid-resistant mutant of Staphylococcus aureus. J Gen Microbiol. 1980;118:267–270. doi: 10.1099/00221287-118-1-267. [DOI] [PubMed] [Google Scholar]
- 17.Greenway D L, Dyke K G. Mechanism of the inhibitory action of linoleic acid on the growth of Staphylococcus aureus. J Gen Microbiol. 1979;115:233–245. doi: 10.1099/00221287-115-1-233. [DOI] [PubMed] [Google Scholar]
- 18.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 19.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982;150:815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovde C J, Hackett S P, Bohach G A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990;220:329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh P C, Siegel S A, Rogers B, Davis D, Lewis K. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc Natl Acad Sci USA. 1998;95:6602–6606. doi: 10.1073/pnas.95.12.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanemasa Y, Yoshioka T, Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureuscultured in medium containing NaCl. Biochim Biophys Acta. 1972;280:444–450. [PubMed] [Google Scholar]
- 24.Kasahara Y, Nakai S, Ogasawara N. Sequence analysis of the 36-kb region between gntZ and trnY genes of Bacillus subtilisgenome. DNA Res. 1997;4:155–159. doi: 10.1093/dnares/4.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Kennelly P J, Potts M. Fancy meeting you here! a fresh look at “prokaryotic” protein phosphorylation. J Bacteriol. 1996;178:4759–4764. doi: 10.1128/jb.178.16.4759-4764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Ketchum, K. A. 5 August 1998. Sequences. [Online.] http://www.tigr.org/. [5 May 1999, last date accessed.]
- 26.Kleckner N, Roth J, Botstein D. Genetic engineering in vivousing translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977;116:125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- 27.Kohno T, Schmid M, Roth J R. Effect of electrolytes on growth of mutant bacteria. Basic Life Sci. 1979;14:53–57. doi: 10.1007/978-1-4684-3725-6_5. [DOI] [PubMed] [Google Scholar]
- 28.Kreiswirth B N, Lofdahl S, Betley M J, O’Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 29.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 30.Ling B, Berger-Bachi B. Increased overall antibiotic susceptibility in Staphylococcus aureus femABnull mutants. Antimicrob Agents Chemother. 1998;42:936–938. doi: 10.1128/aac.42.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson M D, Armitage J P, Hoch J A, Macnab R M. Bacterial locomotion and signal transduction. J Bacteriol. 1998;180:1009–1022. doi: 10.1128/jb.180.5.1009-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, P. K. Unpublished data.
- 33.Massey L K, Sokatch J R, Conrad R S. Branched-chain amino acid catabolism in bacteria. Bacteriol Rev. 1976;40:42–54. doi: 10.1128/br.40.1.42-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 35.Mojumdar M, Khan S A. Characterization of the tetracycline resistance gene of plasmid pT181 of Staphylococcus aureus. J Bacteriol. 1988;170:5522–5528. doi: 10.1128/jb.170.12.5522-5528.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 37.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 40.Pattee P A, Lee H C, Bannantine J P. Genetic and physical mapping of the chromosome of Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH; 1990. pp. 41–58. [Google Scholar]
- 41.Pattee P A, Neveln D S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975;124:201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins J B, Youngman P J. Construction and properties of Tn917-lac, a transposon derivative that mediates transcriptional gene fusions in Bacillus subtilis. Proc Natl Acad Sci USA. 1986;83:140–144. doi: 10.1073/pnas.83.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 44.Quon K C, Yang B, Domain I J, Shapiro L, Marczynski G T. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Roe, B. A. 5 August 1998. Sequences. [Online.] http://www.genome.ou.edu/. [5 May 1999, last date accessed.]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Sanderson K E, Roth J R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988;52:485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder C J, Pattee P A. Transduction analysis of transposon Tn551 insertions in the trp-thy region of the Staphylococcus aureuschromosome. J Bacteriol. 1984;157:533–537. doi: 10.1128/jb.157.2.533-537.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seki T, Yoshikawa H, Takahashi H, Saito H. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J Bacteriol. 1987;169:2913–2916. doi: 10.1128/jb.169.7.2913-2916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seki T, Yoshikawa H, Takahashi H, Saito H. Nucleotide sequence of the Bacillus subtilis phoRgene. J Bacteriol. 1988;170:5935–5938. doi: 10.1128/jb.170.12.5935-5938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw J H, Clewell D B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985;164:782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith C D, Pattee P A. Biochemical and genetic analysis of isoleucine and valine biosynthesis in Staphylococcus aureus. J Bacteriol. 1967;93:1832–1838. doi: 10.1128/jb.93.6.1832-1838.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauch M A, de Mendoza D, Hoch J A. cis-Unsaturated fatty acids specifically inhibit a signal-transducing protein kinase required for initiation of sporulation in Bacillus subtilis. Mol Microbiol. 1992;6:2909–2917. doi: 10.1111/j.1365-2958.1992.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 54.Sun G, Birkey S M, Hulett F M. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:941–948. doi: 10.1046/j.1365-2958.1996.422952.x. [DOI] [PubMed] [Google Scholar]
- 55.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volz K. Structural conservation in the CheY superfamily. Biochemistry. 1993;32:11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- 57.Watson S P, Clements M O, Foster S J. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol. 1998;180:1750–1758. doi: 10.1128/jb.180.7.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White D C, Frerman F E. Fatty acid composition of the complex lipids of Staphylococcus aureusduring the formation of the membrane-bound electron transport system. J Bacteriol. 1968;95:2198–2209. doi: 10.1128/jb.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J, Ohta N, Newton A. An essential, multicomponent signal transduction pathway required for cell cycle regulation in Caulobacter. Proc Natl Acad Sci USA. 1998;95:1443–1448. doi: 10.1073/pnas.95.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong Z, Ge S, Chamberlain N R, Kapral F A. Growth cycle-induced changes in sensitivity of Staphylococcus aureusto bactericidal lipids from abscesses. J Med Microbiol. 1993;39:58–63. doi: 10.1099/00222615-39-1-58. [DOI] [PubMed] [Google Scholar]
- 62.Youngman P, Perkins J B, Losick R. A novel method for the rapid cloning in Escherichia coli of Bacillus subtilis chromosomal DNA adjacent to Tn917insertions. Mol Gen Genet. 1984;195:424–433. doi: 10.1007/BF00341443. [DOI] [PubMed] [Google Scholar]