Poor sleep quality is recognized in one-third of long coronavirus disease (COVID) patients irrespective of acute disease severity [1]. However, information on neuroimaging correlates of poor sleep quality is limited. A recent study from our group demonstrated that COVID-19 survivors had a significant worsening in sleep quality when compared to noninfected individuals [2]. Using that cohort, we sought to assess whether the persistence of long COVID-related poor sleep quality is associated with an increased burden of enlarged basal ganglia-perivascular spaces (BG-PVS). These fluid-filled cavities (referred to as part of the glymphatic system) are thought to be involved in mechanisms of brain interstitial fluid and metabolic waste clearance [3]. There is evidence that such clearance mainly occurs during sleep, explaining BG-PVS enlargement in the setting of sleep disorders [4].
The study was conducted in Atahualpa, a rural Ecuadorian village severely struck by the SARS-CoV-2 pandemic [5]. The above-mentioned cohort included 639 participants. Overall, 454 (71%) were good sleepers before the pandemic (June 2019), 236 of whom (52%) had SARS-CoV-2 infections between May and June 2020, and were eligible for the present study. A total of 143 of 236 candidates did not participate because they either died, emigrated, became infected after June 2020, declined consent after the second round of sleep quality assessment, or did not receive brain MRIs at baseline (2012–2019) and follow-up (May–December 2021). The study protocol and informed consent forms were approved by the Ethics Committee of Universidad Espiritu Santo—Ecuador (FWA: 00028878).
Sleep quality was evaluated by means of the Pittsburgh Sleep Quality Index (PSQI), a widely used instrument that discriminates between good and poor sleepers. This instrument has been used to assess sleep quality in the study population before the pandemic [2]. The maximum score of the PSQI is 21 points, and the cutoff value for defining poor sleep quality is >5 points. Three rounds of tests to ascertain sleep quality (one before and two during the pandemic) were administered by investigators blinded to the results of previous assessments. Both baseline and follow-up MRIs were performed with a Philips Intera 1.5T equipment (Philips Medical Systems, Eindhoven, the Netherlands). Interest focused on enlarged BG-PVS, assessed on the T2-weighted sequence. BG-PVS burden was rated in the single slice with the highest number of these structures in one side. Accordingly, from 1 to 10 enlarged BG-PVS generated a score of 1, 11–20 a score of 2, 21–40 a score of 3, and >40 a score of 4 [6].
All MRIs were independently read by one neurologist and one neuroradiologist blinded to clinical information. In addition, follow-up MRIs were read independently to baseline MRI findings. Kappa coefficients for interrater agreement of categories of BG-PVS burden were 0.84 at baseline and 0.81 at follow-up; discrepancies were resolved by consensus.
Study participants were classified into two groups according to PSQI scores after the start of the pandemic, including individuals with persistent poor sleep quality at the second and third visits (group 1), and those with good sleep quality during the entire follow-up or with transient poor quality at only one of the post-pandemic visits (group 2). Enlarged BG-PVS progression was defined as the increase in at least one grade of the above-mentioned 4-points scale in follow-up MRIs (Figure 1).
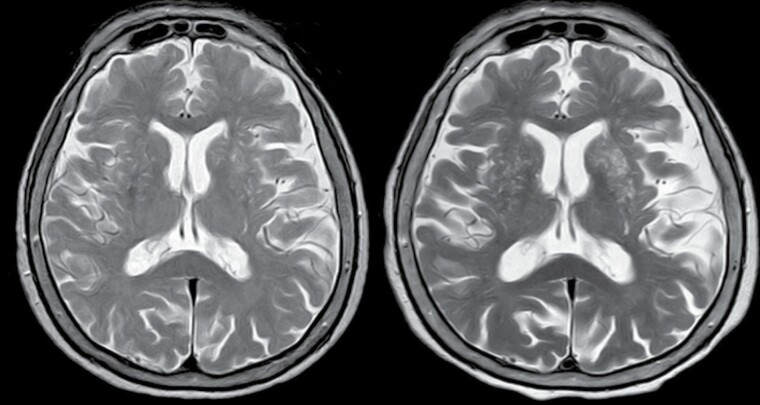
Figure 1.
T2-weighted MRI of a 75-year-old man with long COVID-related persistent poor sleep quality. There is a marked increase in the burden of enlarged basal ganglia-perivascular spaces between baseline (left) and follow-up (right) MRIs.
Data analyses were carried out by using STATA version 17 (College Station, TX, USA). In unadjusted analyses, continuous variables were compared by univariate linear models and categorical variables by the χ 2 or Fisher’s exact test as appropriate. A multivariate Poisson regression model—using dates of SARS-CoV-2 seropositivity and follow-up MRIs as an estimate of exposure time—measured differences in the incidence rate ratio (IRR) of BG-PVS progression according to sleep quality groups (group 1 versus group 2).
The above-described selection process left 93 participants with good sleep quality at baseline, evidence of SARS-CoV-2 infection, and MRIs at baseline and follow-up. Time between SARS-CoV-2 seropositivity and follow-up brain MRIs was 125.6 person-years (95% CI: 122.9–128.4 years), and the median follow-up was 1.4 years (interquartile range: 1.1–1.6 years).
The mean age of participants was 69.4 ± 6.5 years, 56 (60%) were women, 22 (24%) had a body mass index ≥30 kg/m2, and 34 (37%) were hypertensive. Twenty-six individuals had persistent poor sleep quality (group 1) and the remaining 67 had either good sleep quality or only transient poor sleep quality (group 2).
Assessments of enlarged BG-PVS on baseline MRI showed that 77 (83%) participants had a score of 1, 13 (14%) had a score of 2, three (3%) had a score of 3, and none had a score of 4. At follow-up, 60 (65%) individuals had a score of 1, 29 (31%) had a score of 2, one (1%) had a score of 3, and three (3%) had a score of 4. Overall, 20 (22%) individuals had MRI evidence of enlarged BG-PVS progression, which was from score 1 to 2 in 17 cases, from 2 to 4 in one, and from 3 to 4 in the remaining two. Progression was noticed in 42.3% (11/26) of subjects in group 1 and in only 13.4% (9/67) of those in Group 2 (p = 0.006).
A Poisson regression model that took into account the exposure time since SARS-CoV-2 seropositivity as well as age at baseline, sex, body mass index, and blood pressure levels, showed a significant effect of persistent poor sleep quality on BG-PVS progression (IRR: 2.68; 95% CI: 1.08–6.66; p = 0.033). None of the investigated covariates reached independent significance in this model (Table 1).
Table 1.
Poisson regression model showing that the incidence rate ratio (IRR) of enlarged basal ganglia-perivascular spaces (BG-PVS) progression is directly associated with persistent poor sleep quality among long COVID patients
| Enlarged BG-PVS progression | IRR | 95% CI | p |
|---|---|---|---|
| Persistent poor sleep quality | 2.68 | 1.08–6.66 | 0.033* |
| Age at baseline | 1.02 | 0.96–1.09 | 0.497 |
| Being female | 1.11 | 0.43–2.87 | 0.822 |
| Body mass index ≥30 kg/m2 | 0.49 | 0.14–1.80 | 0.289 |
| Blood pressure ≥140/90 mm Hg | 1.71 | 0.65–4.50 | 0.277 |
*Statistically significant result.
Evidence on the association between sleep disorders and progression of enlarged BG-PVS is mostly based on experimental models [7]. A few studies conducted on humans have found an association between breathing and nonbreathing-related sleep disorders and abnormal enlargement of BG-PVS [8, 9]. To the best of our knowledge, however, no study has assessed the relationship between the persistence of sleep disorders among COVID-19 survivors and progressive enlargement of BG-PVS.
The longitudinal prospective design, the rigorous inclusion criteria aimed to homogenize the study population and the systematic evaluation of study participants, are major strengths of our study. The relatively small sample size is a potential limitation. While the PSQI is reliable for evaluation of sleep quality, this instrument is based on the subjective assessment of sleep quality. Another perceived limitation of our study is the use of a visual rating scale instead of volumetric assessment of PVS. Visual rating scales are reliable to ascertain BG-PVS but not centrum semiovale-PVS [9]. However, enlargement of BG-PVS can be used as a reliable surrogate of PVS progression as they often progress together with PVS in other brain regions [4]. In addition, despite the longitudinal design, we cannot determine if there is a causal relationship between the persistence of poor sleep quality and progression of enlarged BG-PVS.
In conclusion, this study suggests induction of morphologic alterations expressed as expansion of BG-PVS in individuals with long COVID persistent poor sleep quality. Whether this is an effect of perivascular neuroinflammation related to high angiotensin-converting enzyme 2 expression in perivascular cells, remains unclear [10].
Trial registration: The Atahualpa Project has been registered at ClinicalTrials.gov; the identifier number is NCT01627600, and the date was: 10/02/2012 https://clinicaltrials.gov/ct2/show/NCT01627600?cond=Atahualpa&draw=2&rank=1
The Sleep Disorders substudy has been registered at ClinicalTrials.gov; the identifier number is NCT01877616, and the date was: 06/13/2013 https://clinicaltrials.gov/ct2/show/NCT01877616?cond=Atahualpa&draw=2&rank=4
Contributor Information
Oscar H Del Brutto, School of Medicine and Research Center, Universidad Espíritu Santo—Ecuador, Samborondón, Ecuador.
Robertino M Mera, Biostatistics/Epidemiology, Freenome, Inc., South San Francisco, CA, USA.
Aldo F Costa, Department of Neurology, Hospital Universitario Reina Sofía, Córdoba, Spain.
Denisse A Rumbea, School of Medicine and Research Center, Universidad Espíritu Santo—Ecuador, Samborondón, Ecuador.
Bettsy Y Recalde, School of Medicine and Research Center, Universidad Espíritu Santo—Ecuador, Samborondón, Ecuador.
Pablo R Castillo, Sleep Disorders Center, Mayo Clinic College of Medicine, Jacksonville, FL, USA.
Disclosure Statement
Financial disclosure: The Atahualpa Project is supported by Universidad Espiritu Santo—Ecuador. The sponsor has no role in the design of the study, nor in the collection or interpretation of data. The authors declare no potential conflicts of interest to disclose.
Nonfinancial disclosure: The authors report no competing conflicts of interest to disclose.
Data Availability
Aggregated data will be available from the corresponding author upon reasonable request.
References
- 1. Deng J, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486(1):90–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Del Brutto OH, et al. Sleep quality deterioration in middle-aged and older adults living in a rural Ecuadorian village severely struck by the SARS-CoV-2 pandemic. A population-based longitudinal prospective study. Sleep. 2021;44(8). doi: 10.1093/sleep/zsab041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gouveia-Freitas K, et al. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63(10):1581–1597. doi: 10.1007/s00234-021-02718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wardlaw JM, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137–153. [DOI] [PubMed] [Google Scholar]
- 5. Del Brutto OH, et al. SARS-CoV-2 in rural Latin America. A population-based study in coastal Ecuador. Clin Infect Dis. 2021;73(2):314–317. doi: 10.1093/cid/ciaa1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Potter GM, et al. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berezuk C, et al. Virchow-Robin spaces: correlation with polysomnography-derived sleep parameters. Sleep. 2015;38(6):853–858. doi: 10.5665/sleep.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramirez J, et al. MRI-visible perivascular space volumes, sleep duration and daytime dysfunction in adults with cerebrovascular disease. Sleep Med. 2021;83:83–88. [DOI] [PubMed] [Google Scholar]
- 10. Bocci M, et al. Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int J Mol Sci. 2021;22(21):11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregated data will be available from the corresponding author upon reasonable request.