Novel therapies for SARS-CoV-2 infection have been a focus of research and enquiry, and many promising compounds tested in phase 1 and 2 trials have emerged. In this multinational clinical trial, one such compound—a designed ankyrin repeat protein, ensovibep—was compared with standard of care to determine whether it improved outcomes among patients hospitalized with COVID-19. After randomly assigning 485 patients, the trial was stopped for early futility because the odds of a more favorable pulmonary outcome were no different in those randomized to treatment versus control. This study highlights that effective antiviral therapies for patients hospitalized with COVID-19 remain an unmet need. Even though this trial was negative, important lessons regarding how to conduct such trials and test promising compounds can be gleaned from this study.
Visual Abstract. Ensovibep for Adults Hospitalized With COVID-19.
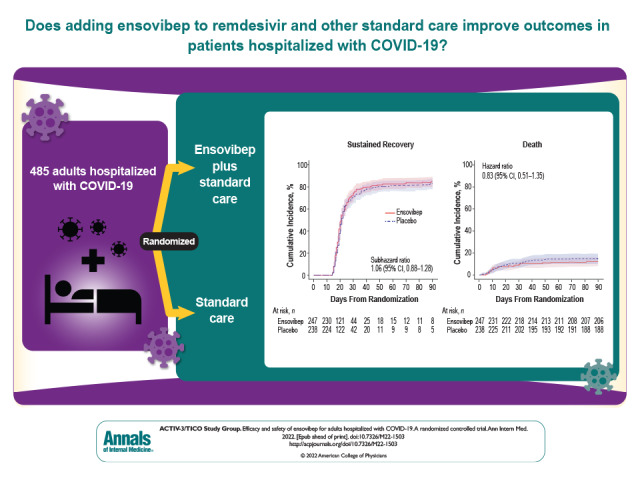
Novel therapies for SARS-CoV-2 infection have been a focus of research and enquiry, and many promising compounds tested in phase 1 and 2 trials have emerged. In this multinational clinical trial, one such compound—a designed ankyrin repeat protein, ensovibep—was compared with standard of care to determine whether it improved outcomes among patients hospitalized with COVID-19. After randomly assigning 485 patients, the trial was stopped for early futility because the odds of a more favorable pulmonary outcome were no different in those randomized to treatment versus control. This study highlights that effective antiviral therapies for patients hospitalized with COVID-19 remain an unmet need. Even though this trial was negative, important lessons regarding how to conduct such trials and test promising compounds can be gleaned from this study.
Abstract
Background:
Ensovibep (MP0420) is a designed ankyrin repeat protein, a novel class of engineered proteins, under investigation as a treatment of SARS-CoV-2 infection.
Objective:
To investigate if ensovibep, in addition to remdesivir and other standard care, improves clinical outcomes among patients hospitalized with COVID-19 compared with standard care alone.
Design:
Double-blind, randomized, placebo-controlled, clinical trial. (ClinicalTrials.gov: NCT04501978)
Setting:
Multinational, multicenter trial.
Participants:
Adults hospitalized with COVID-19.
Intervention:
Intravenous ensovibep, 600 mg, or placebo.
Measurements:
Ensovibep was assessed for early futility on the basis of pulmonary ordinal scores at day 5. The primary outcome was time to sustained recovery through day 90, defined as 14 consecutive days at home or place of usual residence after hospital discharge. A composite safety outcome that included death, serious adverse events, end-organ disease, and serious infections was assessed through day 90.
Results:
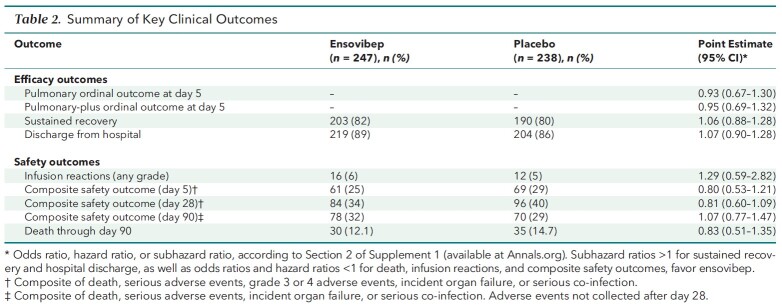
An independent data and safety monitoring board recommended that enrollment be halted for early futility after 485 patients were randomly assigned and received an infusion of ensovibep (n = 247) or placebo (n = 238). The odds ratio (OR) for a more favorable pulmonary outcome in the ensovibep (vs. placebo) group at day 5 was 0.93 (95% CI, 0.67 to 1.30; P = 0.68; OR > 1 would favor ensovibep). The 90-day cumulative incidence of sustained recovery was 82% for ensovibep and 80% for placebo (subhazard ratio [sHR], 1.06 [CI, 0.88 to 1.28]; sHR > 1 would favor ensovibep). The primary composite safety outcome at day 90 occurred in 78 ensovibep participants (32%) and 70 placebo participants (29%) (HR, 1.07 [CI, 0.77 to 1.47]; HR < 1 would favor ensovibep).
Limitation:
The trial was prematurely stopped because of futility, limiting power for the primary outcome.
Conclusion:
Compared with placebo, ensovibep did not improve clinical outcomes for hospitalized participants with COVID-19 receiving standard care, including remdesivir; no safety concerns were identified.
Primary Funding Source:
National Institutes of Health.
Oral antivirals, intravenous remdesivir, and antispike neutralizing antibodies are effective at preventing disease progression in early COVID-19 (1–5). However, for hospitalized patients, finding effective antiviral therapy remains a challenge (6–8). Monoclonal antibody treatments in inpatients have been assessed (6, 7, 9), and although the combination of casirivimab and imdevimab improved clinical outcomes, this was only among patients without detectable antibodies to SARS-CoV-2 at randomization, and before the emergence of the Omicron variant (10).
Designed ankyrin repeat proteins (DARPins) are a new class of engineered protein therapeutics. Derived from naturally occurring ankyrin repeats, they are designed to bind with high affinity and specificity to other proteins (11, 12). Ensovibep (previously MP0420) was selected to bind the SARS-CoV-2 spike protein with in vitro ribosome display based on a physical library with a diversity of approximately 1 trillion DARPin molecules. After a screening process to identify the most potent monovalent DARPin domains that neutralize angiotensin-converting enzyme 2, ensovibep was assembled to generate a multispecific neutralizing candidate against variants of SARS-CoV-2 (13). It consists of 5 linked DARPin domains, 3 of which cooperatively engage the 3 receptor-binding domains of the trimeric SARS-CoV-2 spike protein to inhibit angiotensin-converting enzyme 2 interaction and cellular entry and 2 of which bind to serum albumin for systemic half-life extension, thereby enabling single-dose administration (13, 14).
In a therapeutic hamster model of COVID-19, ensovibep reduced virus replication in both the lower and upper respiratory tract and protected against severe disease (13). In a recently completed phase 2 study (EMPATHY [Randomized, Double-blind, Placebo-controlled, Multicenter Study of Ensovibep in Ambulatory Patients With Symptomatic COVID-19] [15]) in outpatients with mild to moderate COVID-19, ensovibep demonstrated antiviral and clinical efficacy with a 78% (95% CI, 16% to 95%) reduction in hospitalizations, emergency department visits caused by COVID-19, or deaths (15). Here we report results from the TICO (Therapeutics for Inpatients With COVID-19) platform trial comparing ensovibep versus placebo, on a background of remdesivir plus other standard care, among adults hospitalized with COVID-19.
Methods
Trial Design and Oversight
TICO is a master protocol to evaluate the safety and efficacy of multiple investigational agents targeting either the host immune response to SARS-CoV-2 infection or viral control (16). The trial is a phase 3, randomized, double-blind, controlled platform trial. For efficiency, the design of the study allows pooling of control participants from more than 1 concurrent trial therapy.
The study protocol (Supplement 2) was approved by a governing institutional review board for each participating center. All enrolled participants or their legal representative gave written informed consent. All trials done under the master protocol are overseen by an independent data and safety monitoring board (DSMB).
Study Participants and Stratification
Hospitalized adults (aged ≥18 years) were eligible for randomization if they had SARS-CoV-2 infection documented by a nucleic acid amplification test or equivalent and if their COVID-19 symptoms had been present for at most 12 days at the time of randomization. Vaccination against SARS-CoV-2 was not exclusionary. The study protocol excluded persons requiring any of the following interventions at baseline: invasive mechanical ventilation, extracorporeal membrane oxygenation or other forms of mechanical circulatory support, vasopressor therapy, or commencement of renal replacement therapy during admission (a complete list of exclusion criteria is in Supplement 1).
Randomization and Blinding
Eligible participants at each site were randomly assigned in a 1:1 ratio to receive ensovibep or placebo. When possible, placebo controls were shared among investigational agents. The study medication was prepared by unblinded pharmacists at local pharmacies, and all other study staff and recipients remained blinded (Supplement 1).
Interventions and Treatments
Participants were randomly assigned and given their blinded study infusion on study day 0. Ensovibep was administered intravenously over 1 hour in a 1-time infusion containing 600 mg. Supplement 1 describes blinding procedures. Remdesivir was provided to all study participants, including those who had already started receiving this agent, as standard of care unless contraindicated; it was administered as a 200-mg intravenous loading dose followed by a 100-mg intravenous maintenance dose once daily while hospitalized up to a 10-day total course. Dexamethasone or other corticosteroids were administered per the local standard of care.
Study Procedures
Participants were followed per TICO study protocols (6, 7, 16) and assessed for clinical outcomes and adverse events daily from randomization through day 7 and retrospectively on days 14, 28, 60, and 90. Supplement 1 describes adverse event grading and reporting. Blood samples were collected from participants before administration of the study infusion for plasma measurement of neutralizing antibody concentrations against the receptor-binding domain of the SARS-CoV-2 spike protein (GenScript SARS-CoV-2 Surrogate Virus Neutralisation assay; GenScript), total antibody concentration against SARS-CoV-2 nucleocapsid antigen (Bio-Rad Platelia SARS-CoV-2 Total Ab assay; Bio-Rad), and SARS-CoV-2 nucleocapsid antigen concentrations (Quanterix assay; Quanterix). The SARS-CoV-2 RNA load in the nasal swab material was determined using extraction, master mix preparation, and reverse transcriptase polymerase chain reaction as described in the Centers for Disease Control and Prevention's instructions for the 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. The lower limit of quantification for this measurement is 399 copies/mL. Advanced Biomedical Laboratories centrally measured viral RNA. In addition, the presence of the Delta variant versus other variants was determined with a reverse transcriptase polymerase chain reaction assay. Supplement 1 describes these assays in detail.
Outcomes
The initial futility assessment evaluated two 7-category ordinal outcomes collected at day 5 after randomization, the pulmonary and the pulmonary-plus ordinal scales (for details of the futility outcome, see the Trial Design and Treatments section in Supplement 1). The first scale classifies participants according to the intensity of respiratory support, whereas the second also includes extrapulmonary manifestations. These ordinal scales were originally used in influenza studies (17) and have been used in previous COVID-19 studies (18, 19).
The primary efficacy outcome was time to sustained clinical recovery up to day 90, defined as the time from randomization to return to home (the participant's residence or a facility that provided the same or a less intensive level of clinical care before COVID-19) for 14 consecutive days. Mortality through 90 days and time to hospital discharge were also assessed. Supplement 1 details the composite safety outcomes at days 5, 28, and 90, along with all study outcomes.
Statistical Analysis
The planned sample size was 1000 participants; this was intended to coincide with 843 sustained recovery events, which would provide 90% power to detect a subhazard ratio (sHR) of 1.25 comparing the time to sustained recovery between the treatment groups at a 1-sided significance level of 0.025. An independent DSMB reviewed interim data and used prespecified guidelines to assess futility. On 15 November 2021, the DSMB recommended stopping the study for futility (Supplement 1).
After the end of enrollment, all participants were followed for at least 90 days. The analysis population for efficacy and safety outcomes was restricted to participants who received a complete or partial infusion of ensovibep or placebo (modified intention to treat). The distributions of the pulmonary and pulmonary-plus ordinal scales were compared between treatment groups using proportional odds models, as described in the Methods section and Supplement Table 3. Proportional odds models were fitted with the same covariates for the ordinal outcomes at days 1 to 7, 14, and 28.
The cumulative incidences of sustained recovery and hospital discharge were estimated using the Aalen–Johansen estimator, treating death as a competing risk. The cumulative incidence of death was estimated using Kaplan–Meier methods. Subhazard ratios comparing the time to sustained recovery and hospital discharge were estimated using the Fine–Gray model. A Cox proportional hazards model was used to estimate the HR comparing time to death between the treatment groups; models were stratified by study site pharmacy (Supplement Table 3).
The composite safety outcome up to day 5 was compared between groups using logistic regression stratified by study site pharmacy. Times to the composite safety outcomes through days 28 and 90 were analyzed using Cox proportional hazards models, also stratified by study site pharmacy. To assess the consistency of the overall findings for the various outcomes, the following subgroups based on baseline characteristics were considered: pulmonary ordinal scale on day 0, duration of symptoms, age, gender, race and ethnicity, antibody and antigen levels, vaccination status, and immunosuppressive status. For the subgroup analysis based on SARS-CoV-2 antigen levels, baseline antigen levels were dichotomized into those above and below the median value.
All analyses were done using SAS, version 9.4 (SAS Institute), or R, version 4.0 (R Foundation). The master protocol for the TICO study is registered at ClinicalTrials.gov (NCT04501978).
Role of the Funding Source
The funding organizations had no direct involvement in the decisions related to the trial or the drafting or revision of the manuscript.
Results
Study Enrollment and Patient Characteristics
Between 11 June 2021 and 15 November 2021, the study enrolled 496 participants; 255 were assigned to the ensovibep group and 241 to the placebo control. Among the 496 participants, 485 persons from 62 sites in 10 countries received the blinded infusion and are included in the modified intention-to-treat analyses (Figure 1). Sites, enrollment status, and infusion information are detailed in Supplement Tables 1 to 3.
Figure 1. Study flow diagram.
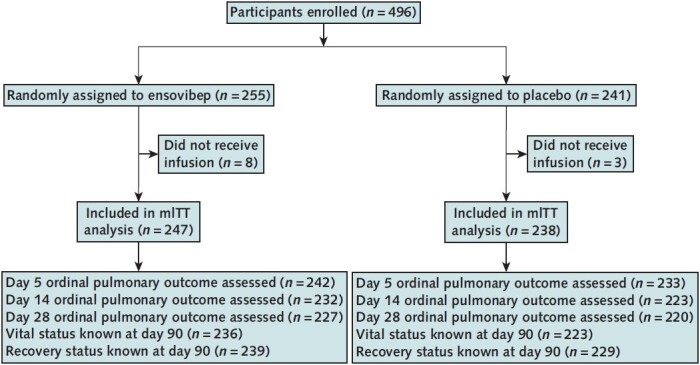
mITT = modified intention-to-treat.
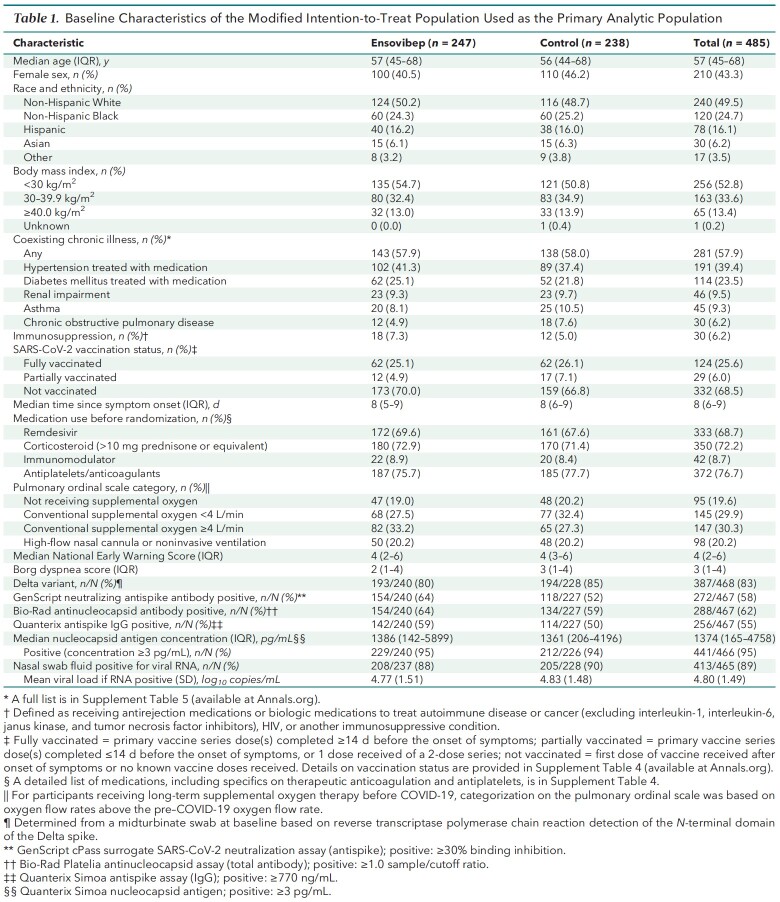
The 2 groups were similar with respect to baseline characteristics (Table 1; Supplement Tables 4 to 7). Overall, the median age was 57 years (IQR, 45 to 68 years), and 49.5% of participants were non-Hispanic White, 24.7% were non-Hispanic Black, and 16.1% were Hispanic. Of note, 47.1% of participants had a body mass index of 30 kg/m2 or greater. The median time between onset of symptoms and randomization was 8 days (IQR, 6 to 9 days).
Table 1.
Baseline Characteristics of the Modified Intention-to-Treat Population Used as the Primary Analytic Population
Participants entered the trial in 1 of the following 4 pulmonary ordinal categories: no supplemental oxygen (19.6%), conventional supplemental oxygen at less than 4 L/min (29.9%), conventional supplemental oxygen at 4 L/min or higher (30.3%), or high-flow nasal oxygen or noninvasive ventilation (20.2%). Corticosteroids (>10 mg of prednisone or equivalent) were used by 72% of participants, 72% had received remdesivir before enrollment, and 68% were unvaccinated. Concomitant use of medication at day 5 and day 28 was also similar between groups (Supplement Table 8).
Efficacy Outcomes
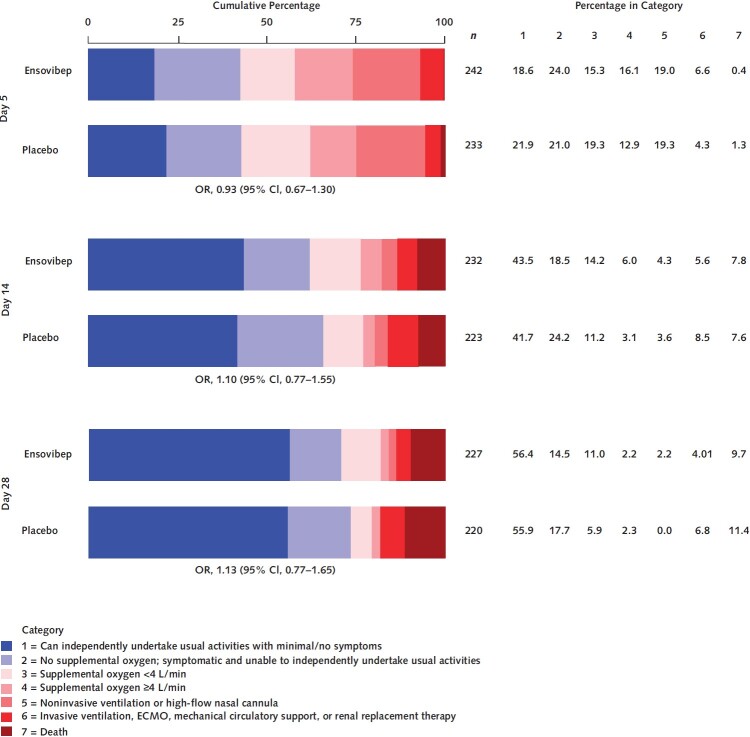
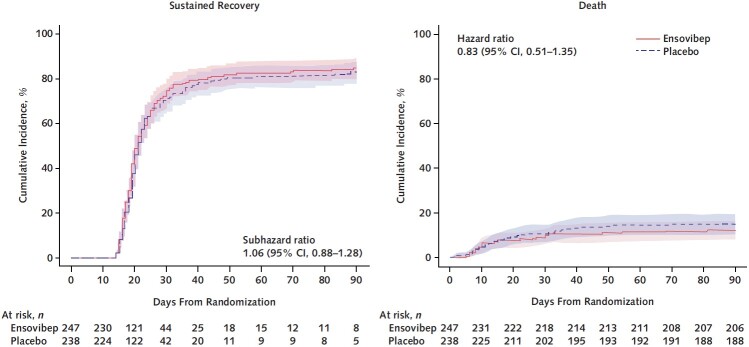
The day 5 ordinal outcome was assessed for 95% of participants, and the sustained recovery outcome at day 90 was known for more than 96% of participants (Figure 1). The adjusted odds ratio (OR) (ensovibep vs. placebo) for participants having a better pulmonary ordinal score at day 5 was 0.93 (CI, 0.67 to 1.30; OR > 1 would favor ensovibep) (Table 2 and Figure 2; Supplement Table 9). Results were similar for the pulmonary-plus ordinal score at day 5 (adjusted OR for ensovibep vs. placebo, 0.95 [CI, 0.69 to 1.32]) (Table 2; Supplement Table 10). One-sided P values were greater than 0.30, the guideline for assessing futility, for both the pulmonary and pulmonary-plus outcomes. The percentage of participants with an improvement in the 7-category ordinal scale between baseline and day 5 was 44.6% in the ensovibep group versus 46.8% in the placebo group (Supplement Table 11). No evidence suggested that the assumption of proportional odds was violated (Supplement Table 12). The adjusted ORs for the pulmonary ordinal outcome for other time periods also showed no evidence of benefit of ensovibep versus placebo (Figure 2; Supplement Tables 13 to 15). Sustained recovery by day 90 was achieved by 82% in the ensovibep group and 80% in the placebo group (sHR, 1.06 [CI, 0.88 to 1.28]) (Table 2 and Figure 3). The sHR for hospital discharge was 1.07 (CI, 0.90 to 1.28; sHR > 1 would favor ensovibep) (Table 2; Supplement Figure 1). Through day 90, a total of 30 participants (12.1%) in the ensovibep group and 35 (14.7%) in the placebo group died (HR, 0.83 [CI, 0.51 to 1.35]) (Table 2 and Figure 3).
Table 2.
Summary of Key Clinical Outcomes
Figure 2. Distribution of patients on the pulmonary ordinal scale on day 5, 14, and 28.
ECMO = extracorporeal membrane oxygenation; OR = odds ratio.
Figure 3. Time to sustained recovery and death through day 90 for ensovibep vs. placebo.
The rate ratios were calculated with Fine–Gray models to account for the competing risk for death and stratified according to study pharmacy. Left. Sustained recovery. Right. Death.
Safety Outcomes
Four participants (2 in each group) had their infusion paused for adverse reactions. There was no evidence of a difference between treatment groups with respect to infusion reactions; incidence or prevalence of adverse events by day 7, 14, or 28; or serious adverse events through day 90 (Supplement Tables 16 to 22). The percentage developing the composite safety outcome (all-cause mortality, serious adverse event, grade 3 or 4 adverse event, organ failure, or serious co-infection) through day 5 was 24.7% in the ensovibep group and 29.0% in the placebo group (OR, 0.80 [CI, 0.53 to 1.21]; OR < 1 would favor ensovibep) (Table 2). Through day 28, these percentages were 34.0% and 40.3%, respectively (HR, 0.81 [CI, 0.60 to 1.09]; HR < 1 would favor ensovibep) (Table 2; Supplement Figure 2). Through day 90, the composite safety outcome (all-cause mortality, serious adverse event, organ failure, or serious co-infection) through day 90 occurred in 78 participants (31.6%) in the ensovibep group and 70 (29.4%) in the placebo group (HR, 1.07 [CI, 0.77 to 1.47]) (Table 2; Supplement Figure 3).
Individual components of the composite safety outcomes also did not differ between treatment groups through day 5, 28, or 90 (Supplement Tables 23 to 25). Incidence of clinical organ failure through day 90, including liver and renal dysfunction and cardiovascular and thromboembolic events, was similar between groups (Supplement Table 26). The most common events were respiratory failure (ensovibep vs. control, 42 vs. 35 events), intercurrent serious infection (26 vs. 19 events), hypotension requiring a vasopressor (19 vs. 25 events), and thromboembolic events (13 vs. 10 events). Rash was a safety event of special interest for this trial and occurred in 7 participants in the ensovibep group and 4 in the placebo group (1.6% and 1.3%, respectively) (Supplement Table 27). In both groups, most of these events did not present with concurrent events (Supplement Table 27).
Subgroup Analyses
Subgroup analyses provided no evidence for heterogeneity in the treatment effect for either efficacy or safety outcomes (Supplement Figure 4 and Supplement Tables 28 to 37).
Discussion
The TICO platform was designed to rapidly assess the safety and efficacy of candidate COVID-19 therapies with an early futility analysis based on 2 pulmonary ordinal outcomes through day 5 (16). Ensovibep was the fifth antiviral agent in the TICO platform trial to be tested in patients hospitalized with COVID-19 and is the first DARPin anti-infective to enter human clinical trials. This DARPin molecule, in contrast with conventional monoclonal antibodies, is designed as a pan-variant antiviral that can be produced efficiently through Escherichia coli fermentation and scaled up more easily (11, 13–15).
Ensovibep did not pass the protocol-defined futility assessment based on day 5 clinical data from 421 participants, and further participant accrual was halted per DSMB recommendation. Because enrollment was stopped for futility, the study was underpowered to assess many outcomes, with a wide 95% CI for the sHR comparing the primary end point of time to sustained recovery across treatment groups (CI, 0.88 to 1.28). The results of this trial are similar to those of trials testing other antiviral agents in the TICO platform, highlighting the difficulty of finding an effective therapy to improve outcomes among patients hospitalized with COVID-19 who are already receiving background remdesivir, corticosteroids, and other immune modulators. Bamlanivimab, sotrovimab, and BRII-196 plus BRII-198 did not pass the early futility assessment when tested in TICO (6, 7) despite having been found to be effective at reducing progression to hospitalization and death in outpatients with early disease (1, 5, 6). For a fourth monoclonal antibody, tixagevimab–cilgavimab, the full trial enrollment was achieved. This agent given with remdesivir as the standard of care was not associated with improved time to sustained recovery but was associated with lower mortality than standard care alone (8).
The data from this trial differ from the preliminary findings of the EMPATHY study of ensovibep versus placebo among outpatients with symptomatic COVID-19 (15). Results from the dose-finding part of this study were recently presented, and ensovibep met the primary end point of viral load reduction from baseline to day 8 in comparison with placebo, with a statistically significant reduction in viral load at all 3 doses tested (75 mg, 225 mg, and 600 mg). The study also showed a 78% (CI, 16% to 95%) reduction in the secondary end point of death, hospitalization, or emergency department visits related to COVID-19. The EMPATHY study enrolled persons within 7 days of symptom onset, and this finding is consistent with the hypothesis that treatments using a passive immunity approach (such as monoclonal antibodies) are more effective when given early and in patients who do not yet have COVID-19 complications necessitating hospitalization (1, 4, 7, 8, 20). Consistent with an antiviral effect of various passive immunotherapies in this situation, small-molecule antivirals have also consistently been shown to reduce risk for hospitalization early in the disease course of ambulatory COVID-19 (3, 5, 21, 22). Taken together with the reported result from the EMPATHY trial, our findings suggest that ensovibep treatment in COVID-19 may be effective at preventing progression rather than treating severe disease in patients with a shorter duration of symptoms.
Of note, the anti–SARS-CoV-2 monoclonal antibodies casirivimab–imdevimab and bamlanivimab (10, 23) were both reported to be more effective in seronegative patients, and as a result, an a priori hypothesis of this trial was that ensovibep would benefit patients who were seronegative for SARS-CoV-2 neutralizing antibodies at baseline. Analysis by major baseline subgroups, including serostatus, for time to sustained recovery and mortality identified no statistically significant interactions between treatment group and subgroup. With its early termination, however, this trial lacks precision in the point estimates for subgroups.
Strengths of this trial include enrollment of a diverse population from 62 sites across 10 countries. Antiviral treatment with remdesivir was standardized, and the trial was run with blinding of the investigational agent and continuous DSMB oversight. Regarding study limitations, as a result of its early termination, this study is underpowered to detect modest benefits from ensovibep, particularly among important subgroups, such as those defined by baseline serostatus, disease severity, or comorbid conditions. These factors should be incorporated into the design of future studies, including the study of ensovibep in ambulatory patients. Because ensovibep was tested in a population of patients who received background remdesivir treatment, the efficacy of ensovibep without remdesivir is unknown. Generalizing the results of this study must be considered in light of the fact that it was done primarily among patients with Delta variant infections, with a minority of patients (26%) fully vaccinated and only 30 (6%) categorized as immunosuppressed.
In conclusion, among patients hospitalized with COVID-19 receiving remdesivir and other standard care, ensovibep did not improve clinical outcomes. Ensovibep was well tolerated, even in seriously ill patients receiving high-flow nasal oxygen, with few hypersensitivity reactions. Overall, a broadly applicable and highly effective antiviral therapy for patients hospitalized with COVID-19 remains a major unmet need.
Supplementary Material
Footnotes
This article was published at Annals.org on 9 August 2022.
* For the writing group members, see end of text. For a list of all members of the ACTIV-3/TICO Study Group, see Supplement 1.
Contributor Information
Christina Barkauskas, Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, Duke Health, Durham, North Carolina.
Eleftherios Mylonakis, Division of Infectious Diseases, Rhode Island Hospital and The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Garyfallia Poulakou, 3rd Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Sotiria General Hospital, Athens, Greece.
Barnaby E. Young, National Centre for Infectious Diseases, Singapore.
David M. Vock, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Lianne Siegel, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Nicole Engen, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Greg Grandits, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Nilima R. Mosaly, Duke University Hospital, Durham, North Carolina.
Andrew M. Vekstein, Duke University Hospital, Durham, North Carolina.
Ralph Rogers, Division of Infectious Diseases, Rhode Island Hospital and The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Fadi Shehadeh, Division of Infectious Diseases, Rhode Island Hospital and The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Matthew Kaczynski, Division of Infectious Diseases, Rhode Island Hospital and The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Evangelia K. Mylona, Division of Infectious Diseases, Rhode Island Hospital and The Miriam Hospital, Alpert Medical School of Brown University, Providence, Rhode Island.
Konstantinos N. Syrigos, 3rd Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Sotiria General Hospital, Athens, Greece.
Vasiliki Rapti, 3rd Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Sotiria General Hospital, Athens, Greece.
David C. Lye, National Centre for Infectious Diseases, Tan Tock Seng Hospital, Lee Kong Chian School of Medicine, Singapore.
Shiau Hui Diong, National Centre for Infectious Diseases, Tan Tock Seng Hospital, Singapore.
Lindsay Leither, Division of Pulmonary and Critical Care, Department of Medicine, Intermountain Medical Center, Salt Lake City, Utah.
Kirk U. Knowlton, Cardiovascular Department, Intermountain Medical Center, Salt Lake City, Utah.
Mamta K. Jain, UT Southwestern Medical Center and Parkland Health and Hospital Systems, Dallas, Texas.
Rubria Marines-Price, UT Southwestern Medical Center and Parkland Health and Hospital Systems, Dallas, Texas.
Alice Osuji, UT Southwestern Medical Center and Parkland Health and Hospital Systems, Dallas, Texas.
J. Scott Overcash, Velocity San Diego, San Diego, California.
Ioannis Kalomenidis, 1st Department of Critical Care and Pulmonary Medicine, Medical School, National and Kapodistrian University of Athens, Evaggelismos General Hospital, Athens, Greece.
Zafeiria Barmparessou, 1st Department of Critical Care and Pulmonary Medicine, Medical School, National and Kapodistrian University of Athens, Evaggelismos General Hospital, Athens, Greece.
Michael Waters, Velocity Chula Vista, San Diego, California.
Karla Zepeda, Velocity Chula Vista, San Diego, California.
Peter Chen, Cedars-Sinai Medical Center, Los Angeles, California.
Sam Torbati, Cedars-Sinai Medical Center, Los Angeles, California.
Francis Kiweewa, Lira Regional Referral Hospital, Lira, Uganda.
Nicholus Sebudde, Lira Regional Referral Hospital, Lira, Uganda.
Eyad Almasri, University of California, San Francisco–Fresno, Fresno, California.
Alyssa Hughes, University of California, San Francisco–Fresno, Fresno, California.
Sanjay R. Bhagani, Royal Free Hospital, London, England.
Alison Rodger, Royal Free Hospital, London, England.
Uriel Sandkovsky, Baylor Scott & White Health, Dallas, Texas.
Robert L. Gottlieb, Baylor Scott & White Health, Dallas, Texas.
Eriobu Nnakelu, Institute of Human Virology Nigeria, Abuja, Nigeria.
Barbara Trautner, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, Texas.
Vidya Menon, NYC Health + Hospitals/Lincoln, Bronx, New York.
Joseph Lutaakome, Medical Research Council/Uganda Virus Research Institute and London School of Hygiene & Tropical Medicine Uganda Research Unit, Entebbe, Uganda.
Michael Matthay, University of California, San Francisco, Medical Center, Fresno, California.
Philip Robinson, Hoag Memorial Hospital Presbyterian, Newport Beach, California.
Konstantinos Protopapas, 4th Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Attikon University General Hospital, Athens, Greece.
Nikolaos Koulouris, 1st Respiratory Medicine Department, Medical School, National and Kapodistrian University of Athens, Sotiria General Hospital, Athens, Greece.
Ivan Kimuli, Makerere University Lung Institute, Kampala, Uganda.
Amiran Baduashvili, Division of Hospital Medicine, University of Colorado Hospital - Anschutz Medical Campus, Aurora, Colorado.
Dominique L. Braun, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich and Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Huldrych F. Günthard, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich and Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Srikanth Ramachandruni, CHRISTUS Spohn Shoreline Hospital, Corpus Christi, Texas.
Robert Kidega, Gulu Regional Referral Hospital, Gulu, Uganda.
Kami Kim, Division of Infectious Diseases, University of South Florida and Global Emerging Diseases Institute, Tampa General Hospital, Tampa, Florida.
Timothy J. Hatlen, Lundquist Institute for Biomedical Innovation, Torrance, California.
Andrew N. Phillips, Institute for Global Health, University College London, England.
Daniel D. Murray, CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark.
Tomas O. Jensen, CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, and Department of Pulmonary and Infectious Diseases, North Zealand University Hospital, Hillerod, Denmark.
Maria L. Padilla, Icahn School of Medicine at Mount Sinai, New York, New York.
Evan X. Accardi, Icahn School of Medicine at Mount Sinai, New York, New York.
Katy Shaw-Saliba, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
Robin L. Dewar, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Marc Teitelbaum, Leidos Biomedical Research, Frederick, Maryland.
Ven Natarajan, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Sylvain Laverdure, Laboratory of Human Retrovirology and Immunoinformatics, National Institutes of Health, Frederick, Maryland.
Helene C. Highbarger, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
M. Tauseef Rehman, Frederick National Laboratory for Cancer Research, Frederick, Maryland.
Susan Vogel, Office of Clinical Research Policy and Regulatory Operations, National Institutes of Health, Bethesda, Maryland.
David Vallée, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
Page Crew, Collaborative Clinical Research Branch, National Institutes of Health, Bethesda, Maryland.
Negin Atri, Office of Clinical Research Policy and Regulatory Operations, National Institutes of Health, Bethesda, Maryland.
Adam J. Schechner, Leidos Biomedical Research, Frederick, Maryland.
Sarah Pett, The Medical Research Council Clinical Trials Unit at University College London, London, England.
Fleur Hudson, The Medical Research Council Clinical Trials Unit at University College London, London, England.
Jonathan Badrock, The Medical Research Council Clinical Trials Unit at University College London, London, England.
Giota Touloumi, Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Samuel M. Brown, Division of Pulmonary and Critical Care Medicine, Intermountain Medical Center, and Department of Internal Medicine, University of Utah, Murray, Utah.
Wesley H. Self, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee.
Crystal M. North, Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts.
Adit A. Ginde, University of Colorado School of Medicine, Aurora, Colorado.
Christina C. Chang, The Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia.
Anthony Kelleher, The Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia.
Stephanie Nagy-Agren, Salem Veterans Affairs Medical Center, Salem, Virginia.
Shikha Vasudeva, Salem Veterans Affairs Medical Center, Salem, Virginia.
David Looney, The Veterans Medical Research Foundation, San Diego, California.
Hien H. Nguyen, Veterans Affairs Northern California Health Care System, Sacramento, California.
Adriana Sánchez, Washington Veterans Affairs Medical Center, Washington, DC.
Amy C. Weintrob, Infectious Diseases Section, Washington Veterans Affairs Medical Center, Washington, DC.
Birgit Grund, School of Statistics, University of Minnesota, Minneapolis, Minnesota.
Shweta Sharma, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Cavan S. Reilly, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Roger Paredes, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Agnieszka Bednarska, Wojewódzki Szpital Zakaźny w Warszawie, Medical University of Warsaw, Warsaw, Poland.
Norman P. Gerry, Advanced Biomedical Laboratories, Cinnaminson, New Jersey
Abdel G. Babiker, The Medical Research Council Clinical Trials Unit at University College London, London, England.
Victoria J. Davey, U.S. Department of Veterans Affairs, Washington, DC.
Annetine C. Gelijns, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York.
Elizabeth S. Higgs, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
Virginia Kan, Infectious Diseases Section, Veterans Affairs Medical Center, Washington, DC.
Gail Matthews, The Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia.
B. Taylor Thompson, Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts.
Philippe Legenne, Molecular Partners, Zurich, Switzerland.
Richa Chandra, Novartis, East Hanover Township, New Jersey.
H. Clifford Lane, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland.
James D. Neaton, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Jens D. Lundgren, CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark.
References
- 1. Gupta A , Gonzalez-Rojas Y , Juarez E , et al; COMET-ICE Investigators. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941-1950. [PMID: ] doi: 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 2. Cohen MS , Nirula A , Mulligan MJ , et al; BLAZE-2 Investigators. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46-55. [PMID: ] doi: 10.1001/jama.2021.8828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreich DM , Sivapalasingam S , Norton T , et al; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. [PMID: ] doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinreich DM , Sivapalasingam S , Norton T , et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238-251. [PMID: ] doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen P , Nirula A , Heller B , et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229-237. [PMID: ] doi: 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group.. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622-635. [PMID: ] doi: 10.1016/S1473-3099(21)00751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lundgren JD , Grund B , Barkauskas CE , et al; ACTIV-3/TICO LY-CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905-914. [PMID: ] doi: 10.1056/NEJMoa2033130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ACTIV-3-Therapeutics for Inpatients with COVID-19 (TICO) Study Group.. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2022. [PMID: ] doi: 10.1016/S2213-2600(22)00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takashita E , Kinoshita N , Yamayoshi S , et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant [Letter]. N Engl J Med. 2022;386:995-998. [PMID: ] doi: 10.1056/NEJMc2119407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. RECOVERY Collaborative Group.. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665-676. [PMID: ] doi: 10.1016/S0140-6736(22)00163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stumpp MT , Dawson KM , Binz HK . Beyond antibodies: the DARPin drug platform. BioDrugs. 2020;34:423-433. [PMID: ] doi: 10.1007/s40259-020-00429-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Binz HK , Amstutz P , Kohl A , et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 13. Rothenberger S, Hurdiss DL, Walser M, et al. Ensovibep, a novel trispecific DARPin candidate that protects against SARS-CoV-2 variants. bioRxiv. Preprint posted online 26 February 2022. doi: 10.1101/2021.02.03.429164 [DOI]
- 14. Walser M, Rothenberger S, Hurdiss DL, et al. Highly potent anti-SARS-CoV-2 multivalent DARPin therapeutic candidates. bioRxiv. Preprint posted online 21 July 2021. doi: 10.1101/2020.08.25.256339 [DOI]
- 15. Kumarasamy N, Abrishamian L, Bonten M, et al. Interim results from the randomised, controlled EMPATHY phase 2/3 study evaluating ensovibep, a DARPin therapeutic, in patients with mild-to-moderate COVID-19 [Abstract]. Presented at 32nd European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, 23–26 April 2022. Abstract no. 05017.
- 16. Murray DD , Babiker AG , Baker JV , et al. Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3). Clin Trials. 2022;19:52-61. [PMID: ] doi: 10.1177/17407745211049829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davey RT Jr, Fernández-Cruz E, Markowitz N, et al; INSIGHT FLU-IVIG Study Group.. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med. 2019;7:951-963. [PMID: ] doi: 10.1016/S2213-2600(19)30253-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection.. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192-e197. [PMID: ] doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beigel JH , Tomashek KM , Dodd LE , et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383:1813-1826. [PMID: ] doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dougan M , Nirula A , Azizad M , et al; BLAZE-1 Investigators. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382-1392. [PMID: ] doi: 10.1056/NEJMoa2102685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Brien MP , Forleo-Neto E , Sarkar N , et al; COVID-19 Phase 3 Prevention Trial Team. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327:432-441. [PMID: ] doi: 10.1001/jama.2021.24939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Montgomery H , Hobbs FDR , Padilla F , et al; TACKLE study group. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022. [PMID: ] doi: 10.1016/S2213-2600(22)00180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundgren JD , Grund B , Barkauskas CE , et al; ACTIV-3/TICO Bamlanivimab Study Group. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels. A randomized controlled trial. Ann Intern Med. 2022;175:234-243. [PMID: ] doi: 10.7326/M21-3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.