The Omicron (B.1.1.529) variant of concern (VOC) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), the virus that causes coronavirus disease 2019 (COVID-19), was first detected in November 2021 in South Africa [1]. It is characterized by several mutations of the spike protein especially in the region that recognizes receptors on human cells, which has increased its transmissibility compared with the wild type and previous VOCs. There is increasing evidence in the general population that Omicron is associated with less severe disease and that the third/booster vaccine dose offers additional protection from severe COVID-19 disease [2, 3]. In patients undergoing kidney replacement therapy (KRT), it is now well established that whilst two doses of a COVID-19 vaccination induce a serological response with formation of anti-S immunoglobulin G antibodies in the majority of dialysis patients, this offers lesser protection against death and hospitalization compared with that offered in people without kidney failure [4–7]. In the UK from mid- to late-December, neutralizing monoclonal antibodies (nMabs) and/or oral antiviral therapy were offered to symptomatic patients receiving KRT (dialysis and transplant) following a positive polymerase chain reaction test irrespective of disease severity. There is currently a lack of data on how the Omicron variant has impacted on outcomes following infection with SARS-CoV-2 in patients receiving KRT.
We investigate the effects of COVID-19 vaccination on incidence and clinical outcomes of COVID-19 following the advent of the Omicron variant as the dominant strain from 17 December 2021, until 27 March 2022 in all patients receiving KRT in Scotland.
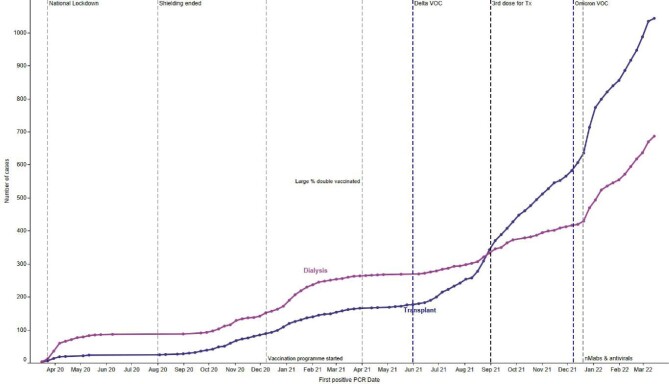
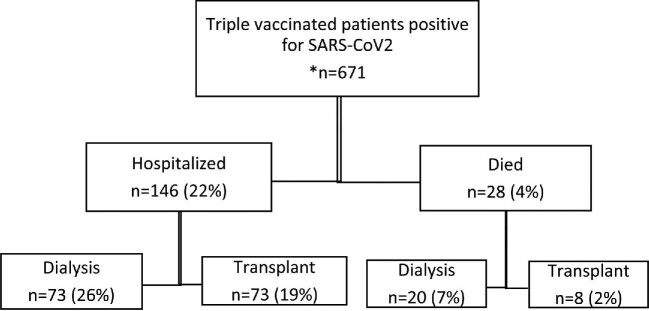
Details of methods are provided in Supplementary Methods. As of 27 March 2022, 4815 patients, which amounts to 90% of the prevalent KRT population in Scotland, had received at least three doses of a SARS-CoV-2 vaccine (88% of dialysis patients and 91% of transplant patients), and 46% a fourth dose. Of those who had received three doses, 97% received a messenger RNA (mRNA) vaccine, the remainder received ChAdOx1 (Oxford–AstraZeneca) as their third dose. This contrasts with the initial two doses where the majority of patients received ChAdOx1 vaccine [5]. Figure 1a shows the timeline of positive cases since the outset of the pandemic in patients receiving KRT in Scotland by modality. There were 792 positive cases of COVID-19 since 17 December 2021 when Omicron was considered the dominant variant in the UK. A total of 671 patients tested positive at least 14 days after a third dose of vaccine. Of these, 389 (58%) were in patients with a transplant and 282 (42%) in patients receiving dialysis. Forty-nine of these were re-infections. Median time to testing positive after third dose was 109 days (interquartile range 83–139). Outcomes of patients who tested positive for SARS-CoV-2 since 17 December 2021 at least 14 days after a third dose of vaccine are shown in Fig. 1b: 146 (22%) were hospitalized; 28 (4%) died. The majority of those who were hospitalized were male (64%). Sixteen percent of triple-vaccinated transplant patients and 23% of triple-vaccinated dialysis patients were hospitalized. The median age of those who died and were triple vaccinated was 71 (interquartile range 63–74) and 61% were male. Overall, 2% of triple-vaccinated transplant patients and 5% of triple-vaccinated dialysis patients died. Supplementary Fig. S1 shows hospital admissions by modality since the beginning of the pandemic, demonstrating a reduction in hospitalizations since administration of a third dose of vaccine and emergence of the Omicron variant. Figure 1c shows the percentage alive at 28 days in those who test positive, by modality.
Figure 1.
a): Timeline of cumulative cases of COVID-19 by KRT modality in Scotland. Purple vertical dashed lines represent date each VOC became the dominant strain. For Delta this was 4 June 2021, while for Omicron this was 17 December 2021 [1, 9].
Figure 2.
b): Flowchart of outcomes for triple-vaccinated patients who tested positive for SARS-CoV-2. *Denominator 4815 triple-vaccinated individuals (1849 dialysis and 2966 transplant).
Figure 3.
c): Percentage of patients alive 28 days following positive COVID-19 polymerase chain reaction shown with total number of SARS-CoV-2 infections for each month, broken down by vaccination status and KRT modality. Black line: % alive at 28 days; bar chart: number of vaccine doses.
We present complete national real-world data demonstrating improvement in both mortality and hospitalization in patients receiving KRT since emergence of the Omicron VOC. In our population we have previously shown that outcomes were extremely poor prior to vaccination, with 26.7% mortality in dialysis patients and 29.2% in transplant patients [8]. This improved following two doses of a COVID-19 vaccine, but mortality was still substantially higher than the general population (7% for dialysis patients and 10% for transplant patients) [5]. We demonstrate further improvement in outcomes with a significant reduction in both hospitalization and mortality over the subsequent period from December 2021 onwards. Whilst the high transmissibility of Omicron has led to a dramatic increase in the number of infections with SARS-CoV-2, the proportion of those being hospitalized and/or dying within 28 days of a positive test is much lower than previous (see Fig. 2a). It remains unclear, however, whether this improvement is due to a third dose of vaccine, the emergence of Omicron as the dominant variant in Scotland or the administration of nMabs and oral antivirals. We do not have genotypic sequence data on the individual cases of SAR2-CoV-2 infection in patients requiring KRT, so we can only comment on the outcomes in patients on KRT during each era when the various VOC were the dominant strain in the general population of Scotland, rather than ascribe the outcomes directly to changes in pathogenicity of each VOC in patients requiring KRT.
Despite increasing transmissibility and dramatic rises in the number patients experiencing SARS-CoV-2 infection, outcomes have improved in those patients requiring KRT with SARS-CoV-2 infection. Whilst it is unclear what the implications of society ‘opening up’ will have for this vulnerable patient group, these data provide some reassurance that outcomes have improved since the onset of the pandemic. Strategies are required to further improve outcomes in patients treated with KRT so that they can safely engage with society, including maintaining social interactions and employment, as society further returns towards pre-pandemic levels of social interaction.
Supplementary Material
Contributor Information
Samira Bell, Division of Population Health and Genomics, School of Medicine, University of Dundee, Dundee, UK; The Scottish Renal Registry, Scottish Health Audits, Public Health Scotland, Glasgow, UK; Renal Unit, Ninewells Hospital, Dundee, UK.
Jacqueline Campbell, The Scottish Renal Registry, Scottish Health Audits, Public Health Scotland, Glasgow, UK.
Chrissie Watters, The Scottish Renal Registry, Scottish Health Audits, Public Health Scotland, Glasgow, UK.
Martin O'Neil, The Scottish Renal Registry, Scottish Health Audits, Public Health Scotland, Glasgow, UK.
Alison Almond, Renal Unit, Dumfries, UK.
Katharine Buck, Renal Unit, Victoria Hospital, Kirkcaldy, UK.
Edward J Carr, Cell Biology of Infection Laboratory, Francis Crick Institute, London, UK.
Zoe Cousland, Renal Unit, Monklands Hospital, Airdrie, UK.
Mark Findlay, Glasgow Renal & Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK.
Nicola Joss, Renal Unit, Raigmore Hospital, Inverness, UK.
Wendy Metcalfe, Department of Renal Medicine, Royal Infirmary of Edinburgh, Edinburgh, UK.
Elaine Spalding, Renal Unit, University Hospital Crosshouse, Crosshouse, UK.
Shona Methven, Department of Renal Medicine, Aberdeen Royal Infirmary, Aberdeen, UK.
Patrick B Mark, Glasgow Renal & Transplant Unit, Queen Elizabeth University Hospital, Glasgow, UK; Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
CONFLICT OF INTEREST STATEMENT
S.B. reports personal fees from AstraZeneca, outside the submitted work. P.B.M. reports grants from Boehringer Ingelheim; personal fees from Astellas, AstraZeneca, Janssen and Novartis; and personal fees and nonfinancial support from Napp, Pharmacosmos and Vifor, outside the submitted work. The results presented in this paper have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly as they are held by Public Health Intelligence, Scotland. Data can be requested from the electronic Data Research and Innovation Service (eDRIS) team, which are part of the Public Health Scotland Public Health Intelligence, Scotland, through phs.edris@phs.scot.
REFERENCES
- 1. Scottish Government; Coronavirus (COVID-19): modelling the epidemic (issue no. 82). Director-General Health and Social Care. 17 December 2021. https://www.gov.scot/publications/coronavirus-covid-19-modelling-epidemic-issue-no-82/. Accessed 25th May 2022. [Google Scholar]
- 2. Wu M, Wall EC, Carr EJet al. . Three-dose vaccination elicits neutralising antibodies against omicron. Lancet 2022;399:715–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolter N, Jassat W, Walaza Set al. . Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet North Am Ed 2022;399:437–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carr EJ, Wu M, Harvey Ret al. . Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet 2022;399:800–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bell S, Campbell J, Lambourg Eet al. . The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland. J Am Soc Nephrol 2022;33:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr EJ, Kronbichler A, Graham-Brown Met al. . Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep 2021;6:2292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu CM, Lacson EK, Manley HJet al. . Seroresponse to third doses of SARS-CoV-2 vaccine among patients receiving maintenance dialysis. Am J Kidney Dis 2022;80:151–153doi: 10.1053/j.ajkd.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bell S, Campbell J, McDonald Jet al. . COVID-19 in patients undergoing chronic kidney replacement therapy and kidney transplant recipients in Scotland: findings and experience from the Scottish renal registry. BMC Nephrol 2020;21:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scottish Government; Coronavirus (COVID-19): state of the epidemic – 4 June 2021. Constitution and Cabinet Directorate; 4 June 2021. Accessed 25th May 2022. https://www.gov.scot/publications/coronavirus-covid-19-state-epidemic-04-june-2021/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly as they are held by Public Health Intelligence, Scotland. Data can be requested from the electronic Data Research and Innovation Service (eDRIS) team, which are part of the Public Health Scotland Public Health Intelligence, Scotland, through phs.edris@phs.scot.