Clinical History and Background
A 6-month-old male presented to the emergency department during the COVID-19 pandemic with 3-day history of fever, nasal congestion, cough, and decreased oral intake. He was not able to sit with support or roll over, and his growth parameters were severely reduced (<1st percentile). He tested negative for COVID-19, and had an unrevealing newborn screen. Severe failure to thrive (FTT) had been evident since his well-child visit at age 2 months. The family was instructed to increase his caloric intake. At the 6 months visit, he was found to have only gained 1 kg since birth and was febrile, prompting emergency department referral.
Initial workup was notable for increased aspartate aminotransferase [567 U/L, reference interval (RI): 20–64 U/L] and alanine aminotransferase (210 U/L, RI: 5–45 U/L), decreased normal total protein (5.6 g/dL, RI: 5.4–7.0 g/dL) and albumin (3.5 g/dL, RI: 3.1–4.2 g/dL), and low thyroid-stimulating hormone (0.22 µIU/ml, RI: 0.70–4.20 µIU/mL) and free thyroxine (0.5 ng/dl, RI: 1.0–1.8 ng/dL). His chest radiograph showed cardiomegaly, and echocardiogram identified a large globally distributed pericardial effusion associated with normal ventricular function.
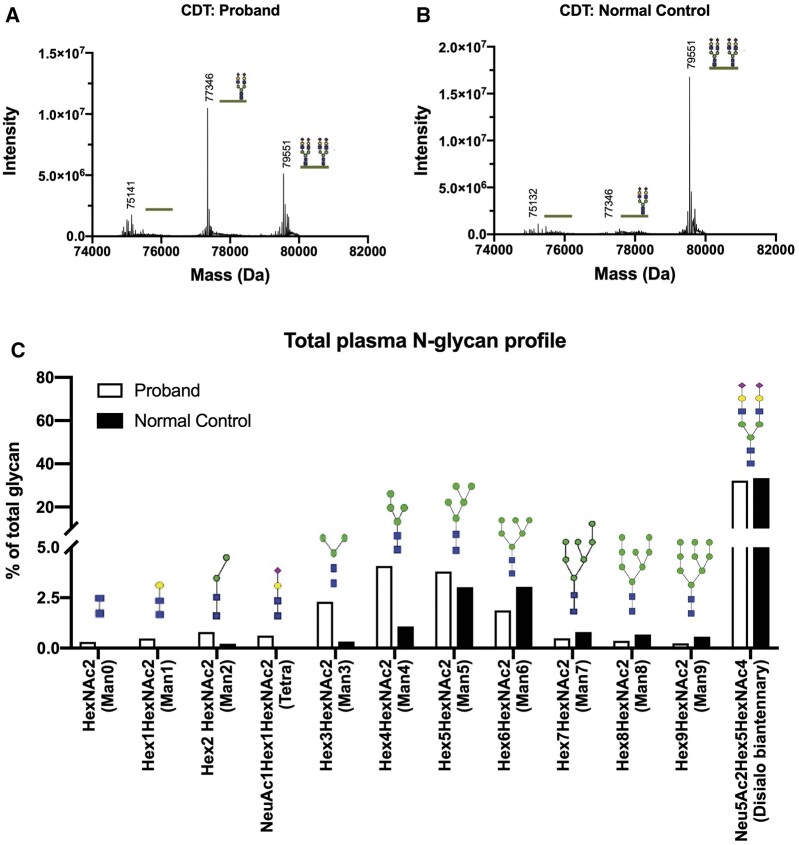
Genetic and metabolism services were consulted for FTT and developmental delay. Hypotonia, poor head control, intermittent esotropia, inverted nipples, bilateral undescended testes, and suprapubic and lateral gluteal fat pads were noted, suggesting a congenital disorder of glycosylation (CDG). Carbohydrate-deficient transferrin analysis revealed a type-I pattern, with profoundly increased hypoglycosylated transferrin glycoforms (Fig. 1, A). Total plasma N-glycan analysis revealed remarkable undermannosylation, with increased mannose-deprived tetrasaccharide and Man0–5GlcNAc2, and reduced Man8–9GlcNAc2 (Fig. 1, B). The biochemical and clinical findings were consistent with phosphomannomutase 2 congenital disorder of glycosylation (PMM2-CDG), confirmed by compound heterozygous pathogenic variants in PMM2: c.368G>A (p.Arg123Gln) and c.691G>A (p.Val231Met). Unfortunately, the patient’s clinical status deteriorated, and he died due to bleeding complications during attempts to surgically address his pericardial effusion.
Fig. 1.
Comparison of carbohydrate-deficient transferrin and N-glycan results for the proband and a normal control.
(A) In the proband, a-glycosylated/di-glycosylated and mono-glycosylated/di-glycosylated transferrin ratios were 0.30 (reference <0.05) and 1.46 (reference <0.05), respectively. (B) In the normal control, the ratios were 0.02 and 0.01 respectively. (C) In total plasma N-glycan profiling, the proband displayed a profound undermannosylation profile with increases in mannose-deprived tetrasaccharide and Man0-5GlcNAc2, along with reduction of Man8-9GlcNAc2; of these findings, the most notable were the increases of mannose-deprived tetrasaccharide and Man3GlcNAc2.
Diagnosis and Summary
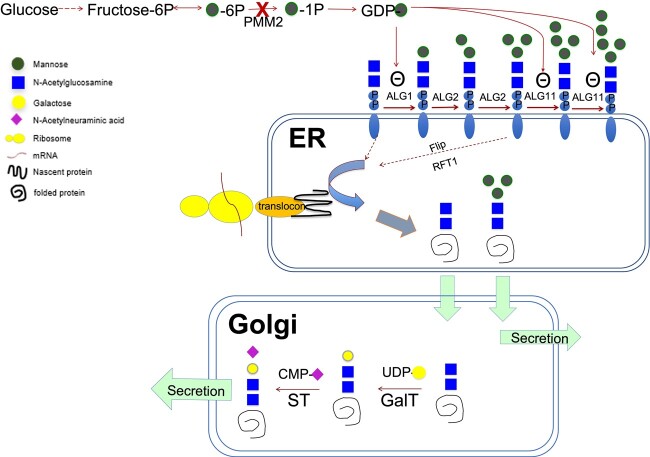
PMM2-CDG is the most common CDG. It is an autosomal recessive condition caused by deficient phosphomannomutase-2 conversion of mannose-6-phosphate to mannose-1-phosphate, precursor for GDP-mannose. As a building block for glycan assembly, the deficiency of GDP-mannose leads to underglycosylation and undermannosylation (Fig. 2). Clinically, PMM2-CDG is associated with variable phenotypes, from severe antenatal presentation with multisystem involvement and high mortality rate to adulthood presentation limited to minor neurological involvement (1).
Fig. 2.
Proposed noncanonical pathway in PMM2-CDG resulting in increase of mannose-deprived tetrasaccharide and Man3GlcNAc2.
The biosynthesis of GDP-mannose is disturbed in PMM2-CDG, leading to accumulation of GlcNAc2 (Man0) and Man3GlcNAc2 (Man3) in endoplasmic reticulum. Man3 is excreted into circulation. Mannose-deprived tetrasaccharide (NeuAc1Gal1GlcNAc2) is produced by further modifications of Man0 in Golgi, with addition of galactose and sialic acid by corresponding glycosyltransferases before secretion.
With emerging new therapies, early diagnosis may improve the survival of PMM2-CDG patients. A functional diagnosis of PMM2-CDG by plasma carbohydrate-deficient transferrin and N-glycan tests can be achieved within 24 h using mass spectrometry-based testing (2, 3). Thus, it is important to recognize the early clinical signs for PMM2-CDG, among which FTT is frequently noted. Hydrops fetalis, cerebellar atrophy, inverted nipples, peculiar fat pads, dysmorphism, cryptorchidism, strabismus, and hypothyroidism (detectable by newborn screen) are also early features that can be detected perinatally. Other signs including hypotonia, seizure, pericardial effusion, ascites, and liver failure usually develop during the infantile period (1). The presence of any subsets of these findings should raise the suspicion for PMM2-CDG. Prenatal evidence for pericardial effusion was previously reported in late gestation at 34 weeks in a PMM2-CDG patient (4). In our case, a fetal echo performed at 21 weeks’ gestation was normal.
Because FTT was evident at 2 months in this child, this case represented a lost opportunity for early diagnosis and management of PMM2-CDG during the COVID-19 pandemic. Several common factors may be attributable to the delay in diagnosis, including an overall reduction in outpatient visits to reduce the risk of transmitting SARS-CoV-2, the limitations of telemedicine in identifying and managing infants with FTT and developmental delay, and also a general lack of awareness of rare genetic and metabolic conditions, which could have prompted earlier referral.
Contributor Information
Xinying Hong, Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Hana Alharbi, Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, Philadelphia, PA, USA; Department of Pediatrics, University of Tabuk, Tabuk, Saudi Arabia.
Daniah Albokhari, Division of Human Genetics,Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, USA; Department of Pediatrics, Taibah University College of Medicine, Medina, Saudi Arabia.
Andrew C Edmondson, Division of Human Genetics,Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Miao He, Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Nonstandard Abbreviations
RI, reference interval; FTT, failure to thrive; CDG, congenital disorders of glycosylation; PMM2, phosphomannomutase 2.
Human Genes
PMM2.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
A. Edmondson, CDG CARE.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
A. Edmondson, U54 NS115198, CDG CARE, Amour Fund; M. He, U54NS115198 from NINDS and NCATS, Rose Gift fund to CHOP.
Expert Testimony
None declared.
Patents
None declared.
References
- 1. Altassan R, Péanne R, Jaeken J, Barone R, Bidet M, Borgel D, et al. International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: diagnosis, treatment and follow up. J Inherit Metab Dis 2019;42:5–28. [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Li X, Edmondson A, Meyers GD, Izumi K, Ackermann AM, et al. Increased clinical sensitivity and specificity of plasma protein N-glycan profiling for diagnosing congenital disorders of glycosylation by use of flow injection-electrospray ionization-quadrupole time-of-flight mass spectrometry. Clin Chem 2019;65:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang W, James PM, Ng BG, Li X, Xia B, Rong J, et al. A novel N-tetrasaccharide in patients with congenital disorders of glycosylation, including asparagine-linked glycosylation protein 1, phosphomannomutase 2, and mannose phosphate isomerase deficiencies. Clin Chem 2016;62:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malhotra A, Pateman A, Chalmers R, Coman D, Menahem S.. Prenatal cardiac ultrasound finding in congenital disorder of glycosylation type 1a. Fetal Diagn Ther 2009;25:54–7. [DOI] [PubMed] [Google Scholar]