Abstract
Background
Little is known about targeted (antiviral or monoclonal antibody) anti-SARS-CoV-2 treatment in immunocompromised patients with COVID-19.
Objectives
To assess the real-life efficacy and tolerance of targeted treatment of COVID-19 in immunocompromised patients.
Patients and methods
Single-centre retrospective case series of immunocompromised patients with COVID-19 between December 2021 and March 2022. We recorded all cases of COVID-19 among immunocompromised patients treatment between 20 December 2021 and 15 March 2022. Choice of treatment was left to the physician’s decision, according to internal treatment protocol, treatment availability and circulating variants. Main outcome was death from COVID-19 after no treatment or targeted treatment.
Results
Sixty-seven immunocompromised patients [38 male; median (IQR) age, 53 (43–63) years], with a median (IQR) follow-up of 60 (47–80) days. Ten patients did not receive any targeted treatment. Targeted treatment consisted of IV curative remdesivir (n = 22), sotrovimab (n = 16), tixagevimab/cilgavimab (n = 13) and casirivimab/imdevimab (n = 1). Ten patients (15%) presented severe COVID-19 and 2 (3%) died from Omicron COVID-19. Comparing patients who received targeted anti-SARS-CoV-2 treatment and no prophylaxis, (n = 42; 81%) with those who did not (n = 10; 19%), death rate was significantly lower in treated patients [n = 0 (0%) versus n = 2 (20%); P = 0.034]. No severe adverse events were reported among treated patients. Among 15 patients who received tixagevimab/cilgavimab as pre-exposure prophylaxis, 6 received an additional curative treatment and none died from COVID-19.
Conclusions
Our results suggest that targeted COVID-19 treatment, including direct antivirals or monoclonal antibodies, is safe and efficient and could be proposed in high-risk immunocompromised patients.
Introduction
Immunocompromised patients are at very high risk of developing severe coronavirus disease 2019 (COVID-19), with high morbidity and mortality rates.1–3 Moreover, low post-vaccinal immune responses have been reported among them, especially in solid organ transplant recipients.4–6 A number of targeted treatments have been developed to prevent and to cure COVID-19 in at-risk patients, and were recently made available in France. Monoclonal antibody combinations casirivimab/imdevimab and tixagevimab/cilgavimab have been used both as pre-exposure prophylaxis (PREP) and as curative treatment, while sotrovimab has been used for early curative treatment only. The direct antiviral remdesivir has also shown its efficacy when prescribed early to prevent severe forms of COVID-19.7–9 However, clinical trials evaluating the efficacy of these preventive and curative treatments have been conducted among non-vaccinated, mostly non-immunocompromised, patients prior to the occurrence of the Omicron variant. While the occurrence of the Omicron variant has been associated with reduced severity of COVID-19,10 recent data show that immunocompromised patients remain at a high risk of morbidity and mortality from COVID-19, possibly due to the lack of vaccine efficacy in this population11,12 and Omicron escape from casirivimab/imdevimab prophylaxis.13 Whether anti-SARS-CoV-2 preventative and curative targeted treatments are well tolerated and are efficient to decrease morbidity and mortality in immunocompromised patients is unknown. Thus, we aimed to evaluate the outcome of immunocompromised patients with COVID-19, who received or did not receive targeted treatments in a real-life setting within our institution.
Patients and methods
Study population
We performed a monocentric observational retrospective study in one university hospital (Hôpital Européen Georges Pompidou, Paris, France). We included all immunocompromised patients aged 18 years or over with laboratory-confirmed (PCR or antigenic test) COVID-19, treated and/or followed in three departments (Nephrology, Internal Medicine and Cardiac Surgery departments) from 20 December 2021 to 15 March 2022. Our aim was to propose targeted curative treatment to all severely immunocompromised patients with COVID-19. Early treatment was defined by treatment less than 10 days after symptom onset, while late treatment was given 10 or more days after symptom onset. Contraindications, availability of the different treatments and efficacy of monoclonal antibodies towards the circulating SARS-CoV-2 variants were also considered in the decision. Choice of treatment was left to the physician’s decision, according to internal treatment protocol, patient characteristics, treatment availability and circulating variants. Specific curative treatment was provided as soon as possible in immunocompromised patients, when a positive diagnosis was confirmed, independently of the presence of symptoms or the reason for testing (symptoms, COVID-19 contact or systematic testing). The study was approved by the hospital’s Internal Review Board (CERAPHP Centre, ref 2022-03-08).
Data collection and definitions
Baseline and follow-up information was collected from the medical file in patients regularly seen at our institution for causes other than COVID-19. Patients for whom follow-up data could not be obtained were contacted by e-mail or by phone.
Data on PREP (history of previous COVID-19, vaccination, monoclonal antibodies), cause of immunosuppression, medical history, details on COVID-19 disease presentation, management and outcomes were collected. Low post-vaccinal immune response was defined as IgG anti-spike (anti-S) ≤264 binding antibody units (BAU)/mL at least 15 days after at least three doses of anti-SARS-CoV-2 vaccination.14 Severe COVID-19 was defined as pneumonia with oxygen requirement. Targeted anti-SARS-CoV-2 treatment included curative treatment by monoclonal antibodies and/or antivirals, and convalescent plasma, and did not include non-specific treatment including tocilizumab and dexamethasone.
Statistical analysis
We conducted descriptive analyses using median (IQR) for quantitative variables and count (percent) for qualitative variables. Comparisons were made using the Mann–Whitney test for quantitative variables and the Fisher exact test for qualitative variables.
Results
From 20 December 2021 to 15 March 2022, 67 immunocompromised patients were treated in our institution (hospital-based or outpatient care) for COVID-19, 70% of whom were solid organ transplant recipients. Variant determination was available for 49 (73%) patients. Among these, 46 (94%) were infected with the Omicron variant, including 34 (69%) BA.1, 3 (6%) BA.2, 9 (18%) undetermined Omicron variant and 3 (6%) Delta variant.
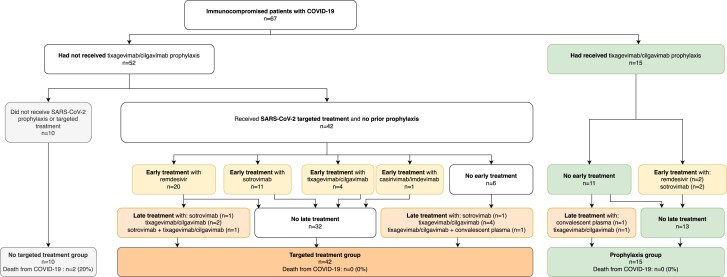
Clinical characteristics and patient management are described in Table S1 (available as Supplementary data at JAC Online) and in Figure 1, and clinical outcomes are provided in Table S2. Eighteen patients (27%) were hospitalized, 10 (15%) presented severe COVID-19 disease and 2 (3%) died. Death from COVID-19 was observed for two solid organ transplant recipients infected with the Omicron BA.1 variant. Both had a low post-vaccinal immune response (anti-S < 30 BAU/mL after three and four doses) and received no specific anti-SARS-CoV-2 treatment.
Figure 1.
Study flowchart. Tixagevimab/cilgavimab prophylaxis was considered effective if administered at least 5 days before symptom onset. Early treatment was defined by targeted treatment administered less that 10 days after symptom onset while late treatment was given at least 10 days after symptom onset. One patient treated with casirivimab/imdevimab, infected by a subsequently identified Omicron variant, was considered non-treated, while one patient in the treated group was treated with casirivimab/imdevimab after identification of a Delta variant. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
A total of 10 patients did not receive tixagevimab/cilgavimab prophylaxis and no targeted treatment either, and a total of 57 patients did receive some treatment: 15 of them received tixagevimab/cilgavimab prophylaxis, 6 of whom also received targeted treatment, and 42 with no prophylaxis received targeted treatment. Causes for the non-administration of specific anti-SARS-CoV-2 treatment are provided in Table S3. Figure 1 provides details on type and time of treatment.
None of the patients who received tixagevimab/cilgavimab prophylaxis or specific anti-SARS-CoV-2 treatment died from COVID-19. The death rate was significantly lower in patients who received specific anti-SARS-CoV-2 treatment compared with those who did not [n = 0 (0%) versus n = 2 (20%); P = 0.034], while patient characteristics were similar between the two groups (Table 1). No severe adverse events were noted among treated patients.
Table 1.
Comparison of patients treated or not treated with targeted curative COVID-19 treatment
| Characteristic | Targeted curative treatment (n = 42; 81%) |
No treatment (n = 10; 19%) |
P value |
|---|---|---|---|
| Age, years, median (IQR) | 52 (45–63) | 55 (36–60) | 0.71 |
| Male sex, n (%) | 23 (55) | 7 (70) | 0.49 |
| Cause of immunosuppression, n (%) | |||
| Solid organ transplantation | 29 (69) | 6 (60) | 0.71 |
| Calcineurin inhibitor | 23 (55) | 6 (60) | 1 |
| Mycophenolate mofetil | 21 (50) | 5 (50) | 1 |
| Everolimus | 11 (26) | 1 (10) | 0.42 |
| Steroids | 36 (86) | 7 (70) | 0.35 |
| Belatacept | 4 (10) | 0 (0) | 0.58 |
| Rituximab | 6 (14) | 3 (30) | 0.35 |
| Other immunosuppressive agent | 2 (5) | 0 (0) | 1 |
| Cyclophosphamide | 5 (12) | 0 (0) | 0.57 |
| Active cancer with chemotherapy | 3 (7) | 0 (0) | 1 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 19 (45) | 3 (30) | 0.49 |
| Chronic heart failure, n (%) | 4 (10) | 0 (0) | 0.58 |
| Chronic respiratory failure, n (%) | 1 (2) | 0 (0) | 1 |
| Obesity, n (%) | 8 (19) | 3 (30) | 0.42 |
| eGFR, mL/min/1.73 m2, median (IQR) | 55 (32–67) | 55 (24–93) | 0.69 |
| End-stage renal disease with dialysis, n (%) | 3 (7) | 1 (10) | 1 |
| COVID-19 prophylaxis, n (%) | |||
| History of COVID-19 | 1 (2) | 0 (0) | 1 |
| At least 3 doses of vaccine | 35 (83) | 7 (70) | 0.38 |
| Anti-S >264 BAU/mL | 1 (3) | 2 (22) | 0.086 |
| Casirivimab/imdevimab PREP | 14 (33) | 4 (40) | 0.72 |
| Tixagevimab/cilgavimab PREP | 0 (0) | 0 (0) | — |
| COVID-19 disease | |||
| Asymptomatic, n (%) | 2 (5) | 2 (20) | 0.16 |
| Fever, n (%) | 26 (62) | 5 (50) | 0.5 |
| Cough, n (%) | 28 (67) | 6 (60) | 0.72 |
| Dyspnoea, n (%) | 15 (36) | 3 (30) | 1 |
| Oxygen requirement, n (%) | 5 (12) | 3 (30) | 0.17 |
| Hospitalization, n (%) | 12 (29) | 3 (30) | 1 |
| Intensive care, n (%) | 2 (5) | 2 (20) | 0.17 |
| Death from COVID-19, n (%) | 0 (0) | 2 (20) | 0.034 |
| Follow-up duration, days, median (IQR) | 63 (55–85) | 59 (46–90) | 0.62 |
Patients who received tocilizumab and other non-SARS-CoV-2-targeted treatments only are listed in the no-treatment column. Patients receiving PREP at least 5 days before symptom onset were excluded from this analysis.
Discussion
Our study reports the COVID-19 outcomes in immunocompromised patients, most of them with low post-vaccinal immune responses, infected by Omicron variant and who received, or did not receive, a targeted anti-SARS-CoV-2 treatment. We show that treatment with specific anti-SARS-CoV-2 treatment was associated with a lower death rate and no reported adverse events.
None of the patients who received curative targeted treatment for COVID-19 died in our cohort. Patients were treated with the antiviral remdesivir as well as monoclonal antibodies, and choice of treatment was constrained by drug availability and kidney function (remdesivir being contraindicated in patients with estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2). Choice of monoclonal antibody was also determined by knowledge of in vitro resistance of the SARS-CoV-2 variant to monoclonal antibodies, requiring regular changes in COVID-19 treatment protocols. For example, Chavarot et al.11 reported clinical efficacy of sotrovimab in kidney transplant recipients with Omicron COVID-19; however, the occurrence of the BA.2 variant, which escapes sotrovimab binding, currently limits the use of sotrovimab in clinical practice.15 In contrast, antivirals have shown persistent in vitro efficacy against COVID-19, independently of the SARS-CoV-2 variant,16 making these drugs a promising alternative for the treatment of COVID-19.
While a small number of patients with COVID-19 had received COVID-19 prophylaxis with tixagevimab/cilgavimab in our study, we found that none of them died from COVID-19. These results are in line with those reported by Bertrand et al.,17 who reported no deaths among 28 patients with Omicron COVID-19 who had received tixagevimab/cilgavimab prophylaxis.
In our study, 20% of patients without targeted treatment died. These results are similar to those reported by Chavarot et al.,11 who found a high mortality rate (approximately 20% at 30 days) from Omicron COVID-19 among untreated kidney transplant recipients including 50% with low post-vaccinal immune response. Bertrand et al.17 also reported a high mortality rate from Omicron COVID-19 in kidney transplant recipients with no or low response to vaccination and no tixagevimab/cilgavimab prophylaxis (5/56; 9%, with no available information on whether or not some of these patients were treated with targeted treatment after infection). In contrast, overall mortality from COVID-19 in our severely immunocompromised cohort was low (n = 2; 3%), with most patients receiving targeted treatment either prophylactically, curatively or both.
A limitation to our retrospective study is the lack of randomization, the relatively small sample size with a low number of events and the fact that choice of treatment was left to the physician’s decision and treatment availability. However, to our knowledge, it is the largest published cohort of immunocompromised patients infected with SARS-CoV-2 during the Omicron era and treated with targeted treatments, using a real-life approach since all treatments were not available at a given time and treatment strategies needed to be adapted to circulating variants. The duration of follow-up is relatively prolonged, allowing for a reliable estimation of morbidity and mortality related to COVID-19.
In conclusion, we found that SARS-CoV-2 infection in immunocompromised patients treated with targeted anti-SARS-CoV-2 treatment was associated with no deaths and a good safety profile, despite low post-vaccinal immune responses. In contrast, patients who did not receive treatment were at high risk of death, similarly to previous reports.11,17 Hence, we propose that early, targeted COVID-19 treatment should be discussed in high-risk immunocompromised patients, and that a treatment strategy can include both antivirals and monoclonal antibodies according to the patient profile, the current SARS-CoV-2 epidemiology and drug availability at a given time and place.
Supplementary Material
Contributor Information
Emmanuel Lafont, Service de Médecine Interne, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France; Université Paris Cité, Paris, France.
Hélène Pere, Université Paris Cité, Paris, France; Service de Microbiologie, Unité de Virologie, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France.
David Lebeaux, Université Paris Cité, Paris, France; Service de Microbiologie, Unité Mobile d’Infectiologie, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France.
Geoffrey Cheminet, Service de Médecine Interne, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France; Université Paris Cité, Paris, France.
Eric Thervet, Université Paris Cité, Paris, France; Service de Néphrologie, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France.
Romain Guillemain, Service de Chirurgie Cardiaque, Hôpital Européen Georges Pompidou, AP-HP. Centre, Université de Paris Cité, Paris, France.
Adrien Flahault, Université Paris Cité, Paris, France; Service de Néphrologie, Hôpital Européen Georges Pompidou, AP-HP Centre, Université Paris Cité, Paris, France.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None of the authors declare any competing interests linked with the present study.
Author contributions
All authors contributed to the manuscript. A.F. and E.L. were responsible for conception and design. A.F. and E.L. were responsible for data collection. A.F. was responsible for analysis. All authors were responsible for the interpretation of data. A.F. and E.L. wrote the first version of the manuscript. All authors critically revised and approved the final version of the manuscript.
Data availability
Data are available upon reasonable request.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Gatti M, Rinaldi M, Bussini L et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect 2022: S1198–743X(22)00116–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avouac J, Drumez E, Hachulla E et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021; 3: e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belsky JA, Tullius BP, Lamb MG et al. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect 2021; 82: 329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hadjadj J, Planas D, Ouedrani A et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseases. Ann Rheum Dis 2022; 81: 720–8. [DOI] [PubMed] [Google Scholar]
- 5. Furer V, Eviatar T, Zisman D et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021; 80: 1330–8. [DOI] [PubMed] [Google Scholar]
- 6. Asderakis A, Khalid U, Koimtzis G et al. An analysis of serological response and infection outcomes following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 vaccines in kidney and kidney pancreas transplants. Transplantation 2022; 106: 1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta A, Gonzalez-Rojas Y, Juarez E et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385: 1941–50. [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb RL, Vaca CE, Paredes R et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386: 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin MJ, Ustianowski A, De Wit S et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med 2022; 386: 2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulloa AC, Buchan SA, Daneman N et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA 2022; 327: 1286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chavarot N, Melenotte C, Amrouche L et al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with omicron infection. Kidney Int 2022: S0085-2538(22)00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al Jurdi A, Gassen RB, Borges TJ et al. Suboptimal antibody response against SARS-CoV-2 omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int 2022; 101:1282–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flahault A, Touchard J, Péré H et al. Breakthrough omicron COVID-19 infections in patients receiving the REGEN-Cov antibody combination. Kidney Int 2022; 101:824–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng S, Phillips DJ, White T et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruel T, Hadjadj J, Maes P et al. Serum neutralization of SARS-CoV-2 omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med 2022; 28: 1297–302. [DOI] [PubMed] [Google Scholar]
- 16. Vangeel L, Chiu W, De Jonghe S et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res 2022; 198: 105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertrand D, Laurent C, Lemée V et al. Efficacy of anti SARS-CoV-2 monoclonal antibodies prophylaxis and vaccination on omicron COVID-19 in kidney transplant recipients. Kidney Int 2022: S0085-2538(22)00382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.