Abstract
The ClpYQ (HslUV) ATP-dependent protease of Escherichia coli consists of an ATPase subunit closely related to the Clp ATPases and a protease component related to those found in the eukaryotic proteasome. We found that this protease has a substrate specificity overlapping that of the Lon protease, another ATP-dependent protease in which a single subunit contains both the proteolytic active site and the ATPase. Lon is responsible for the degradation of the cell division inhibitor SulA; lon mutants are UV sensitive, due to the stabilization of SulA. lon mutants are also mucoid, due to the stabilization of another Lon substrate, the positive regulator of capsule transcription, RcsA. The overproduction of ClpYQ suppresses both of these phenotypes, and the suppression of UV sensitivity is accompanied by a restoration of the rapid degradation of SulA. Inactivation of the chromosomal copy of clpY or clpQ leads to further stabilization of SulA in a lon mutant but not in lon+ cells. While either lon, lon clpY, or lon clpQ mutants are UV sensitive at low temperatures, at elevated temperatures the lon mutant loses its UV sensitivity, while the double mutants do not. Therefore, the degradation of SulA by ClpYQ at elevated temperatures is sufficient to lead to UV resistance. Thus, a protease with a structure and an active site different from those of Lon is capable of recognizing and degrading two different Lon substrates and appears to act as a backup for Lon under certain conditions.
Energy-dependent degradation in Escherichia coli is carried out by a few different ATP-dependent proteases, each with mostly unique substrate specificities. These include Lon, FtsH, and a number of different Clp proteases (reviewed in reference 10). The Clp proteases are composed of two different multimeric components. The smaller subunit (about 19 kDa) is a peptidase, either ClpP (34) or ClpQ (HslV) (38, 43). The larger subunit is an ATPase, ClpA (84 kDa) (24), ClpX (46 kDa) (13), or ClpY (also called HslU) (49 kDa) (38, 43). Either ClpA or ClpX can associate with ClpP to form an ATP-dependent protease (ClpAP or ClpXP); ClpY associates with ClpQ to form an active, energy-dependent protease. While ClpY and ClpX show significant sequence similarity to each other and to ClpA, ClpP and ClpQ/HslV are not related at the sequence level. ClpP is a serine protease, found in many prokaryotes and in the organelles of many eukaryotes; ClpQ has a catalytic amino-terminal threonine residue and shows significant sequence similarity to the eukaryotic proteasome β subunit (46).
The genes encoding ClpQ and ClpY, hslVU, initially were identified as heat shock genes by Chuang et al. (5) and subsequently were selected in searches for multicopy plasmids that perturb heat shock regulation by sigma E and suppress the effects of a mutation in htrC in causing sensitivity to amino acid analogs (38). However, the function of the ClpYQ protease when the genes are present in single copy has remained unknown. One possible function was suggested by work indicating that mutations in hslU (clpY) suppressed a dnaA46 mutation at a high temperature and resulted in smaller cells under some growth conditions (23).
We began the work described here as part of a project to understand whether ClpY differs significantly from ClpA and ClpX in substrate recognition in vivo. While we found some preliminary evidence of overlap in function between the Clp proteases, we uncovered a significant overlap between ClpYQ and the single-component energy-dependent protease, Lon. Our results suggest that one function of ClpYQ is to serve as a secondary protease for some Lon substrates.
MATERIALS AND METHODS
Bacteria and plasmids.
The relevant bacterial strains and plasmids used are listed in Table 1. plac-sulA2 is in the same vector as p21 (49) but contains a missense mutation in sulA. The mutant protein is degraded in a Lon-dependent fashion. The plac-sulA+ plasmid (p21) could not be transformed into the Δlon strains used here, presumably because the basal level of SulA expression was sufficient to kill cells in the absence of Lon. pUC4K (52) was used as the kanamycin-resistant plasmid in our Alp tester strain, in place of those described previously (27). Cells were grown in Luria broth (LB) (47) unless otherwise indicated. Antibiotics were added at the following concentrations, in micrograms per milliliter: ampicillin, 100; tetracycline, 10; and chloramphenicol, 100.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Strains | ||
| DB1255 | recBC sbcB15 hsdR supF8 | 53 |
| JK6214 | Δ(CP54) (SsrA−) Δlon-510 cpsB10::lac | 27 |
| JM101 | Δ(lac-proAB) supE thi (F′ traD36 proAB lacIqlacZΔM15) | 36 |
| OHP3 | Δlon-510 leu::Tn10 ftsZ(SfiB*) | 8 |
| SG1185 | clpY::cat recBC sbcB15 hsdR supF8 | DB1255 + pWF3 (linearized) |
| SG1186 | clpQ::cat recBC sbcB15 hsdR supF8 | DB1255 + pWF2 (linearized) |
| SG12064 | clpQ::cat | C600 + P1 (SG22192) |
| SG12065 | clpY::cat | C600 + P1 (SG22193) |
| SG20780 | Δlon-510 cpsB10::lac-Mu-λ imm | 3 |
| SG20781 | lon+ cpsB10::lac-Mu-λ imm | 3 |
| SG22119 | Δlac lon+ clp+ | Equivalent of SG20250 (15) |
| SG22163 | malP::lacIq | 14 |
| SG22186 | malP::lacIq Δlon rcsA::kan | 14 |
| SG22192 | clpQ::cat | SG22119 + P1 (SG1186) |
| SG22193 | clpY::cat | SG22119 + P1 (SG1185) |
| SG22205 | malP::lacIqclpQ::cat | SG22163 + P1 (SG22192) |
| SG22206 | malP::lacIqclpY::cat | SG22163 + P1 (SG22193) |
| SG22207 | malP::lacIq Δlon rcsA::kan clpQ::cat | SG22186 + P1 (SG22192) |
| SG22208 | malP::lacIq Δlon rcsA::kan clpY::cat | SG22186 + P1 (SG22193) |
| SG22224 | malP::lacIq Δlon rcsA::kan ftsZ(SfiB*) leu::Tn10 | SG22186 + P1 (OHP3) |
| SG22225 | malP::lacIq Δlon rcsA::kan clpQ::cat ftsZ(SfiB*) leu::Tn10 | SG22224 + P1 (SG12064) |
| SG22226 | malP::lacIq Δlon rcsA::kan clpY::cat ftsZ(SfiB*) leu::Tn10 | SG22224 + P1 (SG12065) |
| Plasmids | ||
| pWF1 | clpQ+ clpY+ tetr (pACYC184) | pACYC184 (EcoRI-ScaI) + PCR fragment from pWPC80 |
| pWF2 | clpQ::cat clpY+ tetr | NsiI-cut pWF1 + cat |
| pWF3 | clpQ+ clpY::cat tetr | PstI-cut pWF1 + cat |
| pWF4 | Δ(clpQ-clpY)::cat | NsiI-PstI-cut pWF1 + cat |
| plac::SulA2 | plac-sulA2 Ampr | Derivative of p21 (49) |
| pWPC80 | clpQ+ clpY+ tetr | pACYC184 + EcoRI fragment from Kohara phage 4H12 |
| pUC4K | pUC4 kanr | 52 |
| pCAT19 | pUC19 cat | 9 |
Plasmid constructions.
The procedures used for cloning and restriction enzyme digestion were those described by Sambrook et al. (45). To clone the clpQclpY operon into a plasmid, a 6.5-kb EcoRI DNA fragment containing the clpQ+clpY+ operon and the adjoining genes was first isolated from the DNA of Kohara phage miniset 539 (phage 4H12) (28) and then ligated into the unique EcoRI site of pACYC184; the resulting plasmid was designated pWPC80. A 2.1-kb PCR-generated DNA fragment containing clpQclpY with EcoRI (forward primer, 5′CCGGAATTCCCGGGGGTTGAAACCC3′) and blunt ends (reverse primer, 5′AGCCACGCCTGAGTTCGG3′) was isolated from pWPC80 DNA and subcloned into the EcoRI and ScaI sites of plasmid pACYC184; the resulting plasmid was designated pWF1 (pACYC184 clpQ+ clpY+). PCR amplification was performed with a Gene Amp kit as recommended by the manufacturer (Perkin-Elmer Cetus). Oligonucleotides were purchased from Bioserve Biotechnologies (Laurel, Md.).
To construct a plasmid which would have an interrupted clpQ or clpY sequence, a cassette encoding chloramphenicol acetyltransferase (cat) with its own promoter was isolated by PstI digestion of pCat19 (9), ligated with either NsiI (interrupts clpQ)- or PstI (two sites within clpY)-digested pWF1 DNA (Fig. 1). Chloramphenicol-resistant colonies were selected after transformation of JM101, resulting in pWF2 (pACYC184 clpQ::cat clpY+) and pWF3 (pACYC184 clpQ+ clpY::cat). Either pACYC184 or pWF4 was used as a negative control. pWF4 was constructed by cutting with both NsiI and PstI and ligating in the presence of the cat cassette used above. Therefore, this plasmid has deletions of both clpQ and clpY. The structures of pWF2, pWF3, and pWF4 were confirmed by restriction enzyme analysis. The cat gene is inserted such that the promoter faces in the same direction as clpQclpY operon transcription. Therefore, the clpQ::cat insertion is not polar for clpY, as confirmed by examination of the production of ClpY with anti-ClpY antibody (25) in strains carrying the plasmid (data not shown).
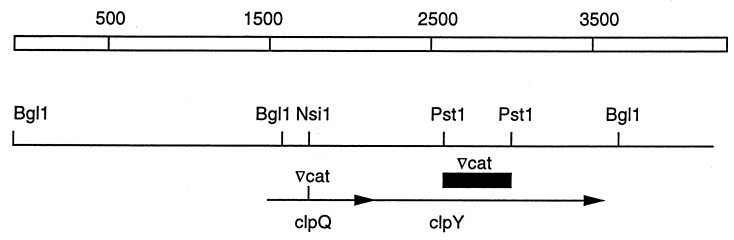
FIG. 1.
Structure of the clpQY operon. The 3,683-bp BglI fragment that contains clpQY is drawn to scale. Restriction enzyme sites referred to in the text are indicated. Arrows show the direction of transcription. The inverted triangle represents the position of insertion of the cat gene, encoding chloramphenicol acetyltransferase, in the clpQ or clpY gene as described in Materials and Methods. The black bar shows the region deleted in the clpY::cat mutant.
Construction and characterization of clpQ::cat and clpY::cat chromosomal mutants.
The cat insertion mutations in clpQ or clpY were transferred to the chromosome by linear transformation into DB1255 with pWF2 (to create SG1186) or pWF3 (to create SG1185) cut with SacII and HindIII as previously described (3). Chloramphenicol-resistant colonies were purified, and the mutations were transduced to the wild-type host SG22119.
The positions of the insertions in the transductants were confirmed in a number of ways. Linkage of chloramphenicol resistance with argE::Tn10 was shown in P1 transductions by selecting for either Arg+ or chloramphenicol resistance and screening for the other marker, to confirm the expected location of clpY and clpQ near minute 89. Linkage was 25% when Arg+ recombinants were selected after transduction with a clpQ::cat arg+ donor and 29% when chloramphenicol-resistant recombinants were selected; therefore, there is no discrimination against clpQ::cat recombinants. Isolates that demonstrated linkage to argE::Tn10 were subjected to Southern blot analysis.
Chromosomal DNA was extracted from wild-type strain SG22119 and insertion derivatives SG22192 (clpQ::cat) and SG22193 (clpY::cat) (47), digested with BglI, and subjected to gel electrophoresis through 1% agarose gels. The resolved DNA fragments in the gels were blotted to N+-bond membranes (Amersham) and subsequently probed separately with biotin-labelled clpQ-, clpY-, and cat-specific DNA probes. After overnight hybridization at 42°C, the membranes were washed with washing buffer several times at 42°C as described in the Amersham Photo-Gene nucleic acid detection system instructions and developed with the Amersham ECL system as described by the manufacturer. As expected (see restriction map in Fig. 1), a single BglI fragment detected in the wild-type DNA was missing from the clpQ::cat and clpY::cat strains and a new fragment that hybridized with the cat marker was detected in these strains (data not shown).
To further confirm that the ClpQ and ClpY proteins were not being produced from the clpQ::cat and clpY::cat strains, respectively, a Western blot assay with anti-ClpQ or anti-ClpY serum was performed. The antibodies used were raised to proteins purified after the expression of ClpQ and ClpY from T7 promoters (25). As expected, the clpQ::cat strain made ClpY but not ClpQ, and the clpY::cat strain expressed ClpQ but not ClpY (data not shown).
Cell growth and temperature sensitivity.
Overnight cultures of the wild type and both single and double mutants grown at 32°C were diluted into medium, regrown to the mid-exponential phase, serially diluted, and spotted on LB agar plates; efficiencies of plating (EOP) were determined as a function of temperature.
MMS sensitivity.
Cells were grown overnight at 30°C in LB, serial dilutions were made in LB or TMG buffer (0.01 M Tris [pH 7.4], 0.01 M MgSO4, 0.01% gelatin), and 10 μl of each dilution was spotted onto LB agar plates with and without 0.025% methyl methanesulfonate (MMS). EOP were determined by dividing the titer of cells forming colonies on the MMS plate by the titer of those on the LB agar plate after overnight incubation at various temperatures. Precise EOP of lon mutant derivatives at higher temperatures varied with the age of the MMS plate, but the general pattern remained the same in all cases.
SulA turnover in vivo.
Cells were grown at 32°C in LB to an optical density at 600 nm of 0.2, collected by centrifugation, and redissolved in a 1/10 volume of 0.01 M Mg2SO4. Cells were exposed to UV irradiation at 20 J/m2 and diluted to the original volume in LB, and growth was continued in the dark. For the measurement of SulA decay at 32°C, growth was continued for 25 min before the addition of spectinomycin (150 μg/ml) to inhibit further protein synthesis; for the measurement of SulA decay at 41.5°C, cells were shifted to 41.5°C immediately after UV treatment and incubated for 10 min before spectinomycin addition. Samples were removed at intervals, and the protein was precipitated with 5% trichloroacetic acid in the cold. Pellets were washed with 80% acetone and resuspended in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer (29). Equal amounts of samples normalized for the original optical density were loaded, electrophoresed by SDS-PAGE (14 or 15% polyacrylamide), blotted to 0.2-μm-pore-size nitrocellulose, and probed with anti-SulA antibody (49). Western blots were developed with the ECL system and quantitated by scanning with an Eagle Eye II (Strategene, La Jolla, Calif.).
Visualization of SulA2 (a mutant form of SulA) from plasmid plac-SulA2 was carried out with cells grown in LB-ampicillin and induced for 30 min with isopropyl-β-d-thiogalactopyranoside (IPTG) before samples were collected and processed as described above.
Enzyme assays.
β-Galactosidase assays were performed as described by Miller (37) with an SDS lysis method. Cells were grown in LB and assayed by the addition of o-nitrophenyl-β-d-galactoside. Assays were performed at least twice with samples from independent cultures.
RESULTS
clpQ+ clpY+-dependent suppression of lon mutant phenotypes.
lon mutants are sensitive to DNA damage and die after exposure to MMS or UV light, due to the accumulation of the unstable Lon substrate SulA, a cell division inhibitor (19, 40). In addition, lon mutations lead to the stabilization and accumulation of RcsA, a positive regulator for the transcription of genes involved in capsule production, resulting in high levels of transcription of the cps genes (capsule synthesis genes) (11, 35, 48; for a review, see reference 12). The clpY+ clpQ+ plasmid pWF1 and the two mutant derivatives, pWF2 (clpQ::cat clpY+) and pWF3 (clpQ+ clpY::cat) were introduced into either SG22186, a Δlon strain, to measure MMS sensitivity or SG20780, a Δlon strain carrying a cpsB10::lac transcriptional fusion. In the latter strain, the levels of β-galactosidase expression reflect RcsA levels, and cells can also be tested for MMS sensitivity.
The results of these tests are shown in Table 2. Both the MMS sensitivity and the capsule overproduction of lon mutants were suppressed by the clpQ+ clpY+ plasmid (lines two and three) but not by clpY or clpQ mutant plasmids (lines four and five). The clpQ+ clpY+ plasmid but not a mutant plasmid also suppressed the mucoidy of a cps+ Δlon strain, JT4000 (49), consistent with an effect of ClpYQ on capsule synthesis and not simply an effect on the cpsB::lacZ transcriptional fusion (data not shown). Therefore, it appears that ClpYQ is capable of recognizing two different Lon substrates, SulA and RcsA. Suppression of the DNA damage sensitivity of lon mutants by plasmids overproducing ClpQ and ClpY (HslV-HslU) was independently demonstrated by Khattar in a search for multicopy plasmids that could render lon mutants resistant to nitrofurantoin, an SOS-inducing agent (26).
TABLE 2.
Overproduction of ClpYQ suppresses two lon mutant phenotypes
| Strain | EOP on MMSa | Relative β-galactosidase levelsb |
|---|---|---|
| lon+ | 1 | 0.01 |
| Δlon-510/pACYC184 | 10−4 | 1 |
| Δlon-510/pACYC184 clpQ+ clpY+ | 0.1 | 0.05 |
| Δlon-510/pACYC184 clpQ::cat clpY+ | 10−4 | 1 |
| Δlon-510/pACYC184 clpQ+ clpY::cat | 10−4 | 1 |
SG22163 (lon+) and SG22186 (Δlon) cells were used as hosts for this assay. EOP on plates containing MMS was compared to that on parallel plates containing LB.
SG20781 (lon+) and SG20780 (Δlon mutant) cells were used as hosts for this assay. In separate tests, the MMS sensitivity of these strains with the various plasmids was similar to that of SG22163 and SG22186. Assay values were normalized to 1 for the Δlon strain carrying the vector (line 2).
To confirm that the MMS-resistant phenotype of Δlon mutant cells carrying the clpQ+ clpY+ plasmid is due to increased degradation of SulA, we measured the accumulation of a mutant form of SulA, SulA2, made from a compatible multicopy plasmid. Western blot analysis with SulA-specific antiserum demonstrated that, while SulA2 could be detected in the lon mutant host (Fig. 2, lane 3), it did not accumulate sufficiently to be detected in either the lon+ host (lane 2) or the lon mutant host carrying the multicopy clpQ+ clpY+ plasmid (lane 4). However, Δlon mutant cells with plasmid pWF2 (clpQ::cat clpY+) or pWF3 (clpQ+ clpY::cat) showed a significant accumulation of SulA2 (Fig. 2, lanes 5 and 6). Therefore, SulA2 accumulates only in cells which are MMS sensitive, supporting a model for the recognition and rapid degradation of both chromosomally encoded SulA (based on the MMS sensitivity test results shown in Table 2) and mutant SulA2 (Fig. 2) in lon+ cells and in Δlon cells overexpressing ClpYQ protease.
FIG. 2.

Accumulation of SulA2 in cells overproducing ClpYQ. Cells transformed with either pACYC184 (lanes 2 and 3) or pWF1 (clpQ+ clpY+), pWF2 (clpQ::cat clpY+), or pWF3 (clpQ+ clpY::cat) were grown at 30°C in LB to an optical density at 595 nm of 0.5 and induced with IPTG at a final concentration of 5 mM for 30 min. Samples were subjected to electrophoresis and Western blotting for SulA as described in Materials and Methods. The lon+ host was SG22163, and the Δlon host was SG22186; both of these strains carry lacIq integrated at the mal locus and the plac::SulA2 plasmid. Lane 1 was a control loaded with purified SulA.
Phenotypes of clpY and clpQ mutants.
The clpQ::cat and clpY::cat insertion mutations were transferred from the plasmid to the chromosome by linear transformation. The structure of the gene carrying the insertion was confirmed by Southern blotting and by Western blotting to demonstrate the absence of the appropriate protein. Linkage to a nearby marker in P1 transduction confirmed that neither clpQ nor clpY is essential for E. coli growth (see Materials and Methods for details). Strains carrying clpQ::cat or clpY::cat either alone or in combination with single mutations in clpA, clpB, clpX, clpP, or lon did not show impaired cell growth at temperatures up to 42°C. It has been reported that hslVU (clpYQ) mutant cells show temperature sensitivity at a very high temperature (44°C) (23, 38). Kanemori et al. have also found that triple mutants (hsl clpP lon) are both cold and temperature sensitive (22). While we did not test our double mutations at temperatures higher than 42°C, one general conclusion from all studies is that ClpYQ is not essential for E. coli growth in the normal temperature range for this organism, except under special circumstances.
The ability of the ClpYQ protease to degrade Lon substrates when overproduced suggested that it might be responsible for the residual degradation of Lon substrates in lon mutants. SulA, RcsA, and lambda N protein are all degraded at significant rates in a lon mutant (21). Isogenic strains carrying either the single clpY, clpQ, or lon mutation or combinations of lon and clp mutations were examined for Lon phenotypes and turnover of SulA. In lon+ hosts, neither the EOP on MMS (a measure of SulA stability) nor the expression of cps::lac fusions (a measure of RcsA stability) was affected by clpQ and clpY mutations (data not shown). Therefore, Lon does not require ClpY or ClpQ to function normally.
lon mutant cells were, as expected, MMS sensitive (EOP at 32°C, 10−5 (Table 3). At elevated temperatures, however, lon mutants were relatively MMS resistant (Table 3). This reduced sensitivity to DNA damage at elevated temperatures is consistent with the results of previous work by Canceill and coworkers (4). However, double mutants containing lon and either clpY or clpQ mutations remained significantly MMS sensitive even at 41°C (Table 3). This finding is consistent with a contribution of physiological levels of ClpYQ to the degradation of SulA at elevated temperatures. The MMS sensitivity of strains was dependent upon SulA inhibition of FtsZ. lon clpY or lon clpQ mutant cells carrying the ftsZ(SfiB*) mutation, an allele of ftsZ that blocks SulA interaction with FtsZ, were fully resistant to MMS at all temperatures (data not shown).
TABLE 3.
ClpY and ClpQ contribute to the MMS sensitivity of lon mutants
| Straina | Genotype | EOP
on MMSb at a temp (°C) of:
|
|||
|---|---|---|---|---|---|
| 32 | 37 | 39 | 41 | ||
| SG22163 | Wild type | 1 | 1 | 1 | 1 |
| SG22186 | Δlon rcsA::kan | 10−5 | 10−3 | 10−2 | 0.03 |
| SG22205 | clpQ::cat | 0.7 | 0.7 | 0.7 | 0.7 |
| SG22206 | clpY::cat | 1.0 | 0.8 | 1.0 | 1.0 |
| SG22207 | Δlon rcsA::kan clpQ::cat | 10−5 | 10−6 | 10−6 | |
| SG22208 | Δlon rcsA::kan clpY::cat | 10−4 | 10−5 | 10−5 | 10−5 |
All strains carry lacIq on the chromosome.
Cells were grown at 30°C, diluted into buffer, spotted on LB or LB-MMS plates, and incubated at the indicated temperatures. EOP on LB at 32 and 41.5°C were essentially identical.
Contribution of ClpYQ to the turnover of SulA, a Lon substrate.
To examine directly the contribution of ClpYQ to the degradation of SulA, we measured the turnover of SulA in lon+, Δlon, Δlon clpQ::cat, and Δlon clpY::cat cells at 32 and 41.5°C. Chromosomally encoded SulA was induced by UV treatment, and decay was measured by Western blotting during a chase period after treatment with spectinomycin to inhibit further protein synthesis. In initial experiments, the turnover of a plasmid-encoded mutant form of SulA, SulA2, was studied. Because the degradation of this protein in lon mutants was more rapid than expected (data not shown), we repeated the experiments with chromosomally encoded SulA either in strains in which SulA was active (ftsZ+) or in strains in which SulA was unable to act due to a mutation [ftsZ(SfiB*)] in the target of SulA inhibition (4).
The results of the experiments examining SulA decay at 32°C are shown in Fig. 3A and C; those obtained at 41.5°C are shown in Fig. 3B and D. In separate experiments, no SulA was detected in Western blots of lon+ hosts with or without clpYQ; therefore, the half-life could not be calculated directly in these experiments. From previous experiments from various laboratories, we expected a half-life of less than 2 min for SulA in the presence of Lon (21, 40). Kanemori et al. have reported a half-life of 1 to 2 min for SulA in lon+ hosts carrying a deletion of hslVU (clpQY) (22).
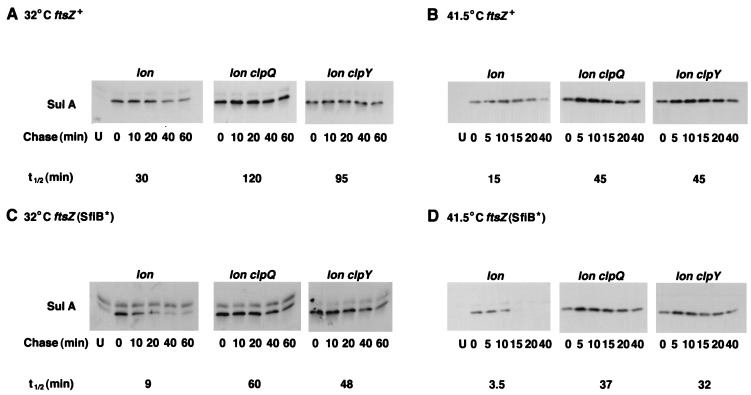
FIG. 3.
Turnover of SulA in lon clpQY mutants. Strains were grown at 32°C in LB and exposed to UV light as described in Materials and Methods. After dilution to the original volume in LB, growth was continued in the dark for 25 min at 32°C (A and C) or for 10 min at 41.5°C (B and D). Spectinomycin (150 μg/ml) was added to inhibit further protein synthesis, and samples were removed and processed for Western blotting with anti-SulA antibody as described in Materials and Methods. (A) ftsZ+ strains SG22186, SG22207, and SG22208 (panels from left to right) at 32°C. (B) ftsZ+ strains at 41.5°C. (C) ftsZ(SfiB*) strains SG22224, SG22225, and SG22226 (panels from left to right) at 32°C. (D) ftsZ(SfiB*) strains at 41.5°C. Lanes U contained lon mutant hosts sampled before UV induction (uninduced) to indicate the position of the UV-inducible SulA band. Half-lives (t1/2) were determined by scanning of the Western blots. Because many of the 5-min spots showed more protein than 0-min samples, presumably due to the delayed action of spectinomycin, the half-lives were calculated with the 5-min samples as time-zero samples.
At 32°C, SulA was relatively stable in a lon host (half-life, 30 min, comparable to that reported previously); however, it became more stable in the lon clpQ and lon clpY double mutants (half-lives, 95 to 120 min; Fig. 3A). When the degradation of SulA was measured at 41.5°C, SulA was significantly more unstable (half-life, 15 min); here, too, the lon clpQ and lon clpY double mutants showed greater SulA stability (half-life, 45 min; Fig. 3B). In initial experiments done with a SulA mutant expressed from a plasmid (data not shown) and in other work (51), we noted that SulA is significantly more unstable when the interaction of SulA with FtsZ is absent. To assess the effect of the ClpYQ protease in that situation, we repeated the SulA turnover experiments with a host carrying a mutation in ftsZ that blocks the SulA interaction, called SfiB* (Fig. 3C and D). As expected, SulA turnover increased in the Δlon host (9-min half-life at 32°C and 3.5-min half-life at 41.5°C). However, this increased instability was primarily due to ClpYQ; in lon clpY and lon clpQ mutants, SulA was once more reasonably stable (48- to 60-min half-life at 32°C and 32- to 37-min half-life at 41.5°C). Therefore, at both low and high temperatures, ClpY and ClpQ are primarily responsible for the Lon-independent degradation of SulA, although at least one other protease must also be able to degrade SulA, particularly at high temperatures.
ClpYQ is not associated with Alp+ function.
We previously reported an activity, called the Alp protease, capable of suppressing Lon (27, 49, 50). Under conditions in which Alp activity is high, both the UV sensitivity and the capsule overproduction of lon mutants are suppressed. Because these results parallel those seen with ClpYQ overproduction, we were interested in determining whether ClpYQ is responsible for Alp activity. Mutations in clpY or clpQ were transduced into the Alp indicator strain JK6214/pUC4K, and the capsule synthesis phenotype of the cells (an indirect assay for RcsA degradation and therefore Alp activity) was compared to that of the parental clpY+ clpQ+ host. If the ClpYQ protease is the only protease degrading RcsA in cells expressing Alp activity, we would expect to abolish suppression of lon in the clpY or clpQ mutant. However, no such change was observed; when cells were scored on MacConkey agar plates, strains with or without ClpYQ showed a Lac− phenotype, consistent with the degradation of RcsA and therefore an Alp+ phenotype.
DISCUSSION
The overproduction of the ClpYQ protease leads to the suppression of two lon mutant phenotypes, MMS sensitivity and capsule overproduction, dependent on two different Lon substrates, SulA and RcsA. Furthermore, MMS resistance correlates with a failure to accumulate SulA, consistent with the rapid degradation of SulA when ClpYQ is overproduced. Both subunits of the ClpYQ protease are necessary for SulA degradation. In vitro, both subunits are required for the ATP-dependent degradation of casein (25). Khattar independently identified ClpYQ in a search for multicopy suppressors of the sensitivity of lon mutants to the SOS inducer nitrofurantoin (26).
The overlap in substrate specificity between Lon and ClpYQ is observed not only for overproduced ClpYQ but also for the protease produced at normal physiological levels. SulA is more stable in lon clpQ and lon clpY double mutants than in the lon single mutant. At elevated temperatures, this additional stability correlates with increased MMS sensitivity. Therefore, SulA is a natural substrate for ClpYQ under physiological conditions but is normally degraded much more rapidly by Lon, so that no MMS sensitivity phenotype is detected for clpY or clpQ mutants unless Lon is also absent. The existence of secondary, energy-dependent proteases that can degrade SulA in the absence of Lon had been noted before (4, 21), but the responsible protease had not been identified. More recently, Kanemori and coworkers found that cells devoid of Lon, ClpP, and HslUV (ClpYQ) become sensitive to both low and high temperatures because of the stabilization of SulA; they found, as we have, that ClpYQ contributes significantly to the degradation of SulA in a lon mutant host (22). They also demonstrated that HslUV can degrade SulA in vitro (22). From the results of both groups, we can conclude that the degradation by ClpYQ is critical both for maintaining a low basal level of SulA in the absence of induction and for returning SulA to the basal level after transient induction.
SulA acts as a cell division inhibitor by interacting with and inhibiting the activity of FtsZ, an essential GTPase necessary for septum formation (6, 7, 41, 42). The synthesis of SulA is dependent upon the induction of the SOS response (18, 39). Under normal (Lon+) conditions, SulA synthesis increases transiently after DNA damage and the accumulating SulA inhibits cell division. After the damage is repaired, new SulA synthesis ceases and SulA is rapidly degraded, allowing cell division to resume. There is evidence that SulA and FtsZ directly interact and that this interaction can modulate the sensitivity of SulA to degradation. The in vivo stability of SulA is increased in the presence of excess FtsZ (1, 20), and specific mutations in FtsZ are resistant to SulA inhibition (19, 31, 32). Recent experiments have demonstrated a direct interaction between SulA and FtsZ (16, 17).
For SulA to inhibit cell division in a fashion that can be reversed once DNA damage is repaired and new SulA synthesis ceases (33), the interaction of SulA and FtsZ must be reversible. This reversal may involve either dissociation of SulA from FtsZ sufficiently to allow protease degradation or degradation of SulA directly from the SulA-FtsZ complex by the protease (or both). Because the half-life of SulA is so short (<2 min) in a lon+ host (21, 40, 49) and no SulA accumulates in lon+ hosts even when SulA is overproduced from a plasmid (Fig. 2), Lon appears able to degrade SulA rapidly whether or not FtsZ interacts with SulA.
For the degradation of SulA by secondary proteases, in particular, ClpYQ, the data suggest that an interaction with FtsZ has a profound effect on the ability of the protease to act. Thus, the half-life of SulA is 30 min in the ftsZ+ host at 32°C but only 9 min in the SfiB* host (Fig. 3A and C). At higher temperatures, there is a similar significant increase in SulA turnover in the absence of FtsZ (Fig. 3B and D). We can imagine a number of explanations. Possibly, ClpYQ recognizes motifs within SulA that are shielded by FtsZ, while Lon recognizes an unshielded motif. Given the overlap of Lon and ClpYQ activities for both SulA and RcsA, it seems to us more likely that both proteases recognize similar motifs. A second possibility is that SulA and FtsZ dissociate transiently during the SulA-dependent inhibition of cell division and that this dissociation is necessary for degradation by any of the proteases. In this case, the affinity of Lon for SulA may be sufficiently high to allow capture and degradation of the released SulA, while the dissociation is too brief for ClpYQ to capture the dissociated SulA before it reassociates with FtsZ. A final explanation is that while similar motifs are recognized by both proteases, Lon is able to actively dissociate SulA from FtsZ, a necessary step for degradation, while ClpYQ cannot remove SulA from the SulA-FtsZ complex. This model predicts a chaperone-like activity for Lon.
The role of ClpYQ in degrading SulA becomes physiologically important in lon mutants at high temperatures. It had been observed by others that lon mutants are relatively resistant to DNA damage at high temperatures (4), and we have confirmed that finding (Table 3). We found a shorter half-life for SulA at high temperatures (15 min rather than 30 min). When the ClpYQ protease is inactivated by a mutation, the SulA half-life increases to 45 min and cells once again become MMS sensitive. Therefore, there is a correlation between a longer SulA half-life and MMS sensitivity. These data suggest that, given that a certain amount of SulA is made during a transient induction period, a half-life of 15 min or less is long enough for the level of SulA to fall below the active threshold in an acceptable period of time; when the half-life is 30 min (as it is at 32°C) or longer (as it is in the lon clpQ double mutant at 41.5°C), SulA is present long enough to be lethal. While Canceill et al. (4) found more rapid degradation of SulA at 42°C than at 30°C, as we did, they found very similar half-lives for SulA overproduced from a lac promoter at 30 and 42°C in an SfiB* host and therefore concluded that protection by FtsZ was at least partially lost at higher temperatures. It is possible that under their conditions, the ClpYQ protease is limiting for SulA degradation.
What is the basis for the shorter half-life of SulA in lon mutants at high temperatures? One obvious possibility is increased synthesis of ClpYQ, a heat shock protease, at higher temperatures (5). This notion assumes that ClpYQ is limiting for SulA degradation at lower temperatures, and it is certainly true that more ClpYQ (presence of a multicopy plasmid in the host) increases SulA degradation and MMS sensitivity even at lower temperatures. Another possibility is that suggested by Canceill et al. (4), a weakening of the SulA-FtsZ interaction. In this case, more rapid degradation might be a secondary consequence of a weaker SulA-FtsZ interaction, providing more opportunity for ClpYQ to degrade the free SulA. However, not all of the FtsZ-SulA interaction can be lost at 41.5°C. If it were, the ftsZ(SfiB*) mutation should have no effect on the half-life of SulA at higher temperatures, and it still has a significant effect (Fig. 3B and D). The expression of ClpYQ under the control of a heterologous promoter would help in sorting out whether changing levels of the protease or changing forms of the substrate are most important at high temperatures.
The multimeric structure and protein sequence of the Lon protease and ClpYQ appear to be very different, making the overlap in substrate specificity somewhat unexpected. While the Lon multimer is composed of a single subunit, containing both an ATP binding site consensus sequence and a proteolytic serine active site, ClpYQ is a composite protease, with a catalytic threonine active site in the peptidase subunit and the ATPase activity in a separate subunit (2, 13, 25, 44, 46, 54). Extrapolating from what is known about the Clp proteases, it seems likely that the amino terminus of Lon, including the ATPase domain, and the ATPase of ClpYQ, ClpY, contain the information for substrate recognition. It has been proposed that the Clp ATPases, the ATPase domain of Lon, and the AAA ATPases (named AAA for ATPases associated with a variety of activities) associated with the eukaryotic proteasome have similar general structures (30). Hopefully, more information on these predicted shared structures will provide insight into the shared substrate specificity.
Although we demonstrate that the ClpYQ protease is capable of degrading Lon targets and serves as a secondary protease for SulA degradation, it seems likely that there exist naturally unstable proteins for which the ClpYQ protease is the primary protease. These targets remain to be found.
ACKNOWLEDGMENTS
We thank William Clark for construction of the pWPC80 plasmid; M. Khattar for communicating results prior to publication; and M. Kanemori, H. Yanagi, and T. Yura for sharing results prior to publication and delaying publication of their paper to allow copublication. We thank members of the Laboratory of Molecular Biology and Michael Maurizi for ongoing discussions and comments on the manuscript.
REFERENCES
- 1.Bi E, Lutkenhaus J. Analysis of ftsZmutations that confer resistance to the cell division inhibitor SulA (SfiA) J Bacteriol. 1990;172:5602–5609. doi: 10.1128/jb.172.10.5602-5609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochtler M, Ditzel L, Groll M, Huber R. Crystal structure of heat shock locus V (HslV) from Escherichia coli. Proc Natl Acad Sci USA. 1997;94:6070–6074. doi: 10.1073/pnas.94.12.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill J A, Quinlan-Walshe C, Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coliK-12. J Bacteriol. 1988;170:2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canceill D, Dervyn E, Huisman O. Proteolysis and modulation of the activity of the cell division inhibitor SulA in Escherichia coli lonmutants. J Bacteriol. 1990;172:7297–7300. doi: 10.1128/jb.172.12.7297-7300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang S-E, Burland I G P B, Daniels D L, Blattner F R. Sequence analysis of four new heat-shock genes constituting the hslTS/ibpAB and hslVU operons in Escherichia coli. Gene. 1993;134:1–6. doi: 10.1016/0378-1119(93)90167-2. [DOI] [PubMed] [Google Scholar]
- 6.Dai K, Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer P A J, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 8.Dervyn E, Canceill D, Huisman O. Saturation and specificity of the Lon protease of Escherichia coli. J Bacteriol. 1990;172:7098–7103. doi: 10.1128/jb.172.12.7098-7103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua W C. An improved chloramphenicol resistance gene cassette for site-directed marker replacement mutagenesis. BioTechniques. 1992;12:223–225. [PubMed] [Google Scholar]
- 10.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman S. Regulation by proteolysis. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1308–1312. [Google Scholar]
- 12.Gottesman S. Roles for energy-dependent proteases in regulatory cascades. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 503–519. [Google Scholar]
- 13.Gottesman S, Clark W P, de Crecy-Lagard V, Maurizi M R. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1993;268:22618–22626. [PubMed] [Google Scholar]
- 14.Gottesman S, Roche E, Zhou Y-N, Sauer R T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottesman S, Trisler P, Torres-Cabassa A S. Regulation of capsular polysaccharide synthesis in Escherichia coliK-12: characterization of three regulatory genes. J Bacteriol. 1985;162:1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashitani A, Ishii Y, Kato Y, Horiuchi K. Functional dissection of a cell-division inhibitor, SulA, of Escherichia coliand its negative regulation by Lon. Mol Gen Genet. 1997;254:351–357. doi: 10.1007/s004380050426. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Cao C, Lutkenhaus J. Interaction between FtsZ and inhibitors of cell division. J Bacteriol. 1996;178:5080–5085. doi: 10.1128/jb.178.17.5080-5085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huisman O, D’Ari R. An inducible DNA-replication-cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- 19.Huisman O, D’Ari R, Gottesman S. Cell division control in Escherichia coli: specific induction of the SOS SfiA protein is sufficient to block septation. Proc Natl Acad Sci USA. 1984;81:4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones C, Holland I B. Role of the SulB (FtsZ) protein in division inhibition during the SOS response in Escherichia coli: FtsZ stabilizes the inhibitor SulA in maxicells. Proc Natl Acad Sci USA. 1985;82:6045–6049. doi: 10.1073/pnas.82.18.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jubete Y, Maurizi M R, Gottesman S. Role of the heat shock protein DnaJ in the Lon-dependent degradation of naturally unstable proteins. J Biol Chem. 1996;271:30798–30803. doi: 10.1074/jbc.271.48.30798. [DOI] [PubMed] [Google Scholar]
- 22.Kanemori M, Yanagi H, Yura T. The ATP-dependent HslVU/ClpQY protease participates in turnover of cell division inhibitor SulA in Escherichia coli. J Bacteriol. 1999;181:3674–3680. doi: 10.1128/jb.181.12.3674-3680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama T, Kubota T, Takata M, Akimitsu N, Sekimizu K. Disruption of the hslU gene, which encodes an ATPase subunit of the eukaryotic 26S proteasome homolog in Escherichia coli, suppresses the temperature-sensitive dnaA46mutation. Biochem Biophys Res Commun. 1996;229:219–224. doi: 10.1006/bbrc.1996.1783. [DOI] [PubMed] [Google Scholar]
- 24.Katayama-Fujimura Y, Gottesman S, Maurizi M R. A multiple-component ATP-dependent protease from Escherichia coli. J Biol Chem. 1987;262:4477–4485. [PubMed] [Google Scholar]
- 25.Kessel M, Wu W-F, Gottesman S, Kocsis E, Steven A C, Maurizi M R. Six-fold rotational symmetry of ClpQ, the E. colihomolog of the 20S proteasome, and its ATP-dependent activator, ClpY. FEBS Lett. 1996;398:274–278. doi: 10.1016/s0014-5793(96)01261-6. [DOI] [PubMed] [Google Scholar]
- 26.Khattar M M. Overexpression of the hslVU operon suppresses SOS-mediated inhibition of cell division in Escherichia coli. FEBS Lett. 1997;414:402–404. doi: 10.1016/s0014-5793(97)01024-7. [DOI] [PubMed] [Google Scholar]
- 27.Kirby J E, Trempy J E, Gottesman S. Excision of a P4-like cryptic prophage leads to Alp protease expression in Escherichia coli. J Bacteriol. 1994;176:2068–2081. doi: 10.1128/jb.176.7.2068-2081.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. colichromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Lupas A, Flanagan J M, Tamura T, Baumeister W. Self-compartmentalizing proteases. Trends Biochem Sci. 1997;22:399–404. doi: 10.1016/s0968-0004(97)01117-1. [DOI] [PubMed] [Google Scholar]
- 31.Lutkenhaus J, Sanjanwala B, Lowe M. Overproduction of FtsZ suppresses sensitivity of lonmutants to division inhibition. J Bacteriol. 1986;166:756–762. doi: 10.1128/jb.166.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutkenhaus J F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983;154:1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguin E, Lutkenhaus J, D’Ari R. Reversibility of SOS-associated division inhibition in Escherichia coli. J Bacteriol. 1986;166:733–738. doi: 10.1128/jb.166.3.733-738.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurizi M R, Clark W P, Katayama Y, Rudikoff S, Pumphrey J, Bowers B, Gottesman S. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- 35.Maurizi M R, Trisler P, Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lonis dispensable. J Bacteriol. 1985;164:1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messing J, Crea R, Seeburg P H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981;9:309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J H. Experiments in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Missiakas D, Schwager F, Betton J M, Georgopoulos C, Raina S. Identification and characterization of HslV HslU (ClpQ ClpY) proteins involved in overall proteolysis of misfolded proteins in Escherichia coli. EMBO J. 1996;15:6899–6909. [PMC free article] [PubMed] [Google Scholar]
- 39.Mizusawa S, Court D, Gottesman S. Transcription of the sulAgene and repression by LexA. J Mol Biol. 1983;171:337–343. doi: 10.1016/0022-2836(83)90097-9. [DOI] [PubMed] [Google Scholar]
- 40.Mizusawa S, Gottesman S. Protein degradation in Escherichia coli: the longene controls the stability of the SulA protein. Proc Natl Acad Sci USA. 1983;80:358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee A, Dai K, Lutkenhaus J. Escherichia colicell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZencodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 43.Rohrwild M, Coux O, Huang H-C, Moerschell R P, Yoo S J, Seol J H, Chung C H, Goldberg A L. HslV-HslU: a novel ATP-dependent protease complex in Escherichia colirelated to the eukaryotic proteasome. Proc Natl Acad Sci USA. 1996;93:5808–5813. doi: 10.1073/pnas.93.12.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrwild M, Pfeifer G, Santarius U, Muller S A, Huang H-C, Engel A, Baumeister W, Goldberg A L. The ATP-dependent HslVU protease from Escherichia coliis a four-ring structure resembling the proteasome. Nat Struct Biol. 1997;4:133–139. doi: 10.1038/nsb0297-133. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 47.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 48.Torres-Cabassa A S, Gottesman S. Capsule synthesis in Escherichia coliK-12 is regulated by proteolysis. J Bacteriol. 1987;169:981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trempy J E, Gottesman S. Alp, a suppressor of lon protease mutants in Escherichia coli. J Bacteriol. 1989;171:3348–3353. doi: 10.1128/jb.171.6.3348-3353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trempy J E, Kirby J E, Gottesman S. Alp suppression of Lon: dependence on the slpAgene. J Bacteriol. 1994;176:2061–2067. doi: 10.1128/jb.176.7.2061-2067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Melderen, L., and S. Gottesman. Substrate sequestration by a proteolytically inactive Lon mutant. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 52.Vieira J, Messing J. An M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 53.Wyman A R, Wolfe L B, Botstein D. Propagation of some human DNA sequences in bacteriophage λ vectors requires mutant Escherichia colihosts. Proc Natl Acad Sci USA. 1985;82:2880–2884. doi: 10.1073/pnas.82.9.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo S J, Him Y K, Seong I S, Seol J H, Kang M S, Chung C H. Mutagenesis of two N-terminal Thr and five Ser residues in HslV, the proteolytic component of the ATP-dependent HslVU protease. FEBS Lett. 1997;412:57–60. doi: 10.1016/s0014-5793(97)00742-4. [DOI] [PubMed] [Google Scholar]