Abstract
Background
We examined the relationship between placental histopathology and transplacental antibody transfer in pregnant patients after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
Differences in plasma concentrations of anti-receptor biding domain (RBD) immunoglobulin (Ig)G antibodies in maternal and cord blood were analyzed according to presence of placental injury.
Results
Median anti-RBD IgG concentrations in cord blood with placental injury (n = 7) did not differ significantly from those without injury (n = 16) (median 2.7 [interquartile range {IQR}, 1.8–3.6] vs 2.7 [IQR, 2.4–2.9], P = 0.59). However, they were associated with lower transfer ratios (median 0.77 [IQR, 0.61–0.97] vs 0.97 [IQR, 0.80–1.01], P = 0.05).
Conclusions
SARS-CoV-2 placental injury may mediate reduced maternal-fetal antibody transfer.
Keywords: COVID-19, pregnancy, placenta, antibody transfer
Since the declaration of the global coronavirus disease 2019 (COVID-19) pandemic in March 2020, the World Health Organization has reported over 418 million cases with 5 million deaths worldwide [1]. Among pregnant persons, the prevalence of people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presenting to labor and delivery ranges from 3% to 20% [2]. Although studies have indicated a robust maternal immune response after SARS-CoV-2 infection in pregnancy, they also demonstrate that the ratio of cord to maternal levels after natural immunity is low [3]. This is important because the neonatal immune system is not fully mature until at least 6 months of age; passive immunity through transplacental transfer of maternal antibodies is an important mechanism of protection against illness during infancy and early childhood [4].
The causal mechanisms driving reduced antibody transfer efficiency is an active area of research, and histopathologic examination of placentas from pregnancies affected by SARS-CoV-2 has provided some insight. Several studies have demonstrated that SARS-CoV-2 infection leads to hypoxemic ischemic injury and histopathologic changes of inflammation including intervillositis, perivillous fibrin deposition, vascular malperfusion, infarcts, and trophoblastic necrosis [5, 6]. These findings contribute to increased risk of adverse perinatal outcomes including fetal growth restriction, stillbirth, and maternal hypertensive disorders of pregnancy [2].
It is not known whether there is an association between these placental lesions and the less than expected antibody transfer efficiency seen after SARS-CoV-2 infection. Previous studies have established the relationship between placental function and extent of transplacental maternal IgG transfer in vaccine-preventable illness [7]. Our study examines the relationship between transplacental antibody transfer and abnormal placental pathology in pregnant patients after infection with SARS-CoV-2. We hypothesize that SARS-CoV-2-associated placental injury correlates with reduced transplacental antibody transfer.
METHODS
We recruited pregnant English- or Spanish-speaking patients aged 18 or older with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection at any point during pregnancy and had available pathology results. This study design has been described previously [3].
Placentas were sent for histologic evaluation at the discretion of the delivering providers. Medical chart abstraction was performed to identify those patients with available placental pathology results. Histopathologic evaluation was performed by hospital pathologists using hospital protocols, independent from our study, and did not include techniques that allow virus detection and localization in placental tissue. Severe acute respiratory syndrome coronavirus 2-induced placental injury was defined as the presence of infarct, histiocytic intervillositis, perivillous fibrin deposition, and trophoblast necrosis [8]. Demographics, including maternal age, race/ethnicity, insurance status, and medical comorbidities such as obesity, chronic hypertension, and pregestational diabetes, as well as information surrounding timing and severity of SARS-CoV-2 infection were abstracted from the medical record.
Maternal venous blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes when the patient was admitted for delivery. Umbilical cord blood was collected either directly into EDTA tubes or into a cord blood kit using sterile technique; all samples were processed to obtain plasma and stored at −8°C.
The levels of anti-SARS-CoV-2 anti-receptor biding domain (RBD) immunoglobulin (Ig)G present in maternal and cord blood were assessed using an enzyme-linked immunosorbent assay developed in collaboration with the Emory Medical Laboratory. Log endpoint titers were calculated by imputation after sigmoidal fitting using GraphPad Prism software under clinically validated optical density cutoffs (0.2 for IgG, 0.15 for IgA, and 0.35 for IgM), determined relative to prepandemic negative controls and to convalescent plasma. Antibody end dilution titers were expressed as log10 (value). The IgG concentrations were log-transformed log(10) before analysis. Median differences in maternal and cord blood median log endpoint antibody titers time in those with and without SARS-CoV-2 induced placental injury were evaluated using non-parametric tests of significance with 2-sided P values where appropriate (Mann-Whitney U test). Data sets that contained primarily null values were converted to binary variables, and differences in presence or absence were analyzed using Fisher’s exact test.
Neutralizing activity in maternal and cord blood was also quantified, using an assay against the Wuhan-Hu-1 strain [9]. To measure SARS-CoV-2 spike-specific neutralizing activity in plasma, a 5-fold dilution series was prepared for each sample and incubated with a standard amount of the SARS-CoV-2 pseudovirus. The 50% inhibitory concentration-dilution (IC50) for each plasma sample tested was determined by normalizing the luminescence signal in each sample dilution to the maximal signal in a pseudovirus alone control. The IC50 log dilutions were then calculated by imputation after sigmoidal fitting of each neutralization curve using GraphPad Prism.
Transplacental transfer ratios were defined as the ratio of cord to maternal antibody concentrations and were calculated by dividing the IgG concentration in cord blood by the IgG concentration in paired maternal blood. The difference in transplacental transfer ratios in placentas with and without evidence of placental injury was analyzed using 2 sample t test with equal variance; the strength of the relationship was analyzed using Hedge’s g for effect size values of 0.2, 0.5, and 0.8 are thresholds for small, medium, and large effects, respectively. A P < .05 was considered statistically significant for all analyses.
This research was approved by the Institutional Review Boards (Study IRB00101931, Study MOD003-STUDY 00000312), and all subjects provided written informed consent for participation.
RESULTS
Data on demographics, medical comorbidities, COVID-19 disease, and perinatal outcomes are presented in Table 1. The total cohort consisted of 32 singleton pregnancies with PCR-confirmed SARS-CoV-2 infection from April through December 2020, 23 of whom with available pathology results were included in this study. Included women were Non-Hispanic Black (65%), or Hispanic (35%), publicly insured (91%), with dominant comorbidities including obesity (56%) and hypertension (43%). Median gestational age at delivery was 38 (IQR, 36–40) weeks, although 6 (26%) delivered preterm. Among preterm deliveries, 4 delivered for severe preeclampsia, 1 delivered for non-reassuring fetal heart tracing, and 1 delivered for oligohydramnios.
Table 1.
Maternal Demographic, Clinical Characteristics, Obstetric, and Infectious Outcomes in Pregnant Patients With SARS-CoV-2 Infection
| Characteristic | SARS-CoV-2 Placental Injury, n = 7 (%) | SARS-COV-2 Without Placental Injury, n = 16 (%) |
|---|---|---|
| Demographic | ||
| Maternal age at delivery, mean [SD], years | 27.3 [5.4] | 25 [5.9] |
| Race/Ethnicity | ||
| Non-Hispanic Black | 4 (57) | 11 (69) |
| Non-Hispanic White | 0 | 0 |
| Hispanic | 3 (43) | 5 (31) |
| Medical | ||
| Nulliparous | 3 (43) | 5 (31) |
| Comorbidities | ||
| Asthma | 2 (29) | 4 (25) |
| Diabetes | 0 | 2 (13) |
| Hypertension | 4 (57) | 6 (38) |
| Mental health | 0 | 5 (31) |
| Obesity | 5 (71) | 8 (50) |
| Pregravid Body Mass Index (kg/m2, Missing n = 4) | ||
| BMI 18–24.9 | 0 | 4 (25) |
| BMI 25–29.9 | 3 (43) | 3 (19) |
| BMI 30.0–39.9 kg/m2 | 1 (14) | 6 (38) |
| BMI ≥40 kg/m2 | 2 (29) | 2 (13) |
| SARS-CoV-2 Infection | ||
| Gestational age at infection, median [IQR], weeks | 19 [16–39] | 34 [15–38] |
| Indications for Testing | ||
| Asymptomatic testing | 4 (57) | 8 (50) |
| Person under investigation | 3 (43) | 8 (50) |
| First trimester infection | 1 (14) | 2 (13) |
| Second trimester infection | 4 (57) | 3 (19) |
| Third trimester infection | 2 (29) | 11 (69) |
| COVID-19 Disease Severity | ||
| Asymptomatic/mild | 7 (100) | 13 (81) |
| Moderate | 0 | 3 (19) |
| Severe | 0 | 0 |
| Perinatal Outcomes | ||
| Obstetric | ||
| Gestational age at delivery, median [IQR], weeks | 39 [38–39] | 38 [35–39] |
| Obstetric Complications | ||
| Hypertensive disorder of pregnancy | 4 (57) | 7 (44) |
| Gestational Diabetes | 1 (14) | 2 (13) |
| Fetal growth restriction | 1 (14) | 0 |
| Stillbirth | 0 | 0 |
| Preterm birth | 1 (14) | 5 (31) |
| Mode of Delivery | ||
| Vaginal | 2 (29) | 9 (56) |
| Cesarean | 5 (71) | 7 (44) |
| Neonatal | ||
| Infant birthweight, mean (SD), grams | 3281 (674) | 2950 (432) |
| SARS-CoV-2 Test Result (4 Tested) | ||
| Negative | 1 | 3 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
NOTE: Indication for cesarean in those with SARS-CoV-2-induced placental injury included nonreassuring fetal heart tones (n = 2), malpresentation (n = 1), labor arrest (n = 1), failed trial of labor after cesarean (n = 1).
Median gestational age of infection was 31 (IQR, 15–38) weeks. Eleven (48%) underwent SARS-CoV-2 testing for symptoms, whereas the remainder were tested via a universal screening protocol when admitted to the hospital for delivery. Of the 11 symptomatic patients, 3 (13%) had moderate disease as defined by National Institutes of Health disease severity criteria [10] and the remainder had mild disease. Four infants born to SARS-CoV-2-infected mothers were tested in the immediate neonatal period and were negative.
Severe acute respiratory syndrome coronavirus 2-induced injury was present in 7 placentas (7 of 23, 30%). The median gestational age at the time of infection was 19 (IQR, 16–39) weeks. Three had mild disease, and the remainder had asymptomatic disease. Of these 7 patients, 4 had chronic hypertension, 2 of whom went on to develop superimposed preeclampsia.
All maternal sera contained detectable anti-RBD IgG antibodies with the median log end dilution titer (EDT) of 3.0 (IQR, 2.7–3.34). Twelve (52%) of the samples demonstrated maternal neutralizing activity, with median IC50 log EDT of 3.0 (IQR, 2.6–3.3). Anti-RBD IgG was present in 19 (83%) of cord blood sera (median log EDT of 2.9; IQR, 2.4–3.1), and neutralizing activity was detected in 5 (22%) (median IC50 log EDT of 2.4; IQR, 2.3–2.6). The cord to maternal anti-RBD IgG ratio was 0.87, representing a total cohort transplacental antibody transfer efficiency of 87%.
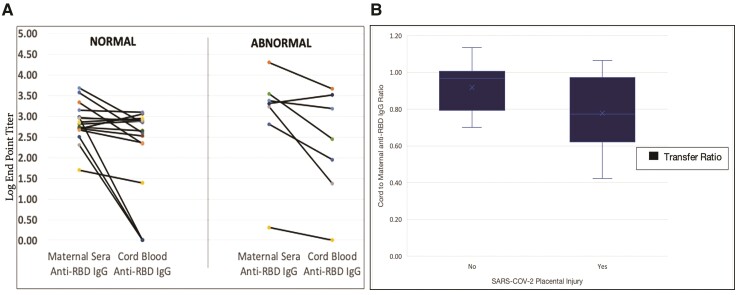
There was no statistically significant difference in the cord blood anti-RBD IgG concentrations in patients with and without SARS-CoV-2-induced placental injury (log EDT 2.7 [IQR, 1.8–3.6] vs 2.7 [IQR, 2.4–2.9], P = .59, respectively) (Figure 1A). However, there was reduced antibody transfer efficiency in placentas with SARS-COV-2-induced placental injury compared with normal placentas (median log EDT of 0.77 [IQR, 0.61–0.97] vs 0.97 [IQR, 0.80–1.01], P = .05). Although not significant, there was strong correlation between placental injury and antibody transfer efficiency (Hedges’ g = 1.17) (Figure 1B).
Figure 1.
(A)Absolute anti-receptor biding domain (RBD) immunoglobulin (Ig)G concentrations in cord blood compared with maternal sera according to presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placental injury. All maternal sera and 83% (n = 19) cord blood samples had detectable anti-RBD IgG. There was no statically significant difference in the absolute cord blood anti-RBD IgG concentrations in patients with and without SARS-CoV-2-induced placental injury (log end dilution titer 2.7 [interquartile range {IQR}, 1.8–3.6] vs 2.7 [IQR, 2.4–2.9], P = .59). (B) Anti-SARS-CoV-2 RBD IgG cord-to-maternal ratios grouped according to presence of SARS-CoV-2 placental injury. The cord, maternal ratio, or transplacental transfer efficiency was calculated as the ratio between cord blood and maternal anti-RBD IgG log endpoint titers. The median antibody transfer efficiency in placentas without SARS-CoV-2-induced placental injury was 0.77 (IQR, 0.61–0.97). The median antibody transfer efficiency in placentas with SARS-CoV-2-induced placental injury was 0.97 (IQR, 0.80–1.01; P = .05).
Given the increased frequency of preterm birth in the group without infarcts, we separately analyzed the difference in transplacental antibody transfer according to presence of placental injury among term deliveries. Median antibody transfer ratio in those without infarcts was 0.87 (IQR, 0.65–1.09; range, 0–1.13). Median antibody transfer ratio in those with infarcts was 0.66 (IQR, 0.54–0.81; range, 0–1.07; P = .704).
DISCUSSION
We investigated the association between SARS-CoV-2-associated placental injury and the transplacental antibody transfer ratios of anti-RBD IgG antibodies in a population of pregnant patients after natural infection with SARS-CoV-2. We found that SARS-CoV-2-induced placental injury had no effect on the cord blood anti-RBD IgG concentrations and but may be associated with reduced transplacental antibody transfer efficiency.
Maternal infections have previously been associated with compromised placental antibody transfer, but the pathologic mechanisms underlying this compromised transfer have not been well established. Previous studies have implicated impaired placental kinetics, high levels of viremia, and gestational age as factors contributing to reduced transfer efficiency [11]. The implications of placental injury after SARS-CoV-2 infection is an active area of investigation. A recent study by Schwartz et al [12] compiled an analysis of pathologic placental characteristics from 11 cases of patients infected by SARS-CoV-2 during pregnancy to identify pathologic sequelae of the viral infection. The authors found that affected placentas all had evidence of chronic histiocytic intervillositis and necrosis of the syncytiotrophoblast [12]. Chronic histiocytic intervillositis is a rare inflammatory lesion of the placenta that has previously been associated with poor obstetric outcomes including miscarriage, fetal demise, intrauterine growth restriction, and preterm delivery before the emergence of COVID-19 [12]. Whether and how placental lesions after SARS-CoV-2 infection drive poor placental transfer kinetics needs to be determined.
Transplacental transport of maternal IgG antibodies is dependent on numerous factors, including placental function, which we suggest is inhibited by placental injury after natural infection. In contrast, vaccination provides an efficient strategy to confer neonatal protection through passive placental antibody transfer. Halasa et al [13] quantified the antibody response in infants born to mothers after either a natural infection of SARS-CoV-2 or receipt of 1 of the 3 available COVID-19 vaccines in the United States. In the vaccinated group, 94% of infants had detectable anti-S IgG at 2 months, and 60% had detectable antibody at 6 months. In contrast, only 8% of infants born to women infected with SARS-CoV-2 in pregnancy had detectable anti-S IgG at the 6-month timepoint [13]. In addition, Collier et al [14] compared antibody concentrations in neonates exposed to maternal mRNA vaccination versus naturally derived immunity and found that not only were binding, neutralizing, and functional nonneutralizing antibody responses higher in vaccinated women compared with natural infection, there was also enhanced CD4 and CD8 T-cell responses. Therefore, we emphasize the critical importance of vaccination against SARS-CoV-2 to provide robust protection to pregnant persons and their infants.
There are several limitations to this study. The placentas that were sent to pathology for evaluation were up to the discretion of the delivering clinician, which means that placentas from pregnancies with adverse outcomes are overrepresented within our cohort. In addition, it was difficult to determine differences within the placentas in our study that had coexisting preeclampsia in addition to SARS-CoV-2 infection. The INTERCOVID study highlights that SARS-CoV-2 infection during pregnancy is strongly associated with preeclampsia independent of any risk factors or preexisting conditions, and therefore these effects may be collinear [15]. We were unable to assess viremia within the placentas, and therefore we are unable to estimate the relationship between viral load and pathologic injury. Finally, given the small sample size, we were unable to independently estimate the contribution of gestational age on transfer kinetics. However, the magnitude of difference in transfer ratios suggests an interaction between placental infarct and antibody transfer, which should be further explored.
CONCLUSIONS
These data demonstrate that within this small sample of pregnant patients, SARS-CoV-2-induced placental injury did not demonstrate a difference in cord blood anti-RBD IgG titers. However, cord to maternal blood ratios appeared lower in patients whose placentas demonstrated injury. Thus, SARS-CoV-2-induced placental injury may decrease the efficiency of transplacental antibody transfer. Further studies are needed to assess the role in the timing of infection in the severity of placental pathology and subsequent transfer efficiency.
Contributor Information
Patience Timi, Emory University School of Medicine, Atlanta, Georgia, USA.
Sarah E Kellerhals, Department of Obstetrics and Gynecology, University of Arizona College of Medicine, Phoenix, Arizona, USA.
Naima T Joseph, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA.
Carolynn M Dude, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Hans P Verkerke, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Les’Shon S Irby, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Alicia K Smith, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Sean R Stowell, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Denise J Jamieson, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Martina L Badell, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Notes
Financial support. This research was supported by funding from the Emory Medical Care Foundation (to C. M. D., N. T. J., and M. L. B.) and an Emory University School of Medicine Woodruff Health Sciences Synergy Award (to M. L. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. WHO Coronavirus Dashboard. Available at: https://covid19.who.int/. Accessed 1 April 2022.
- 2. Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol 2022; 226:177–86. doi: 10.1016/j.ajog.2021.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph NT, Dude CM, Verkerke HP, et al. Maternal antibody response, neutralizing potency, and placental antibody transfer after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol 2021; 138:189–97. doi: 10.1097/AOG.0000000000004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albrecht M, Arck PC. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front Immunol 2020; 11:555. doi: 10.3389/fimmu.2020.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shook LL, Brigida S, Regan J, et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J Infect Dis 2022; 25:754–8. doi: 10.1093/infdis/jiac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020; 101:13–29. doi: 10.1016/j.placenta.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albrecht M, Pagenkemper M, Wiessner C, et al. Infant immunity against viral infections is advanced by the placenta-dependent vertical transfer of maternal antibodies. Vaccine 2022; 40:1563–71. doi: 10.1016/j.vaccine.2020.12.049 [DOI] [PubMed] [Google Scholar]
- 8. Watkins JC, Torous VF, Roberts DJ. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med 2021; 145:1341–9. doi: 10.5858/arpa.2021-0246-SA [DOI] [PubMed] [Google Scholar]
- 9. Crawford KHD, Eguia R, Dingens AS, et al. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses 2020; 12:513. doi: 10.3390/v12050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov. Accessed 27 February 2022. [PubMed]
- 11. Atyeo C, Pullen KM, Bordt EA, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021; 184:628–42.e10. doi: 10.1016/j.cell.2020.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz DA, Baldewijns M, Benachi A, et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med 2021; 145:517–28. doi: 10.5858/arpa.2020-0771-SA [DOI] [PubMed] [Google Scholar]
- 13. Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med 2022; 387:109–119. doi: 10.1056/NEJMoa2204399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021; 325:2370–80. doi: 10.1001/jama.2021.7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol 2021; 225:289.e1–e17. doi: 10.1016/j.ajog.2021.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]