Abstract
Background
Acceleration of negative respiratory conversion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with coronavirus disease 2019 (COVID-19) might reduce viral transmission. Nirmatrelvir/ritonavir is a new antiviral agent recently approved for treatment of COVID-19 that has the potential to facilitate negative conversion.
Methods
A cohort of hospitalized adult patients with mild-to-moderate COVID-19 who had a high risk for progression to severe disease were studied. These patients presented with COVID-19 symptoms between 5 March and 5 April 2022. The time from positive to negative upper respiratory reverse transcription-polymerase chain reaction (RT-PCR) conversion was assessed by Kaplan-Meier plots and Cox proportional hazards regression with the adjustment for patients’ baseline demographic and clinical characteristics.
Results
There were 258 patients treated with nirmatrelvir/ritonavir and 224 nontreated patients who had mild-to-moderate COVID-19. The median (interquartile range) time for patients who converted from positive to negative RT-PCR was 10 days (7–12 days) in patients treated ≤5 days after symptom onset and 17 days (12–21 days) in nontreated patients. The proportions of patients with a negative conversion at day 15 were 89.7% and 42.0% in treated patients and nontreated patients, corresponding to a hazard ratio of 4.33 (95% confidence interval, 3.31–5.65). Adjustment for baseline differences between the groups had little effect on the association. Subgroup analysis on treated patients suggests that time to negative conversion did not vary with the patients’ baseline characteristics.
Conclusions
This cohort study of high-risk patients with mild-to-moderate COVID-19 found an association between nirmatrelvir/ritonavir treatment and accelerated negative RT-PCR respiratory SARS-CoV-2 conversion that might reduce the risk of viral shedding and disease transmission.
Keywords: nirmatrelvir/ritonavir, SARS-CoV-2, COVID-19, negative conversion rate, high-risk patients
High-risk patients with mild-to-moderate COVID-19 treated with nirmatrelvir/ritonavir have more rapid negative conversion of nasopharyngeal or oropharyngeal SARS-CoV-2 reverse-transcription polymerase chain reaction positivity that might attenuate viral transmission.
Management of coronavirus disease 2019 (COVID-19) has mostly relied on nonpharmacologic and vaccination interventions [1, 2]. Neither of these provide relief of disease for patients who have active COVID-19. Because there is a paucity of effective antiviral agents, once a patient is infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it runs its natural course with the patient at risk for shedding virus and potentially infecting others from symptom onset or earlier to 15 days, depending on disease severity [3–6]. Antiviral agents are needed that can actively treat SARS-CoV-2 infection to reduce the burden of the disease and also to limit the spread of infection [7].
Recently, nirmatrelvir has been introduced as a treatment for COVID-19 [8]. Nirmatrelvir is a potent small-molecule inhibitor that binds to the main protease (Mpro, also known as 3CLpro) of SARS-CoV-2 [8], which is an important mediator of viral replication [9]. The sequence of Mpro is highly conserved in coronaviruses [9], such that nirmatrelvir has similar efficacy against wild-type SARS-CoV-2 and its subsequent variants [10–12]. To maximize the therapeutic benefit of nirmatrelvir, the CYP3A inhibitor ritonavir is coadministered in a low dose (100 mg) to reduce metabolism of nirmatrelvir [8, 13]. Coadministration of nirmatrelvir plus ritonavir reduces hospitalization and death rates from COVID-19 when given in an outpatient setting [14]. Little is known regarding nirmatrelvir/ritonavir treatment of COVID-19 and its ability to accelerate positive to negative conversion of upper respiratory reverse transcription-polymerase chain reaction (RT-PCR) testing that, in turn, would suggest it might reduce viral transmission [15], which consequently leads to mitigated pressures on the healthcare system and society.
The First Hospital of Jilin University was granted emergency use approval for nirmatrelvir/ritonavir to treat hospitalized COVID-19 patients [16, 17]. Patients treated at our facility undergo daily RT-PCR testing for SARS-CoV-2 to determine when they no longer present a risk for airborne disease transmission. Nirmatrelvir/ritonavir is usually administered in outpatient settings. The current study capitalizes on the drug’s use in inpatients in China and information obtained from them while they are hospitalized to better understand how nirmatrelvir/ritonavir affects the presence of SARS-CoV-2 in the upper respiratory tract. The purpose of the current study is to determine if nirmatrelvir/ritonavir accelerates positive to negative conversion of upper respiratory SARS-CoV-2 RT-PCR in hospitalized patients who have mild-to-moderate COVID-19 and are at risk for severe disease.
METHODS
Ethical Review of the Study
The study was approved by the Institutional Review Board of the First Hospital of Jilin University and informed consent was obtained from all patients (number AF-IRB-032-06).
Patients and Settings
In China, all people who test positive for COVID-19 are referred to medical providers for observation or treatment. This was a cohort study investigating a series of patients referred for care to the First Hospital of Jilin University after contracting COVID-19. The study coincided with a wave of Omicron variant infections occurring during this time period in our region of China. This study focused on hospitalized patients aged >18 years who had mild-to-moderate COVID-19 and had a high risk for developing severe disease.
Symptom severity was classified using the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 9) released by the China National Health Commission [18]. Mild COVID-19 refers to patients who have 1 or more mild symptoms including fever, cough, sore throat, fatigue, muscle aches, or loss of smell, and no concomitant pneumonia. Moderate COVID-19 was defined as patients having the same types of symptoms as for mild disease but with radiologic evidence of pneumonia. “High risk” was considered as age ≥60 years; a history of diabetes, hypertension, cardiovascular diseases, cerebral infarction, chronic liver or kidney diseases, cancers, smoking, or obesity [19–22].
Patients with Omicron variant COVID-19 were referred to our facility beginning on 5 March 2022. On 24 March 2022, nirmatrelvir/ritonavir became available at our facility for the treatment of eligible patients who had COVID-19. Eligible patients treated with nirmatrelvir/ritonavir were compared with similar patients treated at our facility before the drug was available (Supplementary Figure 1). The study included patients whose symptom onset date was between 5 March 2022 and 5 April 2022. None of them had prior COVID-19 infection. All patients were followed for RT-PCR negative conversion for at least 15 days after symptom onset and 10 days after treatment initiation. All patients received standard medical care in accordance with the Diagnosis and Treatment Protocol for COVID-19 (ie, Trial Version 9) [18].
Patient Characteristics
Patients’ baseline characteristics were assessed and collected at the time of hospital admission, including sex, age, clinical classification (ie, symptom severity), comorbidities, risk factors for progression to severe disease (defined above), and vaccination. Days from symptom onset to hospitalization and to treatment were calculated. Baseline specimens were collected from patients’ upper respiratory tract within 3 days after hospitalization by either nasopharyngeal or oropharyngeal swabs. The specimens were tested by real-time fluorescence quantitative RT-PCR assay to assess the presence of SARS-CoV-2 virus. The targets for the RT-PCR assay were open reading frame 1ab (ORF) and nucleocapsid protein gene (N gene). The cycle threshold (Ct) value (ie, number of cycles that are needed to replicate the viral nucleic acid to detectable level) was recorded for both targets. A lower Ct number indicates a larger amount of viral RNA, implying high viral load [23]. Ct values <35 for either ORF or N gene target was considered as a positive test for the presence of SARS-CoV-2.
Intervention
Patients in the treatment group received 300 mg nirmatrelvir orally, together with 100 mg ritonavir every 12 hours for 5 consecutive days (10 doses in total). All patients in our facility received standard medical care that included bed rest, vital signs monitoring, oxygen saturation measurement, routine blood chemistry, and urine analysis (by Sysmex XN and DIRUI FUS3000), biochemical indicators such as liver and myocardial enzymes and renal function (by Beckman Coulter AU5800), coagulation parameters (by STA-R MAX), arterial blood gas analysis (GEM3500), chest imaging (by Brilliance CT 64 Channel) and cytokine detection (by Roche-Cobas 8000 e801), and oxygen therapy if needed. Patients included in this analysis were not treated with any other COVID-19–specific medications such as remdesivir or monoclonal antibodies.
Primary Outcome
The primary outcome was the time to conversion from symptom onset (also with a positive RT-PCR test for upper respiratory SARS-CoV-2) to a negative test. Because we had limited quantitative information about the positivity of prehospitalization COVID-19 testing in our patients, the first test considered as baseline characteristic in this analysis was the one the patients had within 3 days after they were hospitalized. China’s COVID-19 diagnosis and treatment protocol (ie, Trial Version 9) [18, 24], consider RT-PCR tests as negative if Ct values are ≥35 for both ORF and N SARS-CoV-2 genes for 2 consecutive RT-PCR tests having an interval of >24 hours. All patients were tested for conversion RT-PCR test through either nasopharyngeal or oropharyngeal swabs on a daily basis beginning on day 3 of their hospitalization until conversion was observed.
Secondary Outcomes
Secondary outcomes included the proportion of patients RT-PCR negative for respiratory SARS-CoV-2 at 15 days following symptom onset. Treatment side effects and subgroup analysis of the time to conversion to RT-PCR negative status for SARS-CoV-2 were also examined. The specific subgroup analyses were based on patients’ sex, age, clinical classification, comorbidities and high-risk factors, baseline Ct values, and vaccination history. Safety assessment regarding potential side effects of the drug, such as diarrhea and gastric distress, were collected by healthcare providers (physicians and nurses) through inquiry on a daily basis. Side effect monitoring occurred from the initiation of the treatment until the patient was discharged from the hospital.
Surveillance
A telephone survey was performed between 20 and 26 June 2022 to query the patients about COVID-19 recurrence.
Statistical Analysis
Baseline characteristics were compared between patients treated and not treated with nirmatrelvir/ritonavir using χ2 and Wilcoxon rank-sum tests. Outcomes were assessed by Kaplan-Meier plots and Cox proportional hazards regression. Cox models were created that adjusted for age and sex and also for all risk factors that were different between the groups. Logistic regression was utilized to investigate associations between patients’ baseline characteristics and side effects.
Analyses were conducted using Stata version 17.0 (StataCorp). All statistical tests were 2-sided and the statistical significance level was set to P < .05. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative guideline.
RESULTS
Patient Demographic and Clinical Characteristics
There were 296 eligible patients treated with nirmatrelvir/ritonavir. Of these, 38 (13%) were excluded from the analysis because treatment was discontinued early. Early termination of treatment was usually due to the side effects of dysgeusia and gastric discomfort. The final analytic sample for the treatment group included 258 patients treated with nirmatrelvir/ritonavir who were compared to a control group of 224 untreated patients. The median age was 56 years (interquartile range [IQR], 45–66 years), 282 (58.5%) were men, 435 (90.3%) were classified as mild cases, and 391 (81.1%) had received as least 1 dose of SARS-CoV-2 vaccine. All the patients had received inactivated vaccine (Sinovac), with 374 of 391 (95.7%) patients having 2 or 3 doses administered. For the 364 (93.1%) vaccinated patients who had available information about the last dose date, the median time from last dose to symptom onset was 209 days (IQR, 125–240 days). The most common baseline comorbidities were age ≥60 years (188 [39.0%]), hypertension (172 [35.7%]), diabetes (92 [19.1%]), and current smoking (84 [17.4%]); 203 (42.1%) patients had >1 risk factor. Table 1 shows the baseline characteristics between patients treated with or without nirmatrelvir/ritonavir. Patients in the treatment group were on average 4 years younger and had 1 fewer day of delay between symptom onset and hospitalization. They were more likely to smoke but less likely to have diabetes or hypertension than nontreated patients.
Table 1.
Demographic and Clinical Characteristics of Patients Treated With and Without Nirmatrelvir/Ritonavir
| Characteristics | Patients, No. (%) | ||
|---|---|---|---|
| Treated With Nirmatrelvir/Ritonavir (n = 258) | Nontreated (n = 224) | P Valuea | |
| Age, y, median (IQR) | 54 (41–66) | 58 (50–67) | .01 |
| Sex | |||
| ȃFemale | 104 (40.3) | 96 (42.9) | .57 |
| ȃMale | 154 (59.7) | 128 (57.1) | |
| Days from symptom onset to hospitalization, median (IQR) | 2 (1–3) | 3 (2–5) | <.01 |
| Clinical classification | |||
| ȃMild | 231 (89.5) | 204 (91.1) | .57 |
| ȃModerate | 27 (10.5) | 20 (8.9) | |
| No. of comorbidities and risk factors | |||
| ȃ1 | 160 (62.0) | 119 (53.1) | .08 |
| ȃ2 | 73 (28.3) | 71 (31.7) | |
| ȃ≥3 | 25 (9.7) | 34 (15.2) | |
| Diabetes | |||
| ȃNo | 220 (85.3) | 170 (75.9) | .01 |
| ȃYes | 38 (14.7) | 54 (24.1) | |
| Hypertension | |||
| ȃNo | 181 (70.2) | 129 (57.6) | <.01 |
| ȃYes | 77 (29.8) | 95 (42.4) | |
| Smoking | |||
| ȃNo | 197 (76.4) | 201 (89.7) | <.01 |
| ȃYes | 61 (23.6) | 23 (10.3) | |
| Age ≥60 y | |||
| ȃNo | 164 (63.6) | 130 (58.0) | .21 |
| ȃYes | 94 (36.4) | 94 (42.0) | |
| Baseline N gene Ct value | |||
| ȃ≥30 | 66 (25.6) | 67 (29.9) | .29 |
| ȃ<30 | 192 (74.4) | 157 (70.1) | |
| Baseline ORF Ct value | |||
| ȃ≥30 | 82 (31.8) | 84 (37.5) | .19 |
| ȃ<30 | 176 (68.2) | 140 (62.5) | |
| Vaccination | |||
| ȃNo | 50 (19.4) | 41 (18.3) | .76 |
| ȃYes | 208 (80.6) | 183 (81.7) | |
| No. of vaccinations | |||
| ȃ1 | 8 (3.9) | 9 (4.9) | |
| ȃ2 | 139 (66.8) | 134 (73.2) | .23 |
| ȃ3 | 61 (29.3) | 40 (21.9) | |
Abbreviations: Ct, cycle threshold; IQR, interquartile range; N gene, nucleocapsid protein gene; ORF, open reading frame 1ab.
P value by Pearson χ2 and Wilcoxon rank-sum tests.
Primary Outcome: Time to Negative Conversion
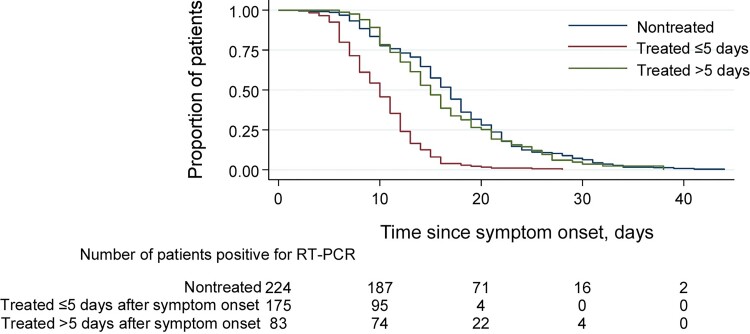
Patients treated with nirmatrelvir/ritonavir within 5 days after symptom onset showed more rapid conversion from positive to negative upper respiratory SARS-CoV-2 RT-PCR testing compared with nontreated patients (median time, 10 days [IQR, 7–12 days] vs 17 days [IQR, 12–21 days]). This difference was not observed for patients whose nirmatrelvir/ritonavir treatment was initiated >5 days after symptom onset (Figure 1). The median time from treatment initiation to negative upper respiratory RT-PCR conversion among all patients in the treatment group was 3 days (IQR, 6–9 days).
Figure 1.
Comparison of conversion from positive to negative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription-polymerase chain reaction (RT-PCR) testing between patients treated with and without nirmatrelvir/ritonavir. Kaplan-Meier plot for the proportion of patients converted from positive SARS-CoV-2 RT-PCR to negative. The median (interquartile range) time for positive to negative RT-PCR conversion was 17 days (12–21 days) in nontreated patients, 10 days (7–12 days) in patients treated with nirmatrelvir/ritonavir ≤5 days after symptom onset, and 15 days (11–21 days) in patients treated >5 days after symptom onset.
Secondary Outcomes
At day 15, 89.7% of patients treated with nirmatrelvir/ritonavir within 5 days of symptom onset had their upper respiratory RT-PCR convert from positive to negative. This day 15 conversion occurred 42.0% of nontreated patients. Of the patients who initiated nirmatrelvir/ritonavir 5 days after symptom onset, 51.8% converted (Table 2). Adjustment for baseline differences between the groups had little effect on the hazard ratio for the effect of nirmatrelvir/ritonavir treatment. The hazard ratio for the day 15 conversion rate for patients treated within 5 days of symptom onset was substantially higher (4.33 [95% confidence interval {CI}, 3.31–5.65) compared with nontreated patients, with the hazard ratio little affected by the adjustment for baseline characteristics (Table 2).
Table 2.
Comparison of Negative Conversion Between Patients Treated With and Without Nirmatrelvir/Ritonavir
| Patients | Total | No. (%) With a Negative Conversiona | Negative Conversion ≤15 Days After Symptom Onseta | |
|---|---|---|---|---|
| Model 1b | Model 2c | |||
| HR (95% CI) | HR (95% CI) | |||
| Without treatment | 224 | 94 (42.0) | Reference | Reference |
| Treated with nirmatrelvir/ritonavir ≤5 d | 175 | 157 (89.7) | 4.33 (3.31–5.65) | 4.85 (3.56–6.61) |
| Treated with nirmatrelvir/ritonavir >5 d | 83 | 43 (51.8) | 1.29 (.90–1.86) | 1.47 (1.02–2.13) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
All patients were followed from symptom onset to negative conversion or day 15, whichever was earliest.
Model 1: adjusted for patient’s age and sex.
Model 2: adjusted for all patients’ baseline characteristics (shown in Table 1).
Subgroup analysis of time to negative conversion of RT-PCR testing in patients receiving nirmatrelvir/ritonavir showed no statistically significant association with a patient’s sex, age, clinical classification, baseline comorbidities, risk factors, or vaccination history in either the minimally or adjusted model (Supplementary Table 1 and Supplementary Figure 2). Patients having lower Ct values before the treatment (eg, larger viral load) were associated with a lower rate of negative RT-PCR conversion within 10 days after the treatment.
Among patients treated with nirmatrelvir/ritonavir, 103 (39.9%) patients experienced at least 1 side effect. The most common side effects were dysgeusia (94 [36.4%]) and diarrhea (14 [5.4%]). Subgroup analysis also showed that elderly patients (ie, aged ≥60 years) were less likely to report side effects, particularly for dysgeusia (odds ratio, 0.48 [95% CI, .26–.87]) (Supplementary Table 2). There was a statistically significant greater risk of experiencing any side effect, in particular dysgeusia in patients who were current smokers, had baseline N gene and ORF Ct values <30, or were vaccinated for SARS-CoV-2; patients in these subgroups were statistically significantly younger than those in the reference group.
Surveillance
COVID-19 recurrence occurred in 2 patients in the treatment group and in 3 patients in the control group (Supplementary Table 3).
DISCUSSION
In this observational cohort study of patients with mild-to-moderate COVID-19 and at high risk for progression to severe disease, nirmatrelvir/ritonavir was associated with more rapid conversion from positive to negative respiratory SARS-CoV-2 RT-PCR status as compared with nontreated patients. The median time for RT-PCR negative conversion was 7 days earlier in patients receiving the treatment within 5 days after symptom onset compared with nontreated patients. Timing of drug administration relative to symptom onset was an important predictor of positive to negative RT-PCR conversion. Patients who received treatment >5 days after symptom onset failed to accelerate RT-PCR negative conversion as compared with the nontreated patients.
The subgroup analysis of time to negative conversion since treatment initiation within the treatment group demonstrated several interesting phenomena. The rate at which RT-PCR conversion became negative was not affected by age, sex, clinical classification, types of comorbidities, or vaccination history. As such, nirmatrelvir/ritonavir should be recommended as treatment for patients irrespective of baseline characteristics, because aside from accelerating their recovery from COVID-19, they will cease shedding virus sooner and reduce the risk of disease transmission [25]. Although medication discontinuation was fairly common because of dysgeusia and gastrointestinal side effects, there were no serious adverse effects attributable to nirmatrelvir/ritonavir.
Our findings extend results from a phase 2/3 randomized controlled trial (RCT) showing that the coadministration of nirmatrelvir/ritonavir significantly reduced the risk of progression to severe COVID-19 for nonhospitalized adults with risk factors for the development of severe disease [14]. Our study shows that this medication can accelerate the time to negative conversion and, therefore, potentially shorten the duration that patients shed virus. The previous RCT studied unvaccinated and nonhospitalized adults of all races [14]. Our study focused on hospitalized, high-risk Asian adult patients, most of whom were vaccinated. The focus of our investigation was to better understand nirmatrelvir/ritonavir's effect on positive to negative upper respiratory conversion rates as a proxy for when it is safe to end isolation intending to limit COVID-19 disease transmission.
Our investigation was of a community sample and might be more generalizable than the RCT results. However, a consequence of the observational nature of our study was having differences between the treatment and control groups that included age, days from symptom onset to hospitalization, and the incidence of diabetes and hypertension. Multivariable Cox regression modeling was used to adjust these potential confounding factors, suggesting that they did not affect the time to negative SARS-CoV-2 RT-PCR conversion.
Rapid mutation of SARS-CoV-2 results in frequent alterations of the spike protein that may attenuate vaccine and neutralizing antibody treatment effectiveness [26, 27]. Nirmatrelvir targets the conserved coronavirus Mpro enzyme, enabling it to remain effective against COVID-19 variants including Omicron [8, 10, 28]. Coadministration of nirmatrelvir plus ritonavir increases the potency and results in improved safety against COVID-19 compared with other currently available antiviral drugs [29, 30].
COVID-19 patients can experience recurrence (rebound) following treatment with nirmatrelvir/ritonavir. In this phenomenon, viral load increases after an initial decrease that occurs after antiviral treatment [31, 32]. In this study, 2 patients in the treatment group and 3 patients in the control group experienced viral rebound; the average time for COVID-19 RT-PCR tests to convert to positive after being negative was 7.0 and 10.6 days in the 2 groups, respectively. There were no symptoms associated with these rebound episodes.
Patients in this clinical observational study were isolated and managed using standard treatment protocols, facilitating more efficient comparisons between treatment groups as well as acquisition of high quality and complete data capture. Being observational, the results are generalizable to actual clinical practice. This study has some limitations. First, it is an observational study investigating a series of patients who received medical care for COVID-19 at our facility. Because of its demonstrated efficacy, when nirmatrelvir/ritonavir became available, all eligible patients treated at our facility received the medication, precluding having a concurrent control group to study. Second, there were imbalances between the 2 groups, but these had little effect on the outcomes following statistical adjustment. Third, the test for COVID-19 was mainly based on RT-PCR and corresponding Ct values. RT-PCR may overestimate active viral replication and shedding, picking up inactive viral fragments as compared with antigen tests, which are likely better at capturing active disease [33]. Fourth, because we did not perform cell-based assays or disease transmission studies, the present study cannot demonstrate a patient’s infectivity or actual transmission of COVID-19 to other patients.
In summary, nirmatrelvir/ritonavir can shorten the time of RT-PCR conversion for high-risk SARS-CoV-2–infected adults, suggesting that this treatment may reduce the risk of viral shedding and disease transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Hongyan Li, Nursing Department, First Hospital of Jilin University, Changchun, China.
Menghan Gao, Department of Hepatobiliary and Pancreatic Surgery, First Hospital of Jilin University, Changchun, China.
Hailong You, Department of Pediatrics, First Hospital of Jilin University, Changchun, China.
Peng Zhang, Department of Infectious Diseases, First Hospital of Jilin University, Changchun, China.
Yuchen Pan, Department of Clinical Epidemiology, First Hospital of Jilin University, Changchun, China.
Nan Li, Intensive Care Unit, First Hospital of Jilin University, Changchun, China.
Ling Qin, Department of Cardiovascular Medicine, First Hospital of Jilin University, Changchun, China.
Heyuan Wang, Department of Endocrinology and Metabolism, First Hospital of Jilin University, Changchun, China.
Dan Li, Department of Respiratory Medicine, First Hospital of Jilin University, Changchun, China.
Yang Li, Department of Respiratory Medicine, First Hospital of Jilin University, Changchun, China.
Hongmei Qiao, Department of Pediatric Respiratory Medicine, First Hospital of Jilin University, Changchun, China.
Lina Gu, Intensive Care Unit, First Hospital of Jilin University, Changchun, China.
Songbai Xu, Department of Neurosurgery, First Hospital of Jilin University, Changchun, China.
Weiying Guo, Department of Endocrinology and Metabolism, First Hospital of Jilin University, Changchun, China.
Nanya Wang, Cancer Center, First Hospital of Jilin University, Changchun, China.
Chaoying Liu, Department of Respiratory Medicine, First Hospital of Jilin University, Changchun, China.
Pujun Gao, Department of Hepatology, First Hospital of Jilin University, Changchun, China.
Junqi Niu, Department of Hepatology, Center of Infectious Disease and Pathogen Biology, Key Laboratory of Organ Regeneration and Transplantation of the Ministry of Education, State Key Laboratory of Zoonotic Disease, First Hospital of Jilin University, Changchun, China.
Jie Cao, Department of Neurology, First Hospital of Jilin University, Changchun, China.
Yang Zheng, Department of Cardiovascular Medicine, First Hospital of Jilin University, Changchun, China.
Notes
Author contributions. H. L., M. G., J. N., and Y. Z. designed the study. M. G. performed statistical analysis and drafted the manuscript. M. G., H. Y., P. Z., J. N., J. C., and Y. Z. interpreted the data. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
Acknowledgments. The authors thank the patients for their consent to be involved in the study, as well as all front-line medical staff. Professor Songling Zhang is appreciated for coordinating and guiding the study. We acknowledge Professor Jing Jiang and Associate Professor Yuning Zhang for advice on study design and data management.
References
- 1. He G, Zeng F, Xiao J, et al. . When and how to adjust non-pharmacological interventions concurrent with booster vaccinations against COVID-19—Guangdong, China, 2022. China CDC Wkly 2022; 4:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chowdhury R, Luhar S, Khan N, Choudhury SR, Matin I, Franco OH. Long-term strategies to control COVID-19 in low and middle-income countries: an options overview of community-based, non-pharmacological interventions. Eur J Epidemiol 2020; 35:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020:371:m3862. [DOI] [PubMed] [Google Scholar]
- 4. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol 2020; 41:1100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Kampen JJ, van de Vijver DA, Fraaij PL, et al. . Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021; 12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee C, Kanjilal S, Baker M, Klompas M. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis 2021; 72:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnett ML, Mehrotra A, Landon BE. Covid-19 and the upcoming financial crisis in health care. NEJM Catalyst 2020; 1. [Google Scholar]
- 8. Owen DR, Allerton CM, Anderson AS, et al. . An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021; 374:1586–93. [DOI] [PubMed] [Google Scholar]
- 9. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003; 300:1763–7. [DOI] [PubMed] [Google Scholar]
- 10. Vangeel L, Chiu W, De Jonghe S, et al. . Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res 2022; 198:105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ullrich S, Ekanayake KB, Otting G, Nitsche C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg Med Chem Lett 2022; 62:128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh RSP, Toussi SS, Hackman F, et al. . Innovative randomized phase 1 study and dosing regimen selection to accelerate and inform pivotal COVID-19 trial of nirmatrelvir. Clin Pharmacol Ther 2022; 112:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A 2010; 107:18422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hammond J, Leister-Tebbe H, Gardner A, et al. . Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kotecha P, Light A, Checcucci E, et al. . Repurposing of drugs for Covid-19: a systematic review and meta-analysis. Panminerva Med 2022; 64:96–114. [DOI] [PubMed] [Google Scholar]
- 16. National Medical Products Administration of China. Emergency conditional approval of Pfizer's COVID-19 therapy: nirmatrelvir tablet/ritonavir tablet combination package (i.e., Paxlovid) importation registration [in Chinese]. Available at: https://www.nmpa.gov.cn/yaowen/ypjgyw/20220212085753142.html. Accessed 27 April 2022.
- 17. Lamb YN. Nirmatrelvir plus ritonavir: first approval. Drugs 2022; 82:585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Health Commission of the People’s Republic of China. Diagnosis and treatment protocol for COVID-19 (trial version 9) [in Chinese]. Available at: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=b74ade1ba4494583805a3d2e40093d88. Accessed 20 April 2022.
- 19. Docherty AB, Harrison EM, Green CA, et al. . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim L, Garg S, O’Halloran A, et al. . Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)–associated hospitalization surveillance network (COVID-NET). Clin Infect Dis 2021; 72:e206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thakur B, Dubey P, Benitez J, et al. . A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep 2021; 11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 23. Drew RJ, O’Donnell S, LeBlanc D, McMahon M, Natin D. The importance of cycle threshold values in interpreting molecular tests for SARS-CoV-2. Diagn Microbiol Infect Dis 2020; 98:115130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang R, Liang H, Tang J. Negative conversion rate of SARS-CoV-2 infection. JAMA Intern Med 2021; 181:566. [DOI] [PubMed] [Google Scholar]
- 25. Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther 2020; 9:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA 2022; 327:617–18. [DOI] [PubMed] [Google Scholar]
- 27. Borio LL, Bright RA, Emanuel EJ. A national strategy for COVID-19 medical countermeasures: vaccines and therapeutics. JAMA 2022; 327:215–6. [DOI] [PubMed] [Google Scholar]
- 28. Li P, Wang Y, Lavrijsen M, et al. . SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res 202232:322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim SCL, Hor CP, Tay KH, et al. . Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med 2022182:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gottlieb RL, Vaca CE, Paredes R, et al. . Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charness M, Gupta K, Stack G, Chamess M. Rapid relapse of symptomatic Omicron SARS-CoV-2 infection following early suppression with nirmatrelvir/ritonavir. Research Square [Preprint]. Posted online 26 April2022. 10.21203/rs.3.rs-1588371/v1 [DOI]
- 32. Ranganath N, O’Horo JC, Challener DW, et al. . Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID-19) in high-risk persons. Clin Infect Dis 2023; 76:e537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verna R, Alallon W, Murakami M, et al. . Analytical performance of COVID-19 detection methods (RT-PCR): scientific and societal concerns. Life 2021; 11:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.