Abstract
Measures to limit SARS-CoV-2 transmission in 2020 reduced other viral infections. Among 7 US children’s hospitals, invasive pneumococcal disease cumulative incidence decreased by 46% in 2020 vs 2017-2019. Limited droplet transmission of pneumococci and preceding viral pathogens may be responsible.
Keywords: children, invasive pneumococcal disease, pandemic
Despite the significant decrease in disease burden due to Streptococcus pneumoniae following the introduction of pneumococcal conjugate vaccines, this pathogen is still responsible for significant morbidity and mortality within the pediatric population. As invasive pneumococcal disease (IPD) is often preceded by viral upper respiratory infection [1], the reports of bacterial coinfection in patients affected by SARS-CoV-2 during the pandemic were not unexpected [2]. After the implementation of measures to limit the spread of SARS-CoV-2, such as physical distancing, hand washing, remote school, and mask use, a reduced number of cases of other viral infections transmitted via droplets like RSV (respiratory syncytial virus) and influenza was observed [3]. England’s health department reported a 30% decline in incidence of IPD in all age groups during the pandemic [4]. A multinational surveillance initiative reviewing laboratory data revealed a decrease in the expected number of invasive pneumococcal isolates between March and May 2020 when compared with the previous 2 years [5]. We hypothesized that a similar decrease in the number of IPD cases would be observed at US children’s hospitals during the early phase of the COVID-19 pandemic as a consequence of COVID-19 mitigation efforts.
METHODS
We reviewed all IPD cases from January 2017 through December 2020 at 7 children’s hospitals from the US Pediatric Multicenter Pneumococcal Surveillance Group [6]. IPD was defined by the isolation of S. pneumoniae from normally sterile sites (eg, blood, cerebrospinal, pleural, synovial, or peritoneal fluid). Pneumococcal pneumonia was determined by an abnormal chest radiograph in the presence of a positive blood, pleural fluid, or lung culture. A mastoiditis diagnosis required clinical and radiologic findings in addition to isolation of the organism from the middle ear, subperiosteal abscess, or mastoid bone culture. Serotypes were determined by the capsular swelling method [6]. Hospital admission numbers for each center were obtained for incidence calculations. Statistical analyses were performed using STATA 16 (StataCorp LLC, College Station, TX, USA). A P < .05 was considered significant. The surveillance study was approved by the Institutional Review Board of each institution.
RESULTS
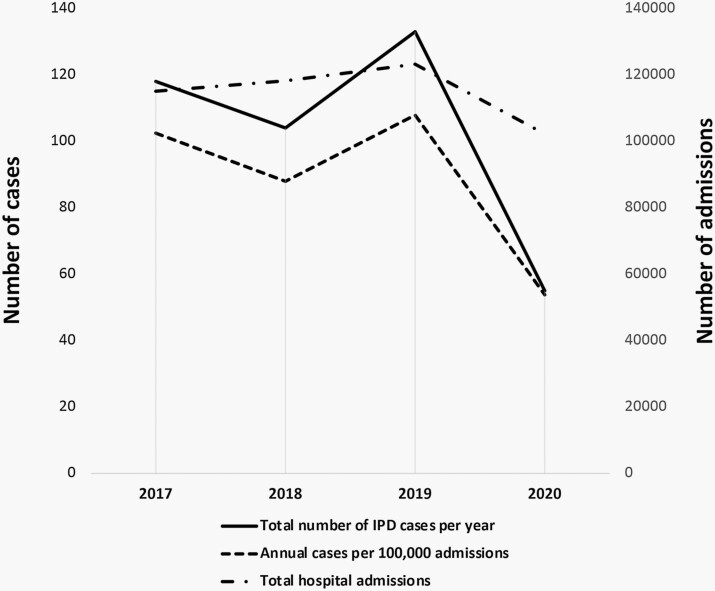
A total of 410 IPD cases were identified. The cumulative incidence of IPD in patients 0-22 years of age decreased from 99.6/100 000 admissions from January 2017 through December 2019 (n = 355) to 53.8/100 000 admissions during 2020 (n = 55, risk ratio 0.54, 95% CI: 0.41-0.72, P < .00001) (Figure 1).
Figure 1.
Total number of IPD cases per year, number of cases per 100 000 admissions, and total number of admissions from 2017 through 2020.
Pneumococcal bacteremia and pneumonia rates decreased significantly in 2020 (risk ratio 0.50, 95% CI: 0.32-0.78, P = .002 and risk ratio 0.49, 95% CI: 0.26-0.92, P = .02, respectively) (Supplemental Figure 1). Although not statistically significant, we observed fewer cases of meningitis and mastoiditis in 2020 compared to previous years (risk ratio 0.58, 95% CI: 0.32-1.07, P = .08 and risk ratio 0.36, 95% CI: 0.11-1.18, P = .08, respectively). Sex, race, age, and the presence of comorbidities were not significantly different for patients in 2020 compared with those seen in 2017-2019.
The most common serotypes isolated in 2020 were 35B, 3, 15B, and 15C. Serotype 3 was predominant during the study period and caused 12% of cases overall (Supplemental Table). Serotype 33F showed a marked decrease during the study period while serotypes 35B, 15B, and 15C increased as a cause of IPD.
As we analyzed the monthly cases of IPD across the study years, we observed a similar pattern in the years 2017-2019 with an overall downtrend in cases over the summer and an uptrend in the last months of the year. In 2020, 33 (60%) of 55 cases of IPD occurred during the first 3 months of the year, with a marked decrease in the number of cases starting in April (Supplemental Figure 2).
DISCUSSION
This surveillance study observed a 46% decrease in the rate of IPD per 100 000 admissions in 7 US children’s hospitals during the first year of the SARS-CoV-2 pandemic when compared with the previous 3 years, similar to reports from other areas of the world. Most cases of IPD (33/55) seen in 2020 presented between January and March, before measures, such as mask use and physical distancing were implemented in most states by early April. This finding suggests that these measures limit the transmission of pneumococcus via droplets. The seasonal pattern of IPD cases observed between 2017 and 2019 (Supplemental Figure 2) overlaps with the pattern seen with some respiratory viral infections [1]. This observation supports the concept that viral infection often precedes IPD and a reduction in RSV and influenza infections in 2020 may have contributed to the decrease in IPD cases. Future prospective studies to assess the use of the different precautions could help determine which measure was most helpful in limiting transmission and may aid in preventing IPD in highly vulnerable populations.
Supplementary Material
Note
Financial support. This study was conducted in part as a collaboration between Baylor College of Medicine and Pfizer. Baylor College of Medicine is the study sponsor. Pfizer had no role in the design and conduct of the study.
Potential conflicts of interest. S. L. K. is the PI for the IPD surveillance study, which is supported by Pfizer. K. G. H. receives support from Pfizer for other pneumococcus-related studies. All other authors have no conflicts of interest to disclose related to this study.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Adriana Sarmiento Clemente, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
Sheldon L Kaplan, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
William J Barson, Department of Pediatrics, Nationwide Children’s Hospital and College of Medicine and Public Health, The Ohio State University, Columbus, Ohio, USA.
Philana Ling Lin, Department of Pediatrics, Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
José R Romero, Department of Pediatrics, Arkansas Children’s Hospital and University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA.
John S Bradley, Department of Pediatrics, Rady Children’s Hospital–San Diego and University of California, San Diego, San Diego, California, USA.
Tina Q Tan, Department of Pediatrics, Ann and Robert H. Lurie Children’s Hospital of Chicago and Feinberg School of Medicine, Northwestern University, Chicago, Illinois, USA.
Pia S Pannaraj, Department of Pediatrics, Children’s Hospital Los Angeles and Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Laurence B Givner, Department of Pediatrics, Brenner Children’s Hospital and Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Kristina G Hultén, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas, USA.
References
- 1. Pelton SI, Jacobs MR. Pneumococcal infections. In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, eds. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 8th ed., Vol. 1. Philadelphia, PA: Elsevier; 2019: 856–893. [Google Scholar]
- 2. Root-Bernstein R. Pneumococcal and influenza vaccination rates and pneumococcal invasive disease rates set geographical and ethnic population susceptibility to serious COVID-19 cases and deaths. Vaccines 2021; 9:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeoh DK, Foley D, Minney-Smith CA, et al. Impact of Coronavirus Disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian Winter. Clin Infect Dis 2021; 72:2199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the COVID-19 pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with SARS-CoV-2: prospective national cohort study, England. Clin Infect Dis 2021; 72:e65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brueggemann AB, Jansen Van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health 2021; 3:e360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan SL, Barson WJ, Lin PL, et al. Invasive pneumococcal disease in children’s hospitals: 2014-2017. Pediatrics 2019; 144:e20190567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.