Abstract
Background
At the initiation of the COVID-19 pandemic, restrictions forced researchers to decide whether to continue their ongoing clinical trials. The PREPARE (Pragmatic Randomized Trial Evaluating Pre-Operative Alcohol Skin Solutions in Fractured Extremities) trial is a pragmatic cluster-randomized crossover trial in patients with open and closed fractures. PREPARE was enrolling over 200 participants per month at the initiation of the pandemic. We aim to describe how the COVID-19 research restrictions affected participant enrollment.
Methods
The PREPARE protocol permitted telephone consent, however, sites were obtaining consent in-person. To continue enrollment after the initiation of the restrictions participating sites obtained ethics approval for telephone consent scripts and the waiver of a signature on the consent form. We recorded the number of sites that switched to telephone consent, paused enrollment, and the length of the pause. We used t-tests to compare the differences in monthly enrollment between July 2019 and November 2020.
Results
All 19 sites quickly implement telephone consent. Fourteen out of nineteen (73.6%) sites paused enrollment due to COVID-19 restrictions. The median length of enrollment pause was 46.5 days (range, 7–121 days; interquartile range, 61 days). The months immediately following the implementation of restrictions had significantly lower enrollment.
Conclusion
A pragmatic design allowed sites to quickly adapt their procedures for obtaining informed consent via telephone and allowed for minimal interruptions to enrollment during the pandemic.
Keywords: COVID-19, Enrollment, Pragmatic trials, PREPARE trial
Trial Registration: clinicaltrials.gov: NCT03523962.
Funding
This work was supported by the Patient-Centered Outcomes Research Institute (PCS-1609-36512) and the Canadian Institutes of Health Research (Foundation Grant).
1. Introduction
On March 13, 2020, the COVID-19 pandemic forced restrictions across the world to slow the spread of the novel coronavirus. In response, many hospitals and research ethics boards introduced policies limiting in-person contact, which prohibited in-person clinical research activities. For clinical trials to continue, researchers had to develop a plan for continuation that demonstrated that there is no increased risk of transmission of COVID-19 as a result of their research activites [1]. As a result, thousands of clinical trials paused or stopped participant enrollment and follow-up [2].
Pragmatic trials evaluate the effectiveness of interventions under real-life, routine conditions. One of the key benefits of pragmatic trials is that the results are more generalizable, which offers higher external validity. The PREPARE trial (Pragmatic Randomized Trial Evaluating Pre-Operative Alcohol Skin Solutions in Fractured Extremities) follows a pragmatic design. Pragmatic aspects of the PREPARE trial include cluster randomization, broad eligibility criteria, consent after surgery, use of interventions that do not require an increase in care delivery and, no additional tests, procedures, or follow-up visits. Most relevant, the PREPARE trial protocol has flexible consent (e.g. in-person and telephone) and follow-up (e.g. in-person, telephone, text, email).
In March 2020, the PREPARE trial was approximately half-way through enrollment and was enrolling over 200 participants a month. We aim to describe how COVID-19 restrictions effected participant enrollment in the PREPARE trial. Specific objectives include: 1) number of hospitals that were able to transition to a telephone consent model; 2) number of hospitals who paused enrollment; and 3) the length of the enrollment pauses and impact on overall enrollment.
2. Methods
2.1. The PREPARE trial
The PREPARE trial is a pragmatic cluster randomized crossover trial that compares iodine povacrylex (0.7% free iodine) in 74% alcohol (DuraPrep™) versus 2% chlorhexidine gluconate in 70% isopropyl alcohol (Chloraprep™) in fracture patients. Each clinical site uses the initially allocated skin preparation solution for a period of two months and subsequently crosses over to the opposite solution for their second recruitment period. This process repeats every two months for 24 months. The primary outcome is surgical site infection (SSI) as defined by the Centers for Disease Control and Prevention (CDC) [3]. The secondary outcome is unplanned fracture-related reoperations within 12 months to manage infection, wound healing problems and fracture healing problems. The PREPARE trial will enroll at least 1,540 patients with open appendicular fractures (open fracture cohort) and 6,280 patients with closed lower extremity and pelvic fractures (closed fracture cohort) at hospitals in North America. These cohorts constitute distinct patient populations with different risks of infection and data from each cohort will be analyzed separately, as described in the protocol [4]. PREPARE is registered on clinicaltrials.gov (NCT03523962) and the protocol has been published [4].
2.2. COVID-19 restrictions
Before the COVID-19 pandemic, all clinical sites obtained informed consent in person at their hospital or fracture clinic. However, after March 13, 2020, COVID-19 restrictions limited in-person consenting at most participating hospitals. Affected clinical sites were encouraged by the trial Methods Centre to transition to telephone consent, which was already included as a consent option in the PREPARE protocol. Prior to implementing telephone consent, clinical sites had to determine local logistics and obtain ethics approval for telephone consent scripts and procedures. Rapid ethics approval to waive the requirement for a participant's written signature on the informed consent form was also obtained from the central ethics committee and local ethics committees (for sites not using the central ethics committee) was also obtained.
2.3. Statistical analysis
For clinical sites that were enrolling participants at the onset of the pandemic, we descriptively evaluated the number of clinical sites that transitioned to telephone consent, the number of clinical sites that had to pause enrollment, and the length of the enrollment pauses.
We evaluated monthly enrollment from July 2019 to November 2020. For this evaluation, we included sites that completed at least eight months of enrollment prior to the onset of the pandemic. Results are stratified by open and closed fracture cohorts and are summarized using descriptive statistics. Statistical analysis using paired t-tests were also used to compare the differences in monthly enrollment. Data analyses were conducted using R (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria).
2.4. Results
At the onset of the pandemic on March 13, 2020, 19 clinical sites were enrolling participants into the PREPARE trial (Table 1). Fourteen (73.6%) clinical sites paused enrollment due to COVID-19 restrictions, while they transitioned to telephone consent models. The median length of enrollment pause was 46.5 days (range, 7–121 days; interquartile range, 61 days). By July 15, 2020, all clinical sites resumed enrollment. Fifteen (78.9%) sites transitioned to telephone consent and four (21.1%) clinical sites continued with in-person consent.
Table 1.
Enrollment pause details.
| Cluster | Location | Enrollment Pause Date | Enrollment Restart Date | Duration of Pause (Days) |
|---|---|---|---|---|
| Duke University | Durham, North Carolina | No Pause | ||
| Hamilton Health Sciences | Hamilton, Ontario | No Pause | ||
| Regional Medical Center of San Jose | San Jose, California | No Pause | ||
| Sanford Health | Sioux-Falls, South Dakota | No Pause | ||
| University of Utah | Salt Lake City, Utah | No Pause | ||
| R Adams Cowley Shock Trauma Center | Baltimore, Maryland | March 15, 2020 | March 22, 2020 | 7 |
| Mississippi Medical Center | Jackson, Mississippi | March 18, 2020 | April 7, 2020 | 20 |
| Dartmouth-Hitchcock Medical Center | Lebanon, New Hampshire | March 18, 2020 | April 13, 2020 | 26 |
| IU Health | Indianapolis, Indiana | March 17, 2020 | April 15, 2020 | 29 |
| Wake Forest Baptist Health | Winston-Salem, North Carolina | March 17, 2020 | April 21, 2020 | 35 |
| Penn Presbyterian Medical Center | Philadelphia, Pennsylvania | March 13, 2020 | April 23, 2020 | 41 |
| Inova Fairfax Medical Campus | Falls Church, Virginia | March 18, 2020 | May 1, 2020 | 44 |
| MetroHealth | Cleveland, Ohio | March 16, 2020 | May 4, 2020 | 49 |
| San Antonio Military Medical Center | San Antonio, Texas | March 20, 2020 | June 1, 2020 | 73 |
| Royal Columbia Hospital | New Westminster, British Columbia | March 17, 2020 | June 4, 2020 | 79 |
| Brigham and Women's Hospital | Boston, Massachusetts | March 16, 2020 | June 16, 2020 | 92 |
| Massachusetts General Hospital | Boston, Massachusetts | March 16, 2020 | June 16, 2020 | 92 |
| University of Maryland Capital Region Health | Cheverly, Maryland | March 17, 2020 | June 15, 2020 | 90 |
| Prisma Health | Greenville, North Carolina | March 16, 2020 | July 15, 2020 | 121 |
Thirteen clinical sites had eight full months of enrollment prior to the COVID-19 pandemic. The average monthly enrollment prior to COVID-19 restrictions was 198 participants in the closed fracture cohort (standard deviation (SD) 22, range: 161–227) and 41 participants in the open fracture cohort (SD 16, range: 22–60). Enrollment was the lowest in April 2020, when 47 participants in the closed fracture cohort and nine participants in the open fracture cohort were enrolled. Enrollment began increasing in May 2020 in the open fracture cohort and in June 2020 in the closed fracture cohort - coinciding with clinical sites resuming enrollment.
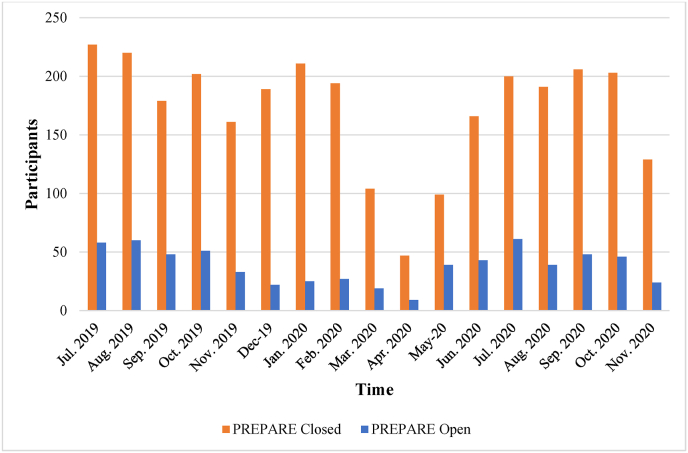
From June 1, 2020, to November 30, 2020, the average monthly enrollment rates were 183 participants in the closed fracture cohort (SD 30, range: 129–206) and 44 participants in the open fracture cohort (SD 12, range: 24–61), which were close to pre-COVID enrollment numbers. Fig. 1 shows enrollment numbers during this period.
Fig. 1.
Prepare trial monthly enrollment.
March, April, and May 2020 had significantly lower enrollment in the closed fracture cohort (p = 0.01) and March and April 2020 had significantly lower enrollment in the open fracture cohort (p = 0.04). Enrollment was similar in all other months.
From July 1, 2019, to February 28, 2020, 78% of eligible patients approached were enrolled into the PREPARE trial. During the period of COVID-19 restrictions (March 13, 2020, to May 31, 2020) where telephone consent was primarily used, this percentage increased to 81%. From June 1, 2020, to November 30, 2020, the percentage of eligible patients approached who were enrolled into the PREPARE trial decreased slightly to 79%.
3. Discussion
The PREPARE trial is a pragmatic high-enrolling clinical trial that was approximately half-way through enrollment at the start of the COVID-19 pandemic. Fourteen of the nineteen clinical sites in the PREPARE trial paused enrollment in response to the initial restrictions. Five clinical sites did not pause enrollment. Reasons for this include a quick transition to telephone consent or local policies which allowed in-person consent to continue with modifications including personal protective equipment and social distancing. Reasons for variation in the duration of the enrollment pause at clinical sites include differences in hospital policies, differences in the prevalence of COVID-19 transmission in each region, changes in research personnel, and differences in the ability of research personnel to adjust to remote work environments.
Key pragmatic features of the PREPARE trial that aided with study continuation during the initial restrictions include the lack of tests, visits, and procedures outside of usual care practices and the flexibility for remote follow-up visits. These features allowed the study to be conducted without exposing participants and study personnel to settings that would increase the risk of COVID-19 transmission. The pragmatic nature of the trial also contributed to the brief duration of the pauses in enrollment. Specifically, the inclusion of telephone consent in the protocol allowed clinical sites to rapidly transition to telephone consent, without protocol amendments. With support from the Methods Centre, clinical sites determined local logistics and obtained rapid ethics approval for telephone consent scripts and procedures. Enrollment decreased from 272 participants in February 2020 to a low of 47 participants in April 2020 in the closed fracture cohort. Enrollment decreased from 32 participants in February 2020 to a low of nine participants in April 2020 in the open fracture cohort. Although enrollment was significantly reduced in March, April and May 2020 in the closed fracture cohort and March and April 2020 in the open fracture cohort, all sites resumed enrollment by July 2020. Despite experiencing changes in the absolute number of participants enrolled from March to May 2020, the use of telephone consent also did not affect the percentage of eligible participants who enrolled in the trial. Given the short duration that enrollment was affected, COVID-19 had minimal impact on overall enrollment and project timelines. We stratified our analyses by open fracture versus closed fracture because these represent two distinct patient populations, and effectiveness of the PREPARE trial interventions will be assessed separately for each cohort, as described in the protocol [4].
Limitations of our findings include the exclusion of some participating clinical sites from our monthly enrollment analysis. To be included, sites must have had at least eight full months of enrollment prior to the COVID-19 pandemic. Additionally, weather and seasonality are associated with differing risks and incidence of fractures [5,6] and can, therefore, influence enrollment. However, no adjustments in our analysis were made to control for these variables. We also acknowledge that this was also a very low-risk study comparing an element of treatment that patients generally do not have a say in determining. The impact of findings related to consent in a study like this may not be generalizable to trials that evaluate new treatments with a higher risk profile.
Pragmatic trials are gaining momentum [7] because they are simple to conduct and their findings are highly generalizable. A secondary and unintended benefit uncovered by conducting a pragmatic trial during a pandemic is that they may be less prone to disruptions in the presence of an epidemic in comparison to explanatory trials. By following a pragmatic design that pre-emptively included telephone consent in the PREPARE protocol, the PREPARE trial experienced minimal interruptions to enrollment during the COVID-19 pandemic. With COVID-19 anticipated to become endemic, pragmatic designs can be used by researchers to mitigate the risk of COVID-19 transmission.
Acknowledgements
*THE PREP-IT Investigators
Executive Committee: Gerard P. Slobogean (Principal Investigator, University of Maryland School of Medicine, Baltimore, MD); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Jeffrey Wells (Patient Representative, Trauma Survivors Network, Falls Church, VA); Mohit Bhandari (Principal Investigator, McMaster University, Hamilton, ON)
Steering Committee: Gerard P. Slobogean (Co-Chair, University of Maryland School of Medicine, Baltimore, MD); Mohit Bhandari (Co-Chair, McMaster University, Hamilton, ON); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); C. Daniel Mullins (University of Maryland School of Medicine, Baltimore, MD); Lehana Thabane (McMaster University, Hamilton, ON); Jeffrey Wells (Trauma Survivors Network, Falls Church, VA); Amber Wood (Association of periOperative Registered Nurses, Denver, CO)
Adjudication Committee: Gregory J. Della Rocca (Chair, University of Missouri, Columbia, MO); Anthony D. Harris, (University of Maryland School of Medicine, Baltimore, MD); Joan Hebden (University of Maryland School of Medicine, Baltimore, MD); Kyle J. Jeray (Greenville Health System, Greenville, SC); Lucas S. Marchand (University of Utah, Salt Lake City, UT); Lyndsay M. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Robert Zura (LSU Health, New Orleans, LA); Christopher Lee (University of California, Los Angeles, CA); Joseph Patterson (University of Southern California, Los Angeles, CA)
Data and Safety Monitoring Committee: Michael J. Gardner (Chair, Stanford University School of Medicine, Palo Alto, CA); Jenna Blasman (Patient Representative, Kitchener, ON); Jonah Davies (University of Washington, Seattle, WA); Stephen Liang (Washington University, St. Louis, MO); Monica Taljaard (Ottawa Hospital Research Institute, Ottawa, ON)
Research Methodology Core: PJ Devereaux (McMaster University, Hamilton, ON); Gordon H. Guyatt (McMaster University, Hamilton, ON); Lehana Thabane (McMaster University, Hamilton, ON); Diane Heels-Ansdell (McMaster University, Hamilton, ON)
Patient Centred Outcomes Core: Debra Marvel (Patient Representative, Baltimore, MD); Jana Palmer (Patient Representative, Baltimore, MD); Jeffrey Wells (Patient, Trauma Survivors Network, Falls Church, VA); Jeff Friedrich (Editor, Slate Magazine, Washington, DC); C. Daniel Mullins (University of Maryland School of Medicine, Baltimore, MD); Nathan N. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Ms. Frances Grissom (Trauma Survivor Network, Baltimore, MD)
Orthopaedic Surgery Core: Gregory J. Della Rocca (University of Missouri, Columbia, MO); I. Leah Gitajn (Dartmouth University, Hanover, NH); Kyle J. Jeray (Greenville Health System, Greenville, SC); Saam Morshed (San Francisco General Hospital, San Francisco, CA); Robert V. O’Toole (University of Maryland School of Medicine, Baltimore, MD); Bradley A. Petrisor (Hamilton Health Sciences, Hamilton, ON)
Operating Room Core: Franca Mossuto (Hamilton Health Sciences, Hamilton, ON)
Infectious Disease Core: Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); Manjari G. Joshi (University of Maryland School of Medicine, Baltimore, MD)
Military Core: Jean-Claude D’Alleyrand (Walter Reed National Military Medical Center, Bethesda, MD); Justin Fowler (United States Army, USA); Jessica Rivera (San Antonio Military Medical Center, San Antonio, TX); Max Talbot (Canadian Armed Forces, Montreal, QC)
McMaster University Methods Center (Hamilton, ON): Sheila Sprague (Principal Investigator); Mohit Bhandari (Principal Investigator); David Pogorzelski (Research Coordinator); Shannon Dodds (Research Coordinator); Silvia Li (Research Coordinator); Alejandra Rojas (Research Coordinator); Gina Del Fabbro (Research Assistant); Olivia Paige Szasz (Research Assistant); Diane Heels-Ansdell (Statistician); Paula McKay (Manager); Alexandra Minea (Research Coordinator); Kevin Murphy (Research Coordinator)
University of Maryland School of Medicine Administrative Center (Baltimore, MD): Gerard P. Slobogean (Principal Investigator); Nathan N. O’Hara (Manager); Andrea Howe (Project Manager); Haley Demyanovich (Project Manager)
University of Maryland School of Pharmacy, The PATIENTS Program (Baltimore, MD): C. Daniel Mullins (Executive Director); Michelle Medeiros (Director of Research); Genevieve Polk (Assistant Director, Dissemination and Research); Eric Kettering (Senior Instructional Technology and Dissemination Specialist); Nirmen Mahal (Program Specialist)
PREP-IT Clinical Sites:
Lead Clinical Site (Aqueous-PREP and PREPARE):
University of Maryland School of Medicine, R Adams Cowley Shock Trauma Center, Baltimore, MD: Robert V. O'Toole, Jean-Claude D'Alleyrand, Andrew Eglseder, Aaron Johnson, Christopher Langhammer, Christopher Lebrun, Jason Nascone, Raymond Pensy, Andrew Pollak, Marcus Sciadini, Gerard P. Slobogean, Yasmin Degani, Haley K. Demyanovich, Andrea Howe, Nathan N. O’Hara, Heather Phipps, Eric Hempen
Aqueous-PREP and PREPARE:
Hamilton Health Sciences – General Site, Hamilton, ON: Brad A. Petrisor, Herman Johal, Bill Ristevski, Dale Williams, Matthew Denkers, Krishan Rajaratnam, Jamal Al-Asiri, Jodi Gallant, Kaitlyn Pusztai, Sarah MacRae, Sara Renaud.
Prisma Health - Upstate, Greenville, SC: Kyle J. Jeray, John D. Adams, Michael L. Beckish, Christopher C. Bray, Timothy R. Brown, Andrew W. Cross, Timothy Dew, Gregory K. Faucher, Richard W. Gurich Jr, David E. Lazarus, S. John Millon, M. Jason Palmer, Scott E. Porter, Thomas M. Schaller, Michael S. Sridhar, John L. Sanders, L. Edwin Rudisill, Jr, Kyle M. Altman, Julia C. Quirion, Markus F. Loeffler, Erin R. Pichiotino, Austin A. Cole, Ethan J. Maltz, Wesley Parker, T. Bennett Ramsey, Alex Burnikel, Michael Colello, Russell Stewart, Jeremy Wise, M. Christian Moody, Matthew Anderson, Joshua Eskew, Benjamin Judkins, James M. Miller, Stephanie L. Tanner, Rebecca G. Snider, Emily Bray, Harper Abbott
IU Health Methodist Hospital, Indianapolis, IN: Roman M. Natoli, Todd O. McKinley, Walter W. Virkus, Anthony T. Sorkin, Jan P. Szatkowski, Brian H. Mullis, Yohan Jang, Luke A. Lopas, Lauren C. Hill, Courteney L. Fentz, Maricela M. Diaz, Krista Brown, Katelyn M. Garst, Emma W. Denari
San Antonio Military Medical Center, San Antonio, TX: Patrick Osborn, Justin Fowler, Sarah Pierrie, Maria Herrera
University of California, San Francisco, San Francisco, CA: Saam Morshed, Theodore Miclau, Meir Marmor, Amir Matityahu, R. Trigg McClellan, David Shearer, Paul Toogood, Anthony Ding, Jothi Murali, Ashraf El Naga, Jennifer Tangtiphaiboontana, Tigist Belaye, Eleni Berhaneselase, Dmitry Pokhvashchev
Aqueous-PREP:
Vanderbilt Medical Center, Nashville, TN: William T Obremskey, Amir Alex Jahangir, Manish Sethi, Robert Boyce, Daniel J. Stinner, Phillip Mitchell, Karen Trochez, Elsa Rodriguez, Charles Pritchett, Natalie Hogan, A. Fidel Moreno
University of Florida, Gainesville, FL: Jennifer E. Hagen, Matthew Patrick, Richard Vlasak, Thomas Krupko, Michael Talerico, Marybeth Horodyski, Chris Koenig, Marissa Pazik, Elizabeth Lossada-Soto
McGovern Medical School at UTHealth Houston, Houston, TX: Joshua L. Gary*, Stephen J Warner, John W. Munz, Andrew M. Choo, Timothy S. Achor, Milton L. “Chip” Routt, Michael Kutzler, Sterling Boutte, Ryan J. Warth
Wright State University, Dayton, OH: Michael Prayson, Indresh Venkatarayappa, Brandon Horne, Jennifer Jerele, Linda Clark
Banner University Medical Center – Tucson, Tucson, AZ: Christina Boulton, Jason Lowe, John T. Ruth, Brad Askam, Andrea Seach, Alejandro Cruz, Breanna Featherston, Robin Carlson, Iliana Romero, Isaac Zarif
The CORE Institute, Phoenix, AZ: Niloofar Dehghan, Michael McKee, Debra L Sietsema, Alyse Williams, Tayler Dykes
Vall d'Hebron University Hospital, Barcelona, Spain: Ernesto Guerra-Farfan, Jordi Tomas-Hernandez, Jordi Teixidor-Serra, Vicente Molero-Garcia, Jordi Selga-Marsa, Juan Antonio Porcel-Vazquez, Jose Vicente Andres-Peiro, Joan Minguell-Monyart, Jorge Nuñez-Camarena, Maria del Mar Villar-Casares, Jaume Mestre-Torres, Pilar Lalueza-Broto, Felipe Moreira-Borim, Yaiza Garcia-Sanchez
Hospital Universitari Parc Tauli, Barcelona, Spain: Francesc Marcano-Fernández, Laia Martínez-Carreres, David Martí-Garín, Jorge Serrano-Sanz, Joel Sánchez-Fernández, Matsuyama Sanz-Molero, Alejandro Carballo, Xavier Pelfort, Francesc Acerboni-Flores, Anna Alavedra-Massana, Neus Anglada-Torres, Alexandre Berenguer, Jaume Cámara-Cabrera, Ariadna Caparros-García, Ferran Fillat-Gomà, Ruben Fuentes-López, Ramona Garcia-Rodriguez, Nuria Gimeno-Calavia, Marta Martínez-Álvarez, Patricia Martínez-Grau, Raúl Pellejero-García, Ona Ràfols-Perramon, Juan Manuel Peñalver, Mònica Salomó Domènech, Albert Soler-Cano, Aldo Velasco-Barrera, Christian Yela-Verdú, Mercedes Bueno-Ruiz, Estrella Sánchez-Palomino, Vito Andriola, Matilde Molina-Corbacho, Yeray Maldonado-Sotoca, Alfons Gasset-Teixidor, Jorge Blasco-Moreu, Núria Fernández-Poch, Josep Rodoreda-Puigdemasa, Arnau Verdaguer-Figuerola, Heber Enrique Cueva-Sevieri, Santiago Garcia-Gimenez
PREPARE:
FRASER HEALTH AUTHORITY/Royal Columbian Hospital, New Westminster, BC: Darius G. Viskontas, Kelly L. Apostle, Dory S. Boyer, Farhad O. Moola, Bertrand H. Perey, Trevor B. Stone, H. Michael Lemke, Ella Spicer, Kyrsten Payne
Inova Fairfax Medical Campus, Falls Church, VA: Robert A. Hymes, Cary C. Schwartzbach, Jeff E. Schulman, A. Stephen Malekzadeh, Michael A. Holzman, Greg E. Gaski, Jonathan Wills, James S. Ahn, Sharmistha Das, Antoinisha D. English, Jaslynn A. N. Cuff
Wake Forest Baptist Health, Winston-Salem, NC: Holly Pilson, Eben A. Carroll, Jason J. Halvorson, Sharon Babcock, J. Brett Goodman, Martha B. Holden, Wendy Williams, Taylor Hill, Ariel Brotherton
MetroHealth Medical Center, Cleveland, OH: Nicholas M. Romeo, Heather A Vallier*, Joanne Fraifogl, Anna Vergon
University of Utah, Salt Lake City, Utah: Thomas F. Higgins, Justin M. Haller, David L. Rothberg, Lucas S. Marchand, Ashley Neese, Zachary M. Olsen, Abby V. McGowan, Sophia Hill, Morgan K. Dauk
University of Mississippi Medical Center, Jackson, MS: Patrick F. Bergin, George V. Russell, Matthew L. Graves, John Morellato, Sheketha L. McGee, Eldrin L. Bhanat, Ugur Yener, Rajinder Khanna, Priyanka Nehete
Sanford Health, Sioux Falls, SD: David Potter*, Robert VanDemark III, Kristi Atkins
Dartmouth-Hitchcock Medical Center, Lebanon, NH: I. Leah Gitajn, Marcus Coe, Kevin Dwyer, Devin S. Mullin, Theresa A. Chockbengboun
Carolinas Medical Center, Atrium Health Musculoskeletal Institute, Charlotte, NC: Kevin Phelps, Michael Bosse, Madhav Karunakar, Laurence Kempton, Stephen Sims, Joseph Hsu, Rachel Seymour, Christine Churchill, Ada Mayfield, Juliette Sweeney
University of Maryland, Capital Region Health: Largo, MD: Todd Jaeblon, Robert Beer, Haley K. Demyanovich, Brent Bauer, Sean Meredith, Caroline Benzel,
University of Wisconsin Madison, Madison, WI: Christopher M. Domes
Duke University Hospital, Durham, NC: Mark J. Gage*, Rachel M. Reilly, Ariana Paniagua, JaNell Dupree
Brigham Women's Hospital, Boston, MA: Michael J. Weaver, Arvind G. von Keudell, Abigail E. Sagona
University of Pennsylvania, Philadelphia, PA: Samir Mehta, Derek Donegan, Annamarie Horan, Mary Dooley
Massachusetts General Hospital, Boston, MA: Marilyn Heng, Mitchel B. Harris, David W. Lhowe, John G. Esposito, Ahmad Alnasser
Bryan Medical Center, Lincoln, Nebraska: Steven F. Shannon*, Alesha N. Scott, Bobbi Clinch, Becky Weber
University of Cincinnati, Cincinnati, OH: Michael J. Beltran, Michael T. Archdeacon, Henry Claude Sagi, John D. Wyrick, Theodore Toan Le, Richard T. Laughlin, Cameron G. Thomson, Kimberly Hasselfeld
Cedars-Sinai Medical Center, Los Angeles, CA: Carol A. Lin, Mark S. Vrahas, Charles N. Moon, Milton T. Little, Geoffrey S. Marecek, Denice M. Dubuclet
University of California, Irvine, Orange, CA: John A. Scolaro, James R. Learned, Philip K. Lim, Susan Demas, Arya Amirhekmat, Yan Marco Dela Cruz
* Individual is no longer actively working on the Aqueous-PREP and / or PREPARE trial
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mitchell E.J., Ahmed K., Breeman S., et al. It is unprecedented: trial management during the COVID-19 pandemic and beyond. Trials. 2020;21(1):784. doi: 10.1186/s13063-020-04711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dorn A. COVID-19 and readjusting clinical trials. Lancet. 2020;396(10250):523–524. doi: 10.1016/S0140-6736(20)31787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) 2017. Surgical Site Infection (SSI) Event. [Google Scholar]
- 4.Slobogean G.P., Sprague S., et al. Effectiveness of iodophor vs chlorhexidine solutions for surgical site infections and unplanned reoperations for patients who underwent fracture repair: the PREP-IT master protocol. JAMA Netw. Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2215. Program of Randomized Trials to Evaluate Pre-operative Antiseptic Skin Solutions in Orthopaedic Trauma (PREP-IT) Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi S., Noda T., Kubo S., et al. Variation in fracture risk by season and weather: a comprehensive analysis across age and fracture site using a National Database of Health Insurance Claims in Japan. Bone. 2019;120:512–518. doi: 10.1016/j.bone.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari H.A., Orav J.E., Barrett J.A., Baron J.A. Effect of seasonality and weather on fracture risk in individuals 65 years and older. Osteoporos. Int. 2007;18(9):1225–1233. doi: 10.1007/s00198-007-0364-6. [DOI] [PubMed] [Google Scholar]
- 7.Patsopoulos N.A. A pragmatic view on pragmatic trials. Dialogues Clin. Neurosci. 2011;13(2):217–224. doi: 10.31887/DCNS.2011.13.2/npatsopoulos. [DOI] [PMC free article] [PubMed] [Google Scholar]