Abstract
Infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant is usually asymptomatic or mild and appears to be poorly immunogenic at least in unvaccinated individuals. Here, we found that health care workers vaccinated with 2 doses of Sputnik V and a booster dose of ChAdOx1 mount a vigorous neutralizing-antibody response after Omicron breakthrough infection.
Keywords: COVID-19, SARS-CoV-2, Omicron, neutralizing antibodies
Breakthrough infections with SARS-CoV-2 Omicron are seen with increasing frequency. We found that Omicron breakthrough infection after heterologous vaccination with 2 doses of Sputnik V and a booster dose of ChAdOx1 induces a strong neutralizing response against Omicron.
The emergence of the variant of concern (VOC) Omicron with 37 amino acid substitutions in the spike protein has challenged the immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) conferred by both vaccination and previous infection [1]. It also severely compromised the therapeutic activity of most monoclonal antibodies directed to the receptor-binding motif of the spike protein [2]. Pioneering studies have reported that plasma from convalescent patients or individuals vaccinated with 2 doses of the different anti–SARS-CoV-2 vaccine platforms (mRNA, viral vectors, and virus inactivated vaccines) show very low levels of neutralizing antibodies or no neutralizing activity when assessed against the Omicron variant [1, 2]. A booster vaccine dose has shown to increase the neutralizing response against Omicron and hence it has been incorporated to the vaccination scheme applied in adults worldwide [3, 4]. In this context, with the emergence of new viral variants and a decline in the memory immune response, rational criteria to guide the administration of additional booster doses is needed. Omicron infection itself could act in a similar way to a booster dose; however, its immunogenicity has not been clearly defined in previously vaccinated individuals. Here we analyzed the immunogenicity of Omicron breakthrough infection in a cohort of health care workers vaccinated with 2 doses of Sputnik V and a booster dose of ChAdOx1.
METHODS
Our study was approved by the Ethics Committee at Hospital Alejandro Posadas, Hospital Central de San Isidro Melchor A. Posse, Hospital de Clínicas José de San Martín, and Hospital de Villa Mercedes Juan Domingo Perón, Argentina, in accordance with the Declaration of Helsinki. Written informed consent was obtained from all donors. Health care workers vaccinated with 2 doses of Sputnik V (dose interval mean, 24 days; range, 18–56 days) in December 2020 or January 2021 were initially recruited in August and September 2021 (n = 113). A serum sample was collected 129 to 225 days after full vaccination. Participants were followed up after receiving a heterologous ChAdOx-1 booster dose in November 2021 (mean interval between second and booster dose was 301 days; range, 215–347 days). Individuals with previous documented infection and/or detectable SARS-CoV-2 nucleocapsid-specific antibodies were excluded from the analysis (n = 28). A second serum sample was collected in February 2022, and samples were grouped according to participants with no record of infection (n = 48) and those with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection (n = 37) between 24 December 2021 and 31 January 2022, a period in which more than 99% of new infections in Argentina were attributed to the VOC Omicron (BA.1 lineage), according to the reports of the National Ministry of Health [5]. Mean age of the cohort was 46.2 years (range, 28–69 years) and 45.5 years (range, 28–64 years) in the uninfected and infected participants, respectively. Sexdistribution between uninfected and infected participants (female to male ratio) was 36:12 and 27:10 respectively. No statistical differences between groups were observed regarding the age, gender distribution, or dose intervals between the second and booster doses. The interval between booster dose and breakthrough infection was 41 days (range, 7–54 days) and the interval between infection (PCR-positive test) and sampling was 38 days (range, 11–76 days). All infections were mild and no participant required hospitalization. Informed consent was obtained from all study participants. Blood samples were collected in dry tubes and serum was separated and stored at −20°C until use. Spike-specific immunoglobulin G (IgG) titers were determined by 2-fold serial dilutions using a 2-step COVIDAR ELISA kit following manufacturer’s instructions [6]. Nucleocapsid-specific IgG was detected using 2-step enzyme-linked immunosorbent assay (ELISA). Serum neutralizing capacity was evaluated using the ancestral SARS-CoV-2 reference strain 2019 B.1 (GISAID accession ID, EPI_ISL_499083) and the VOC Omicron (BA.1 lineage; GISAID accession ID, EPI_ISL_10633761). Vero cells (American Type Culture Collection) were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle’s high glucose medium (DMEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; GIBCO). Serum samples were heat-inactivated (30 minutes, 56°C), and serial dilutions (1/4 to 1/16 384) were incubated for 1 hour at 37°C with SARS-CoV-2 in DMEM 2% FBS. Fifty μL of the mixtures were then incubated with Vero cell monolayers for 1 hour at 37°C (multiplicity of infection = 0.01). Then, the medium was removed and replaced by DMEM 2% FBS. After 72 hours of culture, cells were fixed with paraformaldehyde 4% (4°C for 20 minutes) and stained with crystal violet solution in methanol. The viral cytopathic effect on the monolayer of Vero cells was analyzed and the neutralization titer was defined as the highest serum dilution that prevented any cytopathic effect. Multiple comparisons were analyzed by nonparametric Kruskal-Wallis test and Dunn posttest, and for 2-group comparisons Mann-Whitney test or Wilcoxon pair-matched test were used. Data were analyzed using GraphPad Prism version 8.4.3 software.
RESULTS
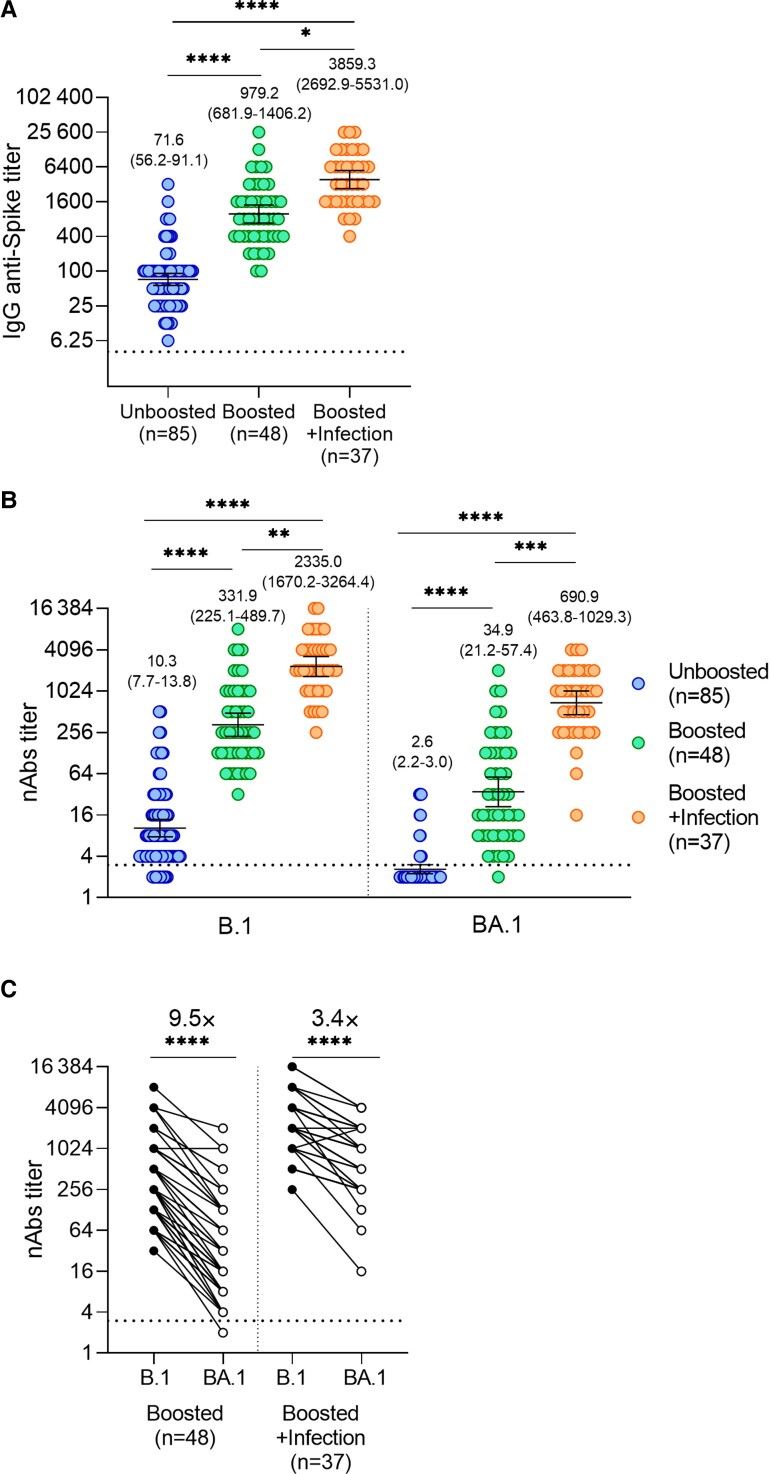
Serum anti-spike IgG titers in health care workers who received 2 doses of Sputnik V (geometric mean titer [GMT] = 71.6) increased 13.7 times after booster vaccination (GMT = 979.2). Breakthrough infection with Omicron further increased IgG titers, reaching values 3.9-fold higher (GMT = 3859.3) compared with uninfected boosted individuals (Figure 1A). We then analyzed neutralizing activity against the original Wuhan (B.1) variant and Omicron (BA.1). Serum neutralizing titers against the Wuhan variant in individuals vaccinated with 2 doses of Sputnik V (GMT = 10.3) increased 32.2 times upon booster vaccination (GMT = 331.9), reaching values 7–fold higher (GMT = 2335.0) after Omicron breakthrough infection (Figure 1B, left panel). Analysis of neutralizing activity against Omicron showed that most of the samples from individuals vaccinated with 2 doses of Sputnik V were seronegative (GMT = 2.6). After receiving the booster dose, neutralizing titers increased more than 13.4 times while Omicron breakthrough infection in boosted individuals further increased neutralizing titers, reaching values 19.8-fold higher compared with uninfected boosted ones (Figure 1B, right panel). Analysis of paired samples further confirmed that Omicron breakthrough infection in boosted individuals reduced Omicron escape fromneutralizing antibodies (Figure 1C).
Figure 1.
Analysis of antibody response induced by Omicron breakthrough infection. A, Titers of serum IgG anti-spike antibodies were analyzed by ELISA. The geometric means with 95% CIs are shown. B, Serum neutralizing titers against the ancestral variant B.1 and Omicron (BA.1) were analyzed using isolated variants. The geometric means with 95% CIs are shown. C, Paired B.1 and BA.1 neutralization titters for each sample. A and B, Kruskal-Wallis test and Dunn test for multiple comparisons were used. C, Wilcoxon pair-matched test was used. *P = .05, **P = .005, ***P = .0005, ****P = .0001. Dotted lines in panels A, B and C represents detection limits. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; nAbs, neutralizing antibodies.
DISCUSSION
The SARS-CoV-2 Omicron variant shows a high ability to infect vaccinated and convalescent individuals; however, booster vaccination has shown to efficiently protect against severe infection [7]. This response appears to be mediated, at least in part, by enhanced production of neutralizing antibodies with increased potency and breadth compared to the response induced after vaccination with 2 doses [8].
While Omicron infection in unvaccinated individuals appears to induce a poor antibody response that shows little cross-reactivity with the earlier variants [9, 10], recent studies have analyzed the immunogenicity of breakthrough infections in individuals vaccinated with either mRNA vaccines (RNA-1273 and BNT162b2) or Ad26.COV2.S. By studying individuals infected with Omicron sublineage BA.1 at 2–3 weeks after infection, with or without previous vaccination with Pfizer-BNT162b2 or Ad26.CoV2.S, Khan and coworkers reported that previously vaccinated individuals mount a strong neutralizing response not only against BA.1, but also against other variants including Omicron BA.2, Delta, Beta, and the ancestral virus [10]. By contrast, only limited cross-protection was observed in infected unvaccinated individuals [10]. Not only breakthrough infections with Omicron but also with Delta have been shown to result in a marked increase in the antibody response against different VOCs, as reported by Kitchin and coworkers in individuals previously vaccinated with Ad26.CoV2.S [11]. In this regard, however, the observations reported by Servellita and coworkers should be mentioned that showed Omicron breakthrough infections appear to be less immunogenic than those produced by Delta [12]. Interestingly, breakthrough infections by Omicron in boosted vaccinated individuals have also been shown to result in a marked increase in the serum neutralizing activity against Omicron as well as the VOCs Alpha, Beta, and Delta, as reported by Woldemeskel and coworkers [13].
In agreement with these observations, by studying health care workers vaccinated with a heterologous scheme that included 2 doses of Sputnik V and a third dose of ChAdOx1, we found that breakthrough infection markedly increased serum neutralizing titers against both the original Wuhan variant and Omicron. Because breakthrough infections occurred in a period in which more than 99% of new infections in Argentina were attributed to Omicron (BA.1 lineage), we assume that this lineage was responsible for the infection of all the individuals recruited in our study. However, we recognize that the inability to confirm Omicron infection by sequence analysis represents a limitation of the study. Further studies are needed to establish the durability of the antibody response induced by Omicron breakthrough infection and whether this response is actually associated with better protection against both infection with different Omicron lineages and progression to severe COVID-19.
Contributor Information
Augusto Varese, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Bianca Mazzitelli, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Fernando Erra Díaz, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
María Victoria Kjolhede, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Diego Ojeda, Fundación Instituto Leloir, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Alejandra Vellicce, Hospital de Clínicas “José de San Martín”, Buenos Aires, Argentina.
Penélope Arto, Hospital de Clínicas “José de San Martín”, Buenos Aires, Argentina.
Carla Cicero, Hospital de Clínicas “José de San Martín”, Buenos Aires, Argentina.
María Pascowsky, Hospital “Alejandro Posadas”, Buenos Aires, Argentina.
Laura Figueras, Hospital Central de San Isidro “Melchor A. Posse”, Buenos Aires, Argentina.
Bárbara Broese, Hospital Central de San Isidro “Melchor A. Posse”, Buenos Aires, Argentina.
Rosa Dávila, Hospital de Villa Mercedes “Juan Domingo Perón”, San Luis, Argentina.
Rocío Zarlenga, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Federico Rivelli, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Camila Verruno, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Valeria Silenzi, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Ivana Beltrán, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Andrea Gamarnik, Fundación Instituto Leloir, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Ana Ceballos, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Carlos Zala, Hospital Central de San Isidro “Melchor A. Posse”, Buenos Aires, Argentina.
Adelina Badolati, Hospital “Alejandro Posadas”, Buenos Aires, Argentina.
Jorge Geffner, Facultad de Medicina, Universidad de Buenos Aires, Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina.
Notes
Acknowledgments. We thank Facundo Di Diego García, Ignacio Mazzitelli, Ana Paletta, and Dr Mauricio Carobene for isolation and characterization of SARS-CoV-2 Omicron VOC. We thank all team members of the Hospital de Clínicas José de San Martín, Hospital Alejandro Posadas, Hospital Central de San Isidro Melchor A. Posse, and Hospital de Villa Mercedes Juan Domingo Perón, Argentina. Most of all, we are indebted to all health care workers that were participants in our study.
Financial support. This work was supported by the Fondo Nacional para la Investigación Científica y Tecnológica (grant numbers PICT 2017-1616 and PICT 2018–02844 to J. G.); and the Universidad de Buenos Aires (grant number 20020170100573BA to J. G.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022; 602:664–70. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022; 602:657–63. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pajon R, Doria-Rose NA, Shen X, et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med 2022; 386:1088–91. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 2022; 28:477–80. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voto C, Baumeister E, Campos J, et al. COVID-19 situación de nuevas variantes SARS-CoV-2 en Argentina-SE8/2022 [in Spanish]. https://www.argentina.gob.ar/sites/default/files/2022/04/vigilancia_genomica-se14.pdf. Accessed 9 June 2022.
- 6. Ojeda DS, Ledesma MMGL, Pallarés HM, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog 2021; 17:e1009161. doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrews N, Stowe J, Kirsebom F, et al. Covid;19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muecksch F, Wang Z, Cho A, et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost [published online ahead of print 21 April 2022]. Nature. doi: 10.1038/s41586-022-04778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rössler A, Knabl L, von Laer D, Kimpel J. Neutralization profile after recovery from SARS-CoV-2 Omicron infection. N Engl J Med 2022; 386:1764–6. doi: 10.1056/NEJMc2201607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan K, Karim F, Cele S, et al. Omicron infection enhances Delta antibody immunity in vaccinated persons [published online ahead of print 6 May 2022]. Nature 2022. doi: 10.1038/s41586-022-04830-x. [DOI] [PMC free article] [PubMed]
- 11. Kitchin D, Richardson SI, van der Mescht MA, et al. Ad26.COV2.S breakthrough infections induce high titers of neutralizing antibodies against Omicron and other SARS-CoV-2 variants of concern. Cell Rep Med 2022; 3:100535. doi: 10.1016/j.xcrm.2022.100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Servellita V, Syed AM, Morris MK, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and delta variants. Cell 2022; 185:1539–48.e5. doi: 10.1016/j.cell.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woldemeskel BA, Garliss CC, Aytenfisu TY, et al. SARS-CoV-2–specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge. JCI Insight 2022; 7:e159474. doi: 10.1172/jci.insight.159474. [DOI] [PMC free article] [PubMed] [Google Scholar]