Abstract
Aims
To investigate the impact of coronavirus disease 2019 lockdown on trajectories of arterial pulse-wave velocity in a large population of users of connected smart scales that provide reliable measurements of pulse-wave velocity.
Methods and results
Pulse-wave velocity recordings obtained by Withings Heart Health & Body Composition Wi-Fi Smart Scale users before and during lockdown were analysed. We compared two demonstrative countries: France, where strict lockdown rules were enforced (n = 26 196) and Germany, where lockdown was partial (n = 26 847). Subgroup analysis was conducted in users of activity trackers and home blood pressure monitors. Linear growth curve modelling and trajectory clustering analyses were performed. During lockdown, a significant reduction in vascular stiffness, weight, blood pressure, and physical activity was observed in the overall population. Pulse-wave velocity reduction was greater in France than in Germany, corresponding to 5.2 month reduction in vascular age. In the French population, three clusters of stiffness trajectories were identified: decreasing (21.1%), stable (60.6%), and increasing pulse-wave velocity clusters (18.2%). Decreasing and increasing clusters both had higher pulse-wave velocity and vascular age before lockdown compared with the stable cluster. Only the decreasing cluster showed a significant weight reduction (−400 g), whereas living alone was associated with increasing pulse-wave velocity cluster. No clusters were identified in the German population.
Conclusions
During total lockdown in France, a reduction in pulse-wave velocity in a significant proportion of French users of connected smart bathroom scales occurred. The impact on long-term cardiovascular health remains to be established.
Keywords: Vascular stiffness, COVID-19, Lockdown, Vascular age, Connected devices, Pulse-wave velocity, Smart bathroom scales
Graphical Abstract
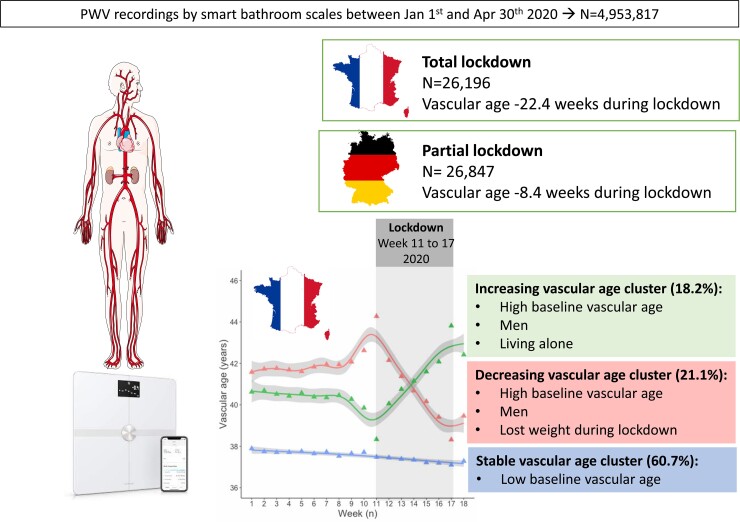
Graphical Abstract.
During COVID19 total lockdown in 2020 in France, a significant reduction in vascular stiffness, recorded by home smart scales, was observed.
See the editorial comment for this article ‘Arterial stiffness on a different scale’, by B. Spronck, https://doi.org/10.1093/ehjdh/ztac036.
Novelty and Significance.
1. What new?
During total lockdown enforced during first COVID-19 pandemic wave in France, around one-fifth improved their vascular stiffness, whereas for another fifth, vascular stiffness was worsened.
Partial lockdown enforced in Germany has no such effect.
2. What is relevant?
Since vascular stiffness is a cause of hypertension, the observed changes may impact its prevalence in the future.
3. Summary—of the conclusions of the study
Social-distancing measures such as total lockdown have a measurable impact on vascular health.
Introduction
Coronavirus disease 2019 (COVID-19) and cardiovascular diseases (CVDs) are tightly interrelated. Pre-existing CVDs are associated with a poor prognosis for COVID-19, and COVID-19 itself can directly or indirectly trigger extensive cardiovascular damage.1 However, trajectories of cardiovascular health might also be inflected in individuals not infected by SARS-CoV-2, for example, as a consequence of interruption of all non-urgent care activities, emergency reallocation of healthcare resources, or social-distancing measures limiting patient access to medical advice and life-saving procedures.2
Nationwide total home confinement, lockdown, was the most striking measure taken to prevent the spread of COVID-19 infection. We previously demonstrated that total lockdown is characterized by a substantial reduction of physical activity, up to 54% compared with pre-pandemic values.3 Reduced physical activity, together with anxiety, financial stress, delay in acute patient referral to appropriate care for myocardial infarction4 related to the spread of COVID-19 and the enforcement of social-distancing measures, might have impaired cardiovascular health in the general population.
On the other hand, lockdown might have had some beneficial effects on cardiovascular health, through reduction in air pollution5–7 increased home cooking and reduced consumption of fast foods and ready-made meals8,9; reduced work-related stress; and better sleep quality due to the suppression of chronic sleep deprivation and social jet lag.10,11 Lockdown has been applied heterogeneously by different countries,2 providing a unique opportunity to compare the impact of different social-distancing measures on cardiovascular health in a real-life experimental scenario in large population samples.
Vascular stiffness, an integrated biomarker of the combined detrimental effect on arteries of inherited and environmental exposure, as well as of cardiovascular risk factors, is considered as a reliable index of vascular age and overall cardiovascular health.12,13 Many studies have shown that the vascular stiffness measured by pulse-wave velocity (PWV) is associated with increased CVD incidence, independently of traditional risk factors.14 The PWV is used to calculate vascular age, a widely recognized index of cardiovascular health, which may be better related to CVD outcomes than chronological age.15
Connected devices monitoring vital parameters for wellbeing and health purposes are increasingly used by the general population, making it possible to obtain seamlessly repeated measures over time in real-life conditions. Very recently, self-measurement and repeated assessment of PWV has been made possible by connected smart bathroom scales, validated against the reference technique.16
The main objective of this study was to investigate PWV trajectories during the lockdown period among Withings Heart Health & Body Composition Wi-Fi Smart Scales users in two representative countries (France and Germany), in which different social-distancing rules were applied.
As changes in body weight, blood pressure (BP), and physical activity might have impacted PWV trajectories, their trajectories over time and any association with PWV trajectories were also investigated.
Methods
An extended method section is available as an online supplement.
Study population
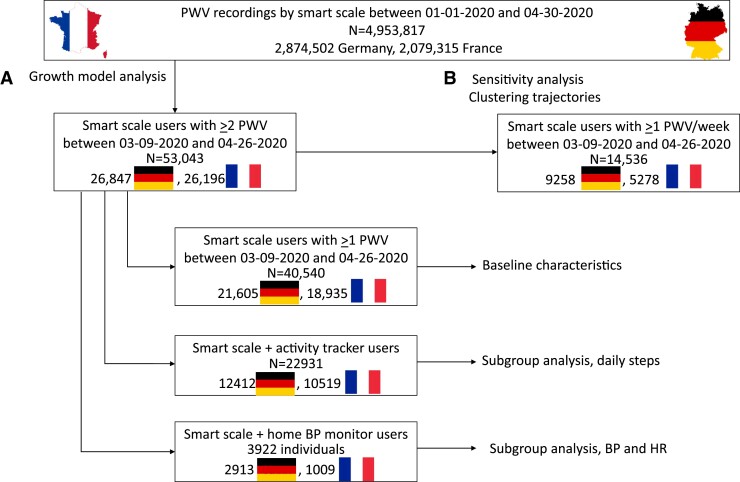
The study population is summarized in Figure 1. The source database included all anonymized PWV recordings obtained by Withings Body Cardio scales users between 1 January 2020 and 30 April 2020 in France (n = 2 079 315) and Germany (n = 2 874 502).
Figure 1.
Flow chart of data extraction and selection of study populations.
From this database, we selected 53 043 individuals having PWV recordings available for at least 2 weeks in the period from Week 11 (the week before total lockdown started in France, starting 9 March 2020) to Week 17 of 2020 (the sixth week of full lockdown in France, ending 26 April 2020; Population A). Data from Weeks 5–8 (before the COVID-19 outbreak) were also extracted and averaged in order to obtain baseline characteristics. We further extracted a subpopulation of individuals with no missing PWV from Week 11 to Week 17 of 2020 for sensitivity analysis and for trajectory cluster analysis (n = 14 536; Population B).
Since several Withings Body Cardio users also own other Withings connected devices, in particular activity trackers (a wristwatch with embedded an accelerometer, 22 931 individuals) and home BP monitors (HBPMs, 3922 individuals), daily steps, BP, and heart values were extracted and analysed.
All Withings connected device users were informed that the anonymized data collected could be used for research purposes, and they provided informed consent before starting to use the devices. They were allowed to withdraw their consent at any time and to request deletion of their individual data.3
Variables
The following variables were analysed:
• Time-invariant variables: age, sex, height, country, region, number of scale users in the household (considered as a proxy of living alone);
• Time-varying variables (variables with longitudinal repeated measurements): PWV, weight, fat mass (whole population); daily steps, systolic and diastolic BP, heart rate (subpopulation).
The bathroom scales use the principle of impedance plethysmography in a single foot combined with ballistocardiography.16 The recorded data were then processed in real time by the device itself and the results sent via Wi-Fi to a secured database. Vascular age was calculated with a methodology already validated for carotid-femoral PWV,15 based on an independent data set of apparently healthy individuals (n = 91), with none of the usual risk factors.16
Statistical analysis
For each participant, weekly averages (ISO 8601 definition of week) of the time-varying variables were calculated. Week 5-to-8 averaged values were considered as baseline values. Absolute differences in time-varying variables (Week 17 minus baseline) were calculated.
In order to investigate the impact of total lockdown vs. partial lockdown on PWV, by comparing it in France and in Germany, latent variable modelling (and in particular linear growth curve modelling) was used, using the R package ‘lavaan’.17 For each model, two latent variables were considered: intercept (PWV Week 11) and slope (weekly rate of PWV change from Week 11 to Week 17), whereas time-varying variables (PWV, vascular age, weight, systolic and diastolic BP, heart rate, daily steps) at each time step from Week 11 to Week 17 were inserted as independent variables and country as a covariate. Separate models in France and Germany were also run.
Trajectory clustering analysis was performed separately on the French and German study populations using the R package traj.18,19 We allowed the number of clusters to vary between 2 and 6, and we calculated 26 clustering validity indices available in the literature (see Supplementary material online, Table S2), using the NbClust R package: the most frequently recommended number was retained.20
Finally, multinomial regression analysis (nnet R package) was used to explore determinants of the different clusters of PWV trajectories.
Results
Characteristics of the study population
The main characteristics of the study population are presented in Table 1. German participants were older than French participants, more frequently men, with higher body mass index (BMI) and fat mass; accordingly, PWV was significantly higher in German than in French participants. Both French and German participants’ vascular age was ∼9 years lower than their chronological age.
Table 1.
Main characteristics of the study population
| Variable | Number of observations | Germany (n = 26 847) | France (n = 26 196) | P-value |
|---|---|---|---|---|
| Age (years) | 53 043 | 49 ± 12 | 44 ± 13 | <0.0001 |
| Men/women, n (%) | 53 043 | 17 643 (65.7%) 9204 (34.3%) | 16 396 (62.6%) 9800 (37.4%) | <0.0001 |
| Sole user in household, n (%) | 53 043 | 4234 (15.8%) | 3784 (14.4%) | <0.0001 |
| Baseline (average Weeks 5–8) | ||||
| PWV (m/s) | 40 540 | 7.48 ± 1.14 | 7.16 ± 1.12 | <0.0001 |
| Vascular age (years) | 40 540 | 41 ± 8 | 38 ± 8 | <0.0001 |
| Weight (kg) | 40 845 | 82.4 ± 17.4 | 75.7 ± 15.5 | <0.0001 |
| BMI (kg/mq) | 40 482 | 26.3 ± 4.5 | 25.2 ± 4.2 | <0.0001 |
| Fat mass (%) | 39 880 | 25.6 ± 9.0 | 24.0 ± 9.0 | <0.0001 |
| Daily steps (n) | 19 162 | 6165 ± 3900 | 5449 ± 3706 | <0.0001 |
| Systolic BP (mmHg) | 4008 | 127 ± 12 | 126 ± 13 | <0.0001 |
| Diastolic BP (mmHg) | 4008 | 80 ± 9 | 79 ± 10 | <0.0001 |
| Heart rate (b.p.m.) | 3828 | 70 ± 11 | 69 ± 13 | <0.0001 |
| Difference (Week 17 − Baseline) | ||||
| Δ PWV (m/s) | 29 373 | −0.01 ± 0.62 | −0.04 ± 0.64 | <0.0001 |
| Δ Vascular age (years) | 28 165 | −0.08 ± 4.4 | −0.30 ± 4.6 | <0.0001 |
| Δ Weight (kg) | 23 322 | −0.45 ± 1.65 | −0.38 ± 1.52 | <0.0001 |
| Δ Fat mass (%) | 22 735 | −0.16 ± 2.24 | −0.16 ± 2.39 | <0.0001 |
| Δ Daily steps (n) | 8874 | 561 ± 3493 | −836 ± 3953 | <0.0001 |
| Δ Systolic BP (mmHg) | 1417 | −1.3 ± 8.7 | −2.1 ± 8.9 | <0.0001 |
| Δ Diastolic BP (mmHg) | 1417 | −0.9 ± 5.8 | −1.3 ± 6.5 | <0.0001 |
| Δ Heart rate (b.p.m.) | 1346 | −0.7 ± 7.4 | −0.3 ± 8.1 | <0.0001 |
Data are expressed as mean ± standard deviation. Baseline values are expressed as mean (average of the mean values from Week 5 to Week 8) ± standard deviation (which corresponds to the dispersion between these four values).
BMI, body mass index; PWV, pulse-wave velocity; BP, blood pressure; Δ, absolute difference during 6-week lockdown (between Weeks 17 and 11).
Trajectories of pulse-wave velocity and physiological variables during lockdown in France and Germany
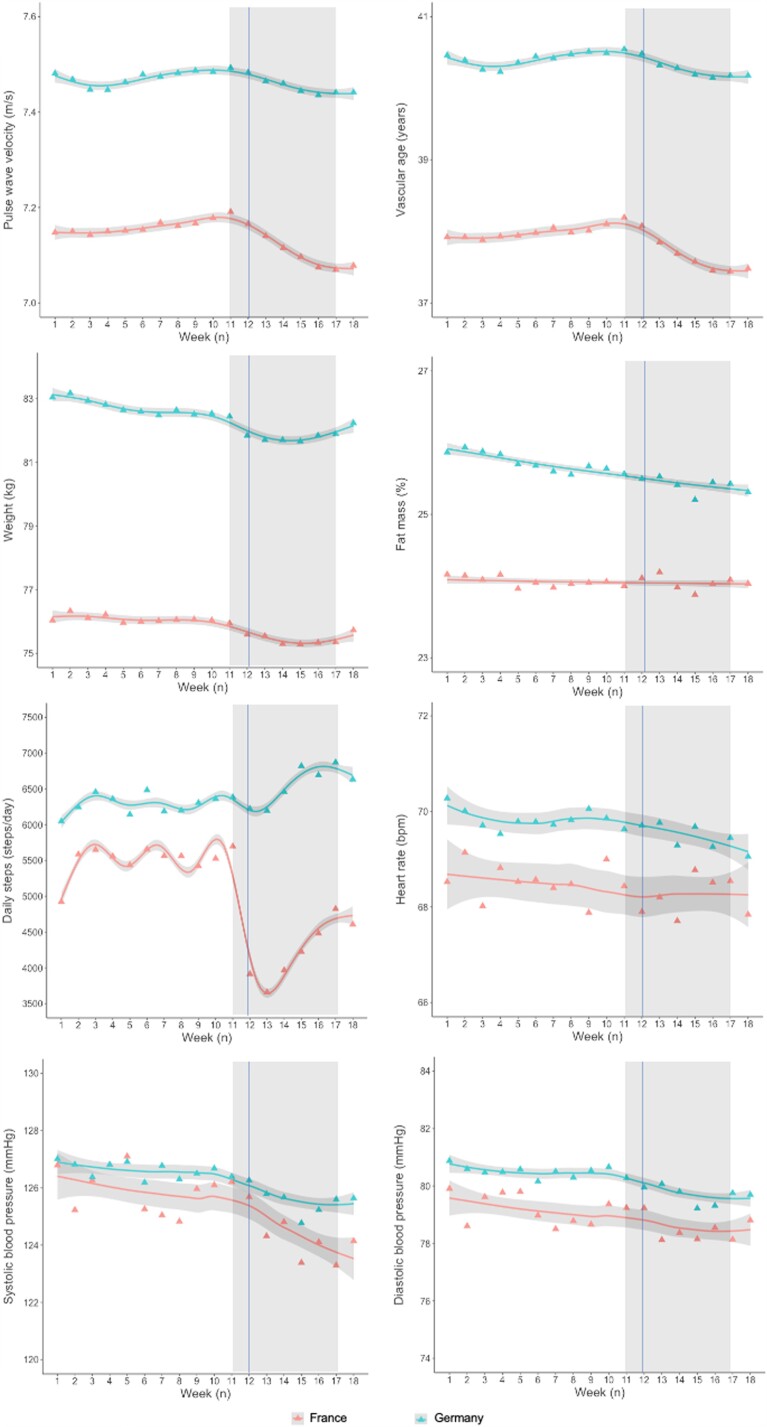
Overall, PWV showed a slight increase from Week 9 to Week 11, corresponding to the period when of COVID-19 spread throughout Europe, followed by a marked linear reduction from Week 11 to Week 17, both in Germany and France (Figure 2). In France, PWV decreased at a rate of 9 mm/s/week, whereas in Germany, it was 4 mm/s/week (see Supplementary material online, Table S2). When combining data from France and Germany and considering country as a covariate, the results confirmed that: (i) the starting PWV was significantly higher in German than in French participants (difference in intercept −0.328 m/s, P < 0.0001); (ii) PWV reduction during lockdown was significantly steeper in France than in Germany (difference in slope 5 mm/s/week, P < 0.0001; Table 2). Vascular age also showed a linear reduction from Week 11 to Week 17 both in the French and the German study populations (Figure 2). However, vascular age reduction during lockdown was steeper in French than in German participants, corresponding to a vascular age reduction of −22.4 weeks vs. −8.4 weeks, respectively (see Supplementary material online, Tables S3 and 2).
Figure 2.
Behaviour of main time-varying variables from 1 January 2020 to 30 April 2020 in the French and German populations. Triangles represent mean weekly values, lines are fitted by general additive models (penalized regression cubic splines, parameter estimation method = restricted maximum likelihood), with 95% confidence intervals in grey. The vertical line represents the date of total lockdown in France (17 March 2020). Rectangles highlight the time interval analysed (Weeks 11–17).
Table 2.
Comparison between time-varying variables in France and Germany by latent growth curve analysis
| France vs. Germany | Number of observations | Estimate | Standard error | P-value |
|---|---|---|---|---|
| Difference in baseline PWV (m/s) | 53 043 | −0.329 | 0.010 | <0.0001 |
| Difference in rate of PWV change (m/s/week) | 53 043 | 0.005 | 0.001 | <0.0001 |
| Difference in baseline vascular age (years) | 53 043 | −2.450 | 0.073 | <0.0001 |
| Difference in rate of vascular age change (years/week) | 53 043 | 0.037 | 0.009 | <0.0001 |
| Difference in baseline weight (kg) | 53 043 | −6.481 | 0.146 | <0.0001 |
| Difference in rate of weight change(kg/week) | 53 043 | 0.030 | 0.003 | <0.0001 |
| Difference in baseline daily steps (n) | 22 931 | −1828 | 49 | <0.0001 |
| Difference in rate of daily steps change (n/week) | 22 931 | 114 | 14 | <0.0001 |
| Difference in baseline SBP (mmHg) | 3922 | −0.342 | 0.473 | 0.470 |
| Difference in rate of SBP change (mmHg/week) | 3922 | 0.328 | 0.071 | <0.0001 |
| Difference in baseline DBP (mmHg) | 3922 | −0.990 | 0.352 | 0.005 |
| Difference in rate of DBP change (mmHg/week) | 3922 | 0.124 | 0.049 | 0.012 |
| Difference in baseline HR (b.p.m.) | 3922 | −1.793 | 0.000 | 0.000 |
| Difference in rate of HR change (b.p.m./week) | 3922 | −0.084 | 0.188 | 0.276 |
Negative estimates indicate lower values in the French than in the German population. PWV, pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Weight trajectories in the two populations were non-linear, with an initial reduction followed by rebound weight gain (Figure 1). French participants showed a slight but greater weight reduction during lockdown than German participants (Figure 2, Tables 1 and 2). In the subpopulation of activity tracker users (n = 22 931), number of daily steps dropped abruptly in France from Week 11 to Week 12 (−59.6%), with a subsequent gradual rise, whereas no reduction, even a small increase in physical activity, was observed in Germany (Figure 2, Tables 1 and 2). In the subpopulation of HBPM users (n = 3922), French participants showed similar baseline systolic BP but lower diastolic BP and heart rate at baseline compared with German participants; a greater reduction in both diastolic and systolic BP was seen in the French participants, while heart rate was unchanged (Figure 2, Tables 1 and 2).
A sensitivity analysis, performed in Population B (without any missing PWV values), substantially confirmed these results (see Supplementary material online, Table S4).
Clusters of pulse-wave velocity trajectories
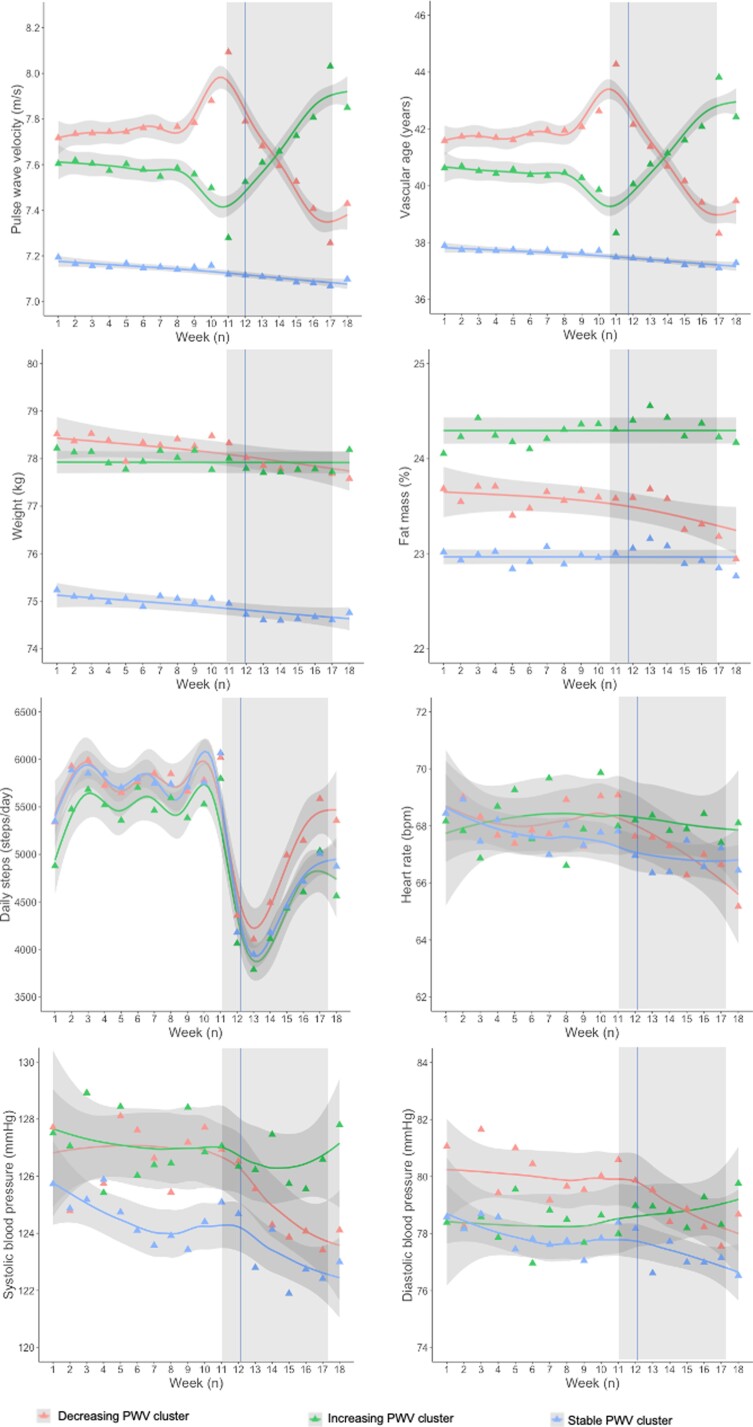
The trajectory clustering analysis was performed on Population B (with no missing PWV values), separately for the French and German populations. Baseline characteristics of individuals with or without missing PWV where substantially similar, except for a greater proportion of German participants and higher age in Population B (see Supplementary material online, Table S5). In the French population, among the 24 measures describing the pattern of PWV behaviour over time, three measures were selected to enter the cluster analysis [Measure 5: Change (PWVWeek17–PWVWeek11); Measure 12: SD of the first differences; Measure 24: Ratio of the mean absolute second difference to the mean absolute first difference; for details on their calculation, please refer to the Online Supplement]. The optimal number of clusters for 13 out of 26 quality criteria tested was 3 (see Supplementary material online, Table S6). Results obtained for two clusters (the preferred option for 8 out of 26 quality criteria tested) were also shown in the online supplement, but considered less relevant from the clinical point of view (see Supplementary material online, Table S7 and Figure S2).
The three clusters (Figure 3) corresponded to: decreasing PWV (Cluster 1, n = 1220 individuals, 21.1%), stable PWV (Cluster 2, n = 3505, 60.6%), and increasing PWV (Cluster 3, n = 1054, 18.2%). Clinical characteristics are shown in Table 3. Noteworthy, the decreasing and increasing PWV clusters showed broadly similar clinical characteristics but were distinct from the stable PWV cluster. They were older, more frequently men with elevated BMI, PWV, and vascular age at baseline compared with the stable PWV cluster. ‘Decreasing’ and ‘increasing’ PWV clusters were both characterized by a higher proportion of sole users in the household compared with Cluster 2. Only individuals in the decreasing PWV cluster showed a significant reduction in body weight (around 400 g), fat mass, and diastolic BP (∼4 mmHg) during lockdown (Table 3).
Figure 3.
Behaviour of main time-varying variables from 1 January 2020 to 30 April 2020 in the French populations, by cluster of PWV trajectory. Cluster 1 (decreasing PWV): red triangles and line; Cluster 2 (stable PWV): green triangles and line: Cluster 3 (increasing PWV): blue triangles and line. Triangles represent mean weekly values, lines are fitted by general additive models (penalized regression cubic splines, parameter estimation method restricted maximum likelihood), with 95% confidence intervals in grey. The vertical line represents the date of total lockdown in France (17 March 2020). Rectangles highlight the time interval analysed (Weeks 11–17).
Table 3.
General characteristics of the clusters of PWV trajectories in the French population
| Variable | Number of observations | Cluster 1, decreasing PWV N = 1220 | Cluster 2, stable PWV N = 3505 | Cluster 3, increasing PWV N = 1054 | P-value |
|---|---|---|---|---|---|
| Baseline (average before lockdown; Weeks 5–8) | |||||
| Age (years) | 5779 | 52 (43–60)*** | 48 (39–57) | 50 (42–60)*** | <0.0001 |
| Vascular age (years) | 4952 | 42 (35–49)***,††† | 36 (32–43) | 40 (34–47)*** | <0.0001 |
| Men, n (%) | 5779 | 937 (77%)***,††† | 2405 (69%) | 783 (74%)*** | <0.0001 |
| Residents in the Paris region, n (%) | 5779 | 607 (50%) | 1711 (49%) | 521 (49%) | 0.833 |
| Sole user in household, n (%) | 5779 | 238 (20%)*** | 573 (16%) | 234 (22%)*** | <0.0001 |
| PWV (m/s) | 5456 | 7.6 (6.9–8.5)***,††† | 6.9 (6.4–7.8) | 7.4 (6.7–8.3)*** | <0.0001 |
| Weight (kg) | 5451 | 77.7 (68.7–87.1)*** | 74.1 (65.4–83.3) | 77.2 (68.8–86.3)*** | <0.0001 |
| BMI (kg/mq) | 5451 | 25.1 (23.0–27.8)*** | 24.3 (22.4–26.8) | 25.8 (23.8–28.5)*** | <0.0001 |
| Fat mass (%) | 5374 | 23.0 (18.2–28.4) | 22.1 (16.9–28.6) | 23.6 (18.4–29.7)*** | <0.0001 |
| Steps (n) | 3065 | 5252 (3103–7710) | 5317 (3300–7721) | 5007 (3204–7368) | 0.260 |
| SBP (mmHg) | 559 | 126 (119–135) | 123 (116–132) | 126 (120–133) | 0.021 |
| DBP (mmHg) | 559 | 79 (75–85)* | 77 (72–83) | 80 (75–84) | 0.009 |
| HR (b.p.m.) | 543 | 67 (61–75) | 67 (61–75) | 68 (62–77) | 0.490 |
| Difference (Week 17 − Baseline) | |||||
| Δ PWV | 5456 | −0.4 (−0.7 to −0.2)***,††† | −0.1 (0.2–0.8) | 0.5 (0.2–0.8)*** | <0.0001 |
| Δ Vascular age | 5127 | −2.9 (−5.4 to −0.09)***,††† | −0.4 (−2.0 to 1.1) | 3.2 (1.1–5.8)*** | <0.0001 |
| Δ Weight (kg) | 5442 | −0.4 (−1.3 to 0.3)* | −0.3 (−1.1 to 0.3) | −0.3 (−1.1 to 0.3) | 0.021 |
| Δ Fat mass (%) | 5326 | −0.2 (−1.2 to 0.6)***,††† | −0.1 (−0.9 to 0.7) | 0.0 (−1.0 to 0.8) | <0.001 |
| Δ Steps (n) | 2397 | −814 (−2888 to 1708) | −968 (−2956 to 960) | −757 (−2565 to 1438) | 0.088 |
| Δ SBP (mmHg) | 296 | −3.3 (−9.2 to 1.1) | −1.9 (−6.9 to 1.2) | −1.1 (−5.1 to 2.7) | 0.141 |
| Δ DBP (mmHg) | 296 | −4.0 (−7.0 to 1.2)††† | −1.1 (−4.0 to 1.2) | 0.4 (−3.8 to 4.5) | 0.001 |
| Δ HR (b.p.m.) | 296 | −1.3 (−5.5 to 3.3) | −0.2 (−4.0 to 2.9) | −0.2 (−5.4 to 3.2) | 0.598 |
Data are expressed as median (25th–75th percentile). Baseline values are expressed as median (median of the mean values from Week 5 to Week 8) and 25th–75th percentile (which corresponds to the dispersion between these four values).
BMI, body mass index; PWV, pulse-wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; Δ, absolute difference between Week 17 and baseline.
P < 0.0001 ‘***’ P < 0.001 ‘**’ P < 0.01 ‘*’ P < 0.05 vs. Cluster 2, stable PWV.
P < 0.0001 ‘†††’ P < 0.001 ‘††’ P < 0.01 ‘†’ P < 0.05 vs. Cluster 3, increasing PWV.
Multinomial regression analysis was used to identify independent determinants of cluster of PWV trajectory during lockdown (Table 4). Older age and male sex were associated with an increased probability of belonging to either the decreasing or increasing PWV cluster when compared with the stable PWV cluster. Weight reduction during lockdown was associated with the decreasing PWV cluster, while being sole user in the household associated with a higher probability of being in the increasing PWV cluster. Similar results were obtained when chronological age was replaced by with vascular age in the model, but with an improved goodness of fit and parsimony of the model (Akaike Information Criterion—AIC 9235 vs. 10118).
Table 4.
Factors associated with cluster of pulse-wave velocity trajectories during lockdown in France
| Model with chronological age | Cluster 1 (decreasing PWV) | Cluster 2 (stable PWV) | Cluster 3 (increasing PWV) |
|---|---|---|---|
| Chronological age | 1.02 (1.01–1.03) | 1.00 | 1.01 (1.01–1.02) |
| Male sex | 1.45 (1.24–1.70) | 1.00 | 1.26 (1.07–1.49) |
| Δ Weight | 0.92 (0.88–0.96) | 1.00 | 0.97 (0.93–1.02) |
| Sole user in household | 1.17 (0.98–1.39) | 1.00 | 1.37 (1.14–1.64) |
| Model with vascular age | Cluster 1 (decreasing PWV) | Cluster 2 (stable PWV) | Cluster 3 (increasing PWV) |
| Vascular age | 1.06 (1.06–1.07) | 1.00 | 1.05 (1.04–1.06) |
| Male sex | 1.54 (1.31–1.82) | 1.00 | 1.31 (1.10–1.55) |
| Δ Weight | 0.91 (0.87–0.95) | 1.00 | 0.96 (0.91–1.01) |
| Sole user in household | 1.11 (0.92–1.33) | 1.00 | 1.28 (1.06–1.55) |
Odds ratios and their 95% confidence intervals were obtained by multinomial logistic regression using the stable PWV cluster as the reference category.
Δ, absolute difference between Week 17 and baseline; PWV, pulse-wave velocity.
In the German population, 2 clusters of trajectories could be identified by 10 out of 26 quality criteria tested (see Supplementary material online, Table S5). However, in the two clusters, the trajectories of the main time-varying variable were visually similar (see Supplementary material online, Figure S1) and no clinically relevant differences were observed in baseline characteristics or Δ changes (see Supplementary material online, Table S6).
Discussion
This study explored the impact on PWV of different national COVID-19 lockdown strategies, by analysing data from connected devices, such as the Withings Body Cardio bathroom scales, activity trackers, and BP monitors, in a large binational real-life population. This innovative approach allowed us to study rapid variations of PWV and PWV-derived vascular age, as proxy of vascular stiffness, assessed by during nationwide total lockdown enforced from the 17 March 2020 in France compared with Germany, a European country adopting less strict lockdown measures.2 Our data also provide information about individual changes in behaviour possibly explaining between- and within-country differences.
The main findings of the study were the following:
• Total lockdown was associated with an overall improvement in cardiovascular health in French users of connected bathroom scales, with a reduction in PWV, body weight, and BP, despite a concomitant reduction in physical activity. In France, the improvement in vascular age during lockdown corresponded to a reduction in vascular age of more than 3 months.
• The phenomenon was only modestly present in Germany, where lockdown was only partial.
• Nevertheless, 18.2% of the French population sample experienced worsening of their PWV (corresponding to impairment of ∼3 years in vascular age), 21.1% showed improvement (corresponding to ∼3 years gain). Both these clusters were older, more frequently men, and with greater vascular stiffness at baseline in comparison with the stable PWV cluster. Notably, PWV improvement during lockdown was associated with concomitant weight reduction. Being the sole user in the household was associated with worsening.
Social-distancing measures are a core component of the response to the COVID-19 pandemic.21 Lockdown has been applied heterogeneously by different governments, while France adopted strict limitations to peoples’ movements, including the shutdown of all non-essential production and commercial activities homogeneously applied throughout the country, in Germany less stringent social-distancing measures were applied.2 This situation has allowed real-life experiments, considered as a unique means of understanding how public policies with large-scale interventions, such as social-distancing measures, might impact the health and exposure to disease of hundreds of millions of people confined to their homes during COVID-19 pandemic.22 Our study is uniquely placed and adequately sized to give novel insights into the link between cardiovascular health and COVID-19 in non-infected individuals.
This study highlighted a marked PWV reduction in France, where strict lockdown was enforced. This corresponded to a ∼3-month reduction in vascular age. Furthermore, we were able to characterize a cluster with decreased PWV, 21.1% of the French population sample, in which the improvement in vascular age was of clinical relevance. Even a small reduction in PWV, if considered over the whole population, might contribute to reducing the burden of CVD.14 In contrast, in Germany, where lockdown was partial, the effect was modest. This suggests a relationship between the impact on daily life of total lockdown and reduction in vascular stiffness.
Such rapid changes in PWV, as those observed in the French population sample, are conceivably attributable to changes in the haemodynamic (haemodynamic load, cardiac contractility) and functional (i.e. endothelial function, sympathetic activation) components rather than structural components of arterial stiffness. Interestingly, these modifiable components of arterial stiffness, while associated with cardiovascular prognosis, are not easily measurable. The day-by-day measurement of arterial stiffness by smart scales may thus provide an opportunity to broadly monitor cardiovascular health.
Thanks to the availability of physiological variables from multiple connected devices, we can suggest some mechanistic explanations for the observed PWV reduction during lockdown. Surprisingly, the two clusters with decreasing and increasing PWV share some common baseline characteristics: older age, higher baseline BMI and vascular stiffness, and predominantly male sex, resulting in more advanced vascular age compared with individuals with stable PWV during lockdown. This might suggest that individuals and populations with unhealthy lifestyles or who already had cardiovascular risk factors were more sensitive and vulnerable to the dramatic societal changes. A context such as lockdown might trigger behavioural changes, with different subgroups of individuals reacting in different ways, some being more resilient and seizing the opportunity to improve their lifestyle. Indeed, weight reduction during lockdown appeared to be a major determinant of a favourable PWV trajectory. Despite the large impact of lockdown on the food supply chain, in a number of different settings, lockdown induced an improvement in dietary habits.8,9 Indeed, a number of surveys have reported that equal proportions of individuals lost and gained weight during lockdown.23–25 Our study, based on nearly 5 million objective weight measurements over time, demonstrated an overall trend towards reduction in body weight during lockdown, possibly contributing to substantial improvement in BP, PWV, and ultimately in cardiovascular health.
However, alongside the cluster with improvement in PWV, 18.2% of our French population sample experienced worsening of PWV during lockdown. While two clusters entered lockdown with elevated vascular age, the cluster with deterioration in PWV did not achieve significant weight reduction during lockdown. Interestingly, individuals belonging to this cluster were more frequently the sole users of the connected scales in their household, a proxy for living alone. It is well known that social isolation (individual or within the community) is associated with increased cardiovascular risk26 and is increasingly recognized as an emerging cardiovascular risk factor to take into account in risk stratification.27 Furthermore, living alone is related to adverse carotid stiffness, independently of potential confounders; and in addition vascular stiffness is related to residing in socially deprived neighbourhoods, but only in men.28 Living alone may thus constitute a disadvantage in a period of crisis such as the COVID-19 pandemic, inhibiting the activation of resiliency mechanisms, especially in men.
Surprisingly, PWV reduction during lockdown in France occurred despite the substantial overall decrease in physical activity levels during lockdown.3,29 Exercise is able to reverse vascular aging,30 whereas even short-term (1–4 weeks) inactivity dramatically accelerates it.31 In our study, no significant difference in daily steps was observed between the different clusters of PWV trajectories. However, the approach used in this study to quantify physical activity (daily steps measured by activity trackers) may have underestimated home-based physical activity including cycling, both widely adopted during lockdown. Furthermore, reduced occupational physical activity during lockdown, which has been suggested as having detrimental effect on vascular health,32 may have contributed to the observed PWV reduction.
The strength of our study resides in the large amount of data collected in two different European countries in respect of data protection regulations and with validated methodologies, allowing us to conduct a real-life comparison of two social-distancing strategies. In addition, to our knowledge, this is the first study analysing self-measured day-by-day PWV by means of connected smart bathroom scales, coupled with objective evaluations of physiological parameters acquired by means of multiple connected devices. This study proposes a novel approach in the field of cardiovascular research in real-life settings.
We acknowledge a number of limitations. The study was by design limited to users of connected scales, a segment of the general population conceivably having, on average, higher socioeconomic status, and better cardiovascular health compared with the general population, as suggested by the observed lower vascular age compared with chronological age. It is likely that the negative cardiovascular consequences of lockdown were amplified in individuals facing financial uncertainty during the COVID-19 pandemic. Interpretation of the cluster analysis might have benefited from information on the reasons why individuals were using smart connected bathroom scales. Finally, since the study was based only on user data from connected devices, no complete information about cardiovascular risk profile and medications could be retrieved, nor data concerning some possible determinants of PWV, such as air pollution or sleep.
Conclusions
In conclusion, social-distancing measures adopted during the COVID-19 pandemic were associated with an overall reduction in PWV and vascular age in French and German smart bathroom scales users. The improvement in vascular age was greater in France, where total lockdown was enforced, than in Germany and it was associated with weight reduction. However, a significant segment of the population experienced marked increase in PWV. Any long-term improvement due to lockdown may appear small compared with the more problematic and enduring effects of this crisis. The negative impact of lockdown in a proportion of the population is of concern. Thus, we plan to follow-up this population over time, in order to study the long-term consequences of the COVID-19 lockdown on cardiovascular health, in particular whether these changes are persistent and their impact in terms of public health. This information will be precious to set up tailored prevention campaigns aimed at preventing a future COVID-19-related CVD pandemic.
Lead author biography
 Rosa Maria Bruno (MD, PhD) is a hypertension specialist, researcher at the Paris Cardiovascular Research Center (PARCC-INSERM) and professor in Clinical Pharmacology at Université Paris Cité (France). Her clinical activity is focused on the management of resistant hypertension, renovascular hypertension, and hypertension in pregnancy. Her main field of research is the non-invasive evaluation of functional and structural vascular alterations in patients with traditional and emerging cardiovascular risk factors, as well as the sympathetic regulation of vascular function in hypertension. She has authored >150 publications in peer-reviewed, PubMed-indexed scientific journals, with an H-index of 31.
Rosa Maria Bruno (MD, PhD) is a hypertension specialist, researcher at the Paris Cardiovascular Research Center (PARCC-INSERM) and professor in Clinical Pharmacology at Université Paris Cité (France). Her clinical activity is focused on the management of resistant hypertension, renovascular hypertension, and hypertension in pregnancy. Her main field of research is the non-invasive evaluation of functional and structural vascular alterations in patients with traditional and emerging cardiovascular risk factors, as well as the sympathetic regulation of vascular function in hypertension. She has authored >150 publications in peer-reviewed, PubMed-indexed scientific journals, with an H-index of 31.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health
Supplementary Material
Acknowledgement
The authors thank Alison Foote (PhD; Grenoble Alpes University Hospital, France) for editing the manuscript.
Contributor Information
Rosa Maria Bruno, Université Paris Cité, Inserm, PARCC, F-75015 Paris, France; Pharmacology Unit, AP-HP, Hôpital Européen Georges Pompidou, F-75015 Paris, France.
Jean Louis Pépin, HP2 Laboratory, INSERM U1042, University Grenoble Alpes, Grenoble, France; EFCR Laboratory, Grenoble Alpes University Hospital, Grenoble, France.
Jean Philippe Empana, Université Paris Cité, Inserm, PARCC, F-75015 Paris, France.
Rui Yi Yang, Withings, Issy les Moulineaux, France.
Vincent Vercamer, Withings, Issy les Moulineaux, France.
Paul Jouhaud, Withings, Issy les Moulineaux, France.
Pierre Escourrou, Centre Interdisciplnaire du Sommeil, 20 rue St Saëns Paris 15, France; Université Paris Saclay, Paris, France.
Pierre Boutouyrie, Université Paris Cité, Inserm, PARCC, F-75015 Paris, France; Pharmacology Unit, AP-HP, Hôpital Européen Georges Pompidou, F-75015 Paris, France.
Funding
None.
Data Availability
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Withings SA at health@withings.com.
References
- 1. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020;116:1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Modesti PA, Wang J, Damasceno A, Agyemang C, Van Bortel L, Persu A, Zhao D, Jarraya F, Marzotti I, Bamoshmoosh M, Parati G, Schutte AE. Indirect implications of COVID-19 prevention strategies on non-communicable diseases: an Opinion Paper of the European Society of Hypertension Working Group on Hypertension and Cardiovascular Risk Assessment in Subjects Living in or Emigrating from Low Resource Settings. BMC Med 2020;18:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pepin JL, Bruno RM, Yang RY, Vercamer V, Jouhaud P, Escourrou P, Boutouyrie P. Wearable activity trackers for monitoring adherence to home confinement during the COVID-19 pandemic worldwide: data aggregation and analysis. J Med Internet Res 2020;22:e19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lantelme P, Couray Targe S, Metral P, Bochaton T, Ranc S, Le Bourhis Zaimi M, Le Coanet A, Courand PY, Harbaoui B. Worrying decrease in hospital admissions for myocardial infarction during the COVID-19 pandemic. Arch Cardiovasc Dis 2020;113:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menut L, Bessagnet B, Siour G, Mailler S, Pennel R, Cholakian A. Impact of lockdown measures to combat COVID-19 on air quality over western Europe. Sci Total Environ 2020;741:140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez-Urrego D, Rodriguez-Urrego L. Air quality during the COVID-19: PM2.5 analysis in the 50 most polluted capital cities in the world. Environ Pollut 2020;266:115042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Son JY, Fong KC, Heo S, Kim H, Lim CC, Bell ML. Reductions in mortality resulting from reduced air pollution levels due to COVID-19 mitigation measures. Sci Total Environ 2020;744:141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deschasaux-Tanguy M, Druesne-Pecollo N, Esseddik Y, de Edelenyi F S, Alles B, Andreeva VA, Baudry J, Charreire H, Deschamps V, Egnell M, Fezeu LK, Galan P, Julia C, Kesse-Guyot E, Latino-Martel P, Oppert J-M, Peneau S, Verdot C, Hercberg S, Touvier M. Diet and physical activity during the COVID-19 lockdown period (March-May 2020): results from the French NutriNet-Sante cohort study. Am J Clin Nutr. 2021;113:924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyes-Olavarria D, Latorre-Roman PA, Guzman-Guzman IP, Jerez-Mayorga D, Caamano-Navarrete F, Delgado-Floody P. Positive and negative changes in food habits, physical activity patterns, and weight Status during COVID-19 confinement: associated factors in the chilean population. Int J Environ Res Public Health 2020;17:5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blume C, Schmidt MH, Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol 2020;30:R795–R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wright KPJ, Linton SK, Withrow D, Casiraghi L, Lanza SM, Iglesia H, Vetter C, Depner CM. Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr Biol 2020;30:R797–R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andres V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol 2020;75:919–930. [DOI] [PubMed] [Google Scholar]
- 13. Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension 2019;74:218–228. [DOI] [PubMed] [Google Scholar]
- 14. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruno RM, Nilsson PM, Engstrom G, Wadstrom BN, Empana JP, Boutouyrie P, Laurent S. Early and supernormal vascular aging: clinical characteristics and association with incident cardiovascular events. Hypertension 2020;76:1616–1624. [DOI] [PubMed] [Google Scholar]
- 16. Campo D, Khettab H, Yu R, Genain N, Edouard P, Buard N, Boutouyrie P. Measurement of aortic pulse wave velocity with a connected bathroom scale. Am J Hypertens 2017;30:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmes FInch W Jr, French BF. Latent Variable Modeling with R. New York and London: Routledge, Taylor and Francis Group; 2015. [Google Scholar]
- 18. Leffondre K, Abrahamowicz M, Regeasse A, Hawker GA, Badley EM, McCusker J, Belzile E. Statistical measures were proposed for identifying longitudinal patterns of change in quantitative health indicators. J Clin Epidemiol 2004;57:1049–1062. [DOI] [PubMed] [Google Scholar]
- 19. Sylvestre MP, McCusker J, Cole M, Regeasse A, Belzile E, Abrahamowicz M. Classification of patterns of delirium severity scores over time in an elderly population. Int Psychogeriatr 2006;18:667–680. [DOI] [PubMed] [Google Scholar]
- 20. Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw 2014;61:1–36. [Google Scholar]
- 21. European Centre for Disease Prevention and Control . Considerations relating to social distancing measures in response to COVID-19—second update. 2020. https://www.ecdc.europa.eu/en/publications-data/considerations-relating-social-distancing-measures-response-covid-19-second (29 May 2022). [DOI] [PubMed]
- 22. Erren TC, Lewis P, Shaw DM. The COVID-19 pandemic: ethical and scientific imperatives for “Natural” experiments. Circulation 2020;142:309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zachary Z, Brianna F, Brianna L, Garrett P, Jade W, Alyssa D, Mikayla K. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract 2020;14:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients 2020;12:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He M, Xian Y, Lv X, He J, Ren Y. Changes in body weight, physical activity, and lifestyle during the semi-lockdown period after the outbreak of COVID-19 in China: an online survey. Disaster Med Public Health Prep 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holt-Lunstad J, Smith TB. Loneliness and social isolation as risk factors for CVD: implications for evidence-based patient care and scientific inquiry. Heart 2016;102:987–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Climie RE, Boutouyrie P, Perier MC, Guibout C, van Sloten TT, Thomas F, Danchin N, Sharman JE, Laurent S, Jouven X, Empana JP. Individual and neighborhood deprivation and carotid stiffness. Hypertension 2019;73:1185–1194. [DOI] [PubMed] [Google Scholar]
- 29. Pecanha T, Goessler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol 2020;318:H1441–H1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhuva AN, D’Silva A, Torlasco C, Jones S, Nadarajan N, Van Zalen J, Chaturvedi N, Lloyd G, Sharma S, Moon JC, Hughes AD, Manisty CH. Training for a first-time marathon reverses age-related aortic stiffening. J Am Coll Cardiol 2020;75:60–71. [DOI] [PubMed] [Google Scholar]
- 31. Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res 2014;190:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Climie RE, Boutouyrie P, Perier MC, Chaussade E, Plichart M, Offredo L, Guibout C, van Sloten TT, Thomas F, Pannier B, Sharman JE, Laurent S, Jouven X, Empana JP. Association between occupational, sport, and leisure related physical activity and baroreflex sensitivity: the Paris Prospective Study III. Hypertension 2019;74:1476–1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Withings SA at health@withings.com.