Abstract
Background
Illness after infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant is less severe compared with previous variants. Data on the disease burden in immunocompromised patients are lacking. We investigated the clinical characteristics and outcomes of immunocompromised patients with coronavirus disease 2019 (COVID-19) caused by Omicron.
Methods
Organ transplant recipients, patients on anti-CD20 therapy, and allogenic hematopoietic stem cell transplantation recipients infected with the Omicron variant were included. Characteristics of consenting patients were collected and patients were contacted regularly until symptom resolution. To identify possible risk factors for hospitalization, a univariate logistic analysis was performed.
Results
114 consecutive immunocompromised patients were enrolled. Eighty-nine percent had previously received 3 mRNA vaccinations. While only 1 patient died, 23 (20%) were hospitalized for a median of 11 days. A low SARS-CoV-2 immunoglobulin G (IgG) antibody response (<300 BAU [binding antibody units]/mL) at diagnosis, being older, being a lung transplant recipient, having more comorbidities, and having a higher frailty score were associated with hospital admission (all P < .01). At the end of follow-up, 25% had still not fully recovered. Of the 23 hospitalized patients, 70% had a negative and 92% had a low IgG (<300 BAU/mL) antibody response at admission. Sotrovimab was administered to 17 of these patients, and 1 died.
Conclusions
While the mortality in immunocompromised patients infected with Omicron was low, hospital admission was frequent and the duration of symptoms often prolonged. In addition to vaccination, other interventions are needed to limit the morbidity from COVID-19 in immunocompromised patients.
Keywords: Omicron, COVID-19, immunocompromised patients, outcome, therapy
Coronavirus disease 2019 (COVID-19)–associated morbidity and mortality in immunocompromised patients is unknown for the severe acute respiratory syndrome coronavirus 2 Omicron variant. This prospective registry demonstrated low COVID-19–associated mortality in these vulnerable patients. However, morbidity remained substantial. Additional interventions to abate COVID-19 severity are needed.
Solid organ transplant recipients (SOTRs), patients treated with B cell–depleting therapy, and allogenic hematopoietic stem cell transplantation recipients are at increased risk of severe coronavirus disease 2019 (COVID-19)–associated morbidity and mortality [1–4]. Several comorbidities that were previously associated with more severe COVID-19 are frequently present in immunocompromised patients, and the use of immunosuppressive agents further increases the risk of a poor outcome. Although vaccination effectively protects against severe COVID-19 disease in the general population, the humoral and cellular immune response after vaccination of immunocompromised patients is lower, and protection from disease therefore is reduced [5, 6].
In November 2021, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant emerged and was rapidly declared a variant of concern (VOC) by the World Health Organization. The high number of mutations observed in the spike protein reduces or completely abolishes the neutralizing capacity of antibodies induced by previous infection or after a standard vaccination regimen [7]. Furthermore, the Omicron variant is more infectious than any previous VOC [7]. In addition to evading preexisting immunity, most monoclonal antibody therapies are ineffective against the Omicron variant [8]. Sotrovimab is the only approved monoclonal antibody that retained activity against the Omicron BA.1 variant [9]. Currently, a second sublineage of the Omicron variant (BA.2) is rapidly replacing BA.1 in most parts of the world, and reduced in vitro activity of sotrovimab against Omicron BA.2 is observed [10, 11].
Infections with the Omicron variant have been associated with diminished morbidity and mortality [12]. To some extent, this can be explained by other immunological correlates, such as cross-reactivity of vaccination- or infection-induced virus-specific T cells [13, 14]. Furthermore, animal models suggest a change in the pathophysiology of Omicron, with a shift toward infection of the upper rather than the lower airways [15]. The uncoupling of the extremely high incidence of Omicron infections and only a moderate increase in hospital and intensive care unit admissions has led several countries to loosen or completely stop public health measures previously implemented. Therefore, the spread of Omicron throughout the population is unpreventable, leading to exposure of immunocompromised patients to this variant. To date, no data are available on the clinical course and outcome of COVID-19 caused by Omicron in immunocompromised patients, in whom protection from preceding infections or vaccinations is probably reduced. Here, we report on the morbidity and mortality of Omicron infections in immunocompromised patients in care at the Erasmus University Medical Center (MC) in the Netherlands.
METHODS
Approximately 200 kidney, 70 liver, 35 lung, and 15 heart transplants as well as 100 allogeneic hematopoietic stem cell transplantations (alloHSCT) are performed annually at Erasmus MC. A prospective registry of SOTRs and patients from the department of hematology and internal medicine (clinical immunology) infected with the Omicron variant was implemented. Patients had been instructed to contact their treating physician when a diagnosis of COVID-19 was suspected or confirmed (eg, self-test or at a public health testing location). Kidney and lung transplant recipients with a positive self-test were invited at the hospital to collect a home monitoring kit that included an oxygen saturation meter and instructions on who to contact when the saturation level declined to 93% or lower. All patients who reported a positive test were instructed to recontact their treating physician if symptoms did not abate within 5 to 7 days or when symptoms worsened. In addition, all patients were contacted 2 weeks after symptom onset. When symptoms had not resolved, they were contacted on a regular basis until 14 March 2022 (date of last contact). When symptoms had resolved, the day of resolution of symptoms was registered. A symptom was considered COVID-19–related if it had started at the time of COVID-19 onset and was not present preceding the infection. Patients who were included fulfilled the following inclusion criteria: a proven SARS-CoV-2 infection with the Omicron variant in the period between 13 December 2021 and 3 February 2022 or a SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR)–based assay or an antigen self-test after 9 January 2022 (when Omicron had become >95% dominant in the Netherlands), SOTRs (kidney, liver, lung, heart, or multiorgan), or immunocompromised due to the use of anti-CD20 therapy for an autoimmune or hematological disease or an alloHSCT recipient on immunosuppressive therapy for the prevention or treatment of graft versus host disease, and follow-up of at least 2 weeks after symptom onset.
Data Collection
Demographic data, medical history, comorbidities, medication use, vaccination status, route to diagnosis (inpatient or outpatient), hospitalization status, clinical features, antibody titer before and after diagnosis (LIAISON SARS-CoV-2 Trimeric Spike immunoglobulin G [IgG]), treatment data, and clinical outcome were collected from all patients from the electronic patient files. The clinical frailty scale (CFS) was used to score the health status of patients by using the clinical assessment of the Canadian Study of Health and Ageing [16]. To evaluate symptom duration, all SARS-CoV-2–infected patients were contacted by telephone up to 14 March 2022.
The SARS-CoV-2 variant and sublineage were determined by detection of variant-specific single-nucleotide polymorphisms (SNPs), including K417, S371, and S373, using PCR and melting-curve analysis (VirSNiP 53-0787-96 and 53-0827-96, TIB MOLBIOL, Berlin, Germany). More information about this SNP analysis is available in the Supplementary Material.
For patients for whom PCR testing was not performed at the Erasmus MC, SARS-CoV-2 infection was diagnosed at the Municipal Health Service (MHS) using reverse-transcription (RT)-PCR or by means of an antigen self-test, and the variant could therefore not be determined. These patients were only included when the onset of symptoms started after 9 January 2022, the date from which the Omicron VOC had become responsible for >95% of the SARS-CoV-2 infections in the Netherlands [17].
Standard Immunosuppressive Regime
Information about the immunosuppressive therapies and regimens used in the different organ transplant recipients is available in the Supplementary Material.
COVID-19 Treatment
Hospitalized COVID-19 patients were treated according to the Dutch COVID-19 guideline, which includes dexamethasone for all patients who require supplemental oxygen, tocilizumab for those with C-reactive protein >74 mg/L, and at least 6 L oxygen/min [18]. Sotrovimab became available on 24 January 2022 in the Netherlands. As the natural course of Omicron in immunocompromised patients was unclear at that time, the vaccination coverage high, and the capacity to treat outpatients with sotrovimab limited, sotrovimab was not implemented as outpatient therapy. However, sotrovimab was used for all immunocompromised patients who required hospital admission as soon as it became available (500 mg intravenously upon admission).
Dutch COVID-19 Vaccination Strategy
According to Dutch guidelines, all SOTRs, B-cell depleted patients, and alloHSCT patients are considered fully vaccinated against COVID-19 when they have received 3 doses of an messenger RNA (mRNA)–based vaccine. This is based on the low response of neutralizing antibodies after 2 vaccinations in these groups. The additional fourth dose is then considered a booster [19].
Statistical Analyses
The primary outcome was hospitalization during the follow-up period. Since this study was not powered or designed to identify independent risk factors for hospitalization, analyses were purely explorative. More information about the statistical analyses is available in the Supplementary Material.
Ethical Approvals
The Erasmus MC Institutional Review Board confirmed that the study does not fall under the Dutch law on research in human subjects. However, all SOTRs provided written informed consent for the use of their clinical data as part of an ongoing quality improvement program, and the non-SOTR group consented for use of their data in the context of this study.
RESULTS
Demographics and Baseline Characteristics
A total of 114 immunocompromised patients with a SARS-CoV-2 infection caused by Omicron were included in the period between December 2021 and February 2022 and were followed for at least 2 weeks after symptom onset. Of these, 100 were SOTRs and 14 were immunocompromised as a result of anti-CD20 therapy for autoimmune or hematological diseases or because they were receiving immunosuppressive therapy after alloHSCT. Of the SOTRs, 43 were kidney, 16 lung, 19 liver, 17 heart, and 5 multiorgan. The demographics and baseline characteristics of the patient cohort are listed in Table 1.
Table 1.
Patient Demographics
| Demographic (n = 114) | Number |
|---|---|
| Age, median (interquartile range), years | 53 (19–84) |
| Age category, n (%), years | |
| ȃ19–44 | 33 (29) |
| ȃ45–64 | 58 (51) |
| ȃ65+ | 23 (20) |
| Sex, n (%), female | 58 (51) |
| Ethnicity, n (%) | |
| ȃCaucasian | 84 (74) |
| ȃAsian | 7 (6) |
| ȃBlack | 7 (6) |
| ȃOther | 16 (14) |
| Solid organ transplanted, n (%) | |
| ȃKidney | 43 (38) |
| ȃLung | 16 (14) |
| ȃLiver | 19 (17) |
| ȃHeart | 17 (15) |
| ȃMultiorgan | 5 (4) |
| Other immunocompromised patients, n (%) | 14 (12) |
| ȃAllogeneic stem cell transplantation on immunosuppressive therapy | 5 (4) |
| ȃAnti-CD20 for underlying hematological disease | 3 (3) |
| ȃAnti-CD20 for autoimmune disease | 5 (4) |
| ȃAnti-CD20 for other disease | 1 (1) |
| Time from transplantation (if transplanted), n (%), years | |
| ȃ<1 | 13 (12) |
| ȃ1–5 | 38 (36) |
| ȃ>5 | 55 (52) |
| Clinical frailty score, n (%) | |
| ȃUnknown | 13 (11) |
| ȃ1–3 | 74 (65) |
| ȃ4–5 | 23 (20) |
| ȃ>5 | 4 (4) |
| Outpatient diagnosis, n (%) | 104 (91) |
| Severe acute respiratory syndrome coronavirus 2 infection, n (%) | |
| ȃProbable Omicron (start of symptoms after 9 January 2022) | 62 (54) |
| ȃProven Omicron | 52 (46) |
| ȃȃBA.1 | 44 (85) |
| ȃȃBA.2 | 2 (4) |
| ȃȃLineage unknown | 6 (11) |
Median age of the immunocompromised patients was 53 years. Half of the patients were female, and most of them were White. Of the transplant recipients, 52% received their transplant more than 5 years ago. The health of most patients was scored as very fit, well, or managing well, and most of them were diagnosed with COVID-19 in the outpatient setting. Omicron was proven to be the causative variant in 46% of the SARS-CoV-2 infections, in which 85% was caused by the BA.1 variant. In the remaining 54% of SARS-CoV-2 infections, the diagnosis was made at the MHS by RT-PCR or by means of an antigen self-test. In these cases, symptom onset started in the period when the Omicron variant was responsible for at least 95% of SARS-CoV-2 infections in the Netherlands and therefore considered Omicron [17]. The most reported symptoms included rhinitis (54%), cough (53%), malaise (46%), fever (40%), headache (40%), sore throat (36%), fatigue (22%), gastrointestinal complaints (14%), and myalgia (10%). Only 1 patient was asymptomatic during the complete follow-up period. The most frequent comorbidities were arterial hypertension (65%), chronic kidney disease defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 (29%), diabetes mellitus type 2 (28%), and atherosclerotic cardiovascular disease or heart failure (15%).
Clinical Features and Outcomes
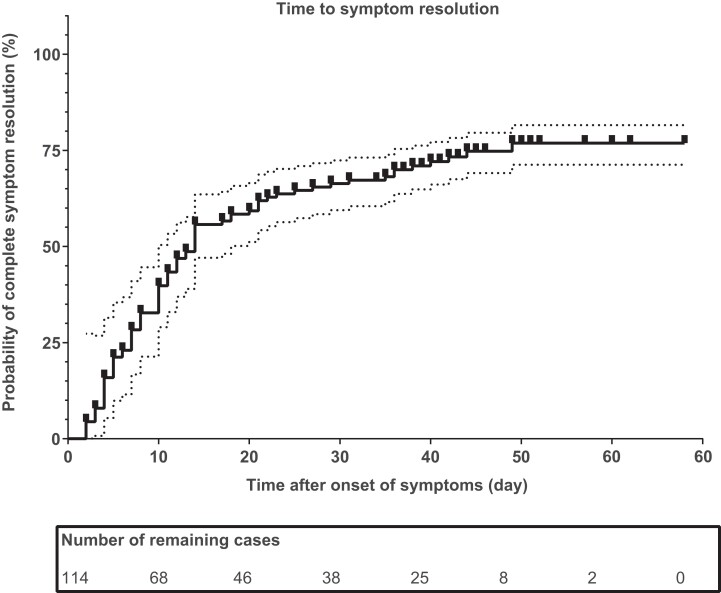
The clinical features and outcomes in the nonhospitalized and hospitalized immunocompromised patients are listed in Table 2. Among 23 hospitalized immunocompromised patients with a median age of 63 years, 57% were male. Of these patients, 35% required supplemental oxygen and 30% required high-flow nasal cannula therapy. Seventy-four percent received sotrovimab, 74% dexamethasone, 35% anti-interleukin 6 therapy, and 13% methylprednisolone pulse therapy. None of the patients required mechanical ventilation. The median duration of hospitalization was 11 days (range, 2–25), and 11 patients (48%) were hospitalized for more than 10 days. In 65% of the hospitalized patients, the COVID-19 diagnosis had been made at least 48 hours before hospital admission. The majority (70%) were seronegative at the time of hospital admission, despite the fact that 78% had been fully vaccinated. Only 5 hospitalized patients (22%) had received their booster (ie, fourth) vaccination. One hospitalized patient died. This was a lung transplant recipient with multiple comorbidities in whom a “do not ventilate” decision had been made. Figure 1 illustrates the time to full symptom resolution. The median duration of symptoms was 14 days, and 25% (28 of 114) had still not fully recovered at the end of the follow-up period (on the last contact date of 11 March 2021), as these 28 patients all continued to have residual symptoms 30 days or more after symptom onset. The most commonly reported residual symptoms at last contact were reduced exercise tolerance (18 patients, 64%), fatigue (10 patients, 36%), cough (9 patients, 32%), dyspnea at rest or during exercise (5 patients, 18%), gastrointestinal complaints (2 patients, 7%), myalgia (2 patients, 7%), sore throat (2 patients, 7%), fever/subfebrile (2 patients, 7%), supplemental oxygen requirement at home (1 patient, 4%), rhinitis (1 patient, 4%), and diagnosed pneumonia (1 patient, 4%).
Table 2.
Clinical Features and Outcomes in Nonhospitalized and Hospitalized Patients
| Clinical characteristic or variable | Nonhospitalized | Hospitalized |
|---|---|---|
| Number, n (%) | 91 (80) | 23 (20) |
| Age, median (IQR), years | 50 (19–84) | 63 (42–74) |
| Age category, n (%), years | ||
| ȃ19–44 | 32 (35) | 1 (4) |
| ȃ45–64 | 48 (53) | 11 (48) |
| ȃ65+ | 11 (12) | 11 (48) |
| Sex, n (%), male | 43 (47) | 13 (57) |
| Ethnicity, non-White, n (%) | 21 (23) | 9 (39) |
| Solid organ transplant recipient, n (%) | ||
| ȃKidney | 34 (37) | 9 (39) |
| ȃLung | 5 (6) | 11 (48) |
| ȃLiver | 19 (21) | 0 |
| ȃHeart | 17 (19) | 0 |
| ȃMultiorgan | 4 (4) | 1 (4) |
| Other immunocompromised patients, n (%) | 12 (13) | 2 (9) |
| Time from transplantation (if transplanted), years | ||
| ȃ<1 | 10 (11) | 3 (13) |
| ȃ1–5 | 29 (32) | 9 (39) |
| ȃ>5 | 46 (51) | 9 (39) |
| Maintenance immunosuppression, n (%) | ||
| ȃCNI monotherapy | 14 (15) | 0 (0.0) |
| ȃCNI + MMF | 27 (30) | 5 (22) |
| ȃPred + CNI | 9 (10) | 0 (0.0) |
| ȃPred + CNI + MMF | 14 (15) | 11 (48) |
| ȃPred + CNI + MMF + EVE | 0 (0.0) | 2 (9) |
| ȃAnti-CD20 monotherapy | 8 (9) | 0 |
| ȃOthera | 19 (21) | 5 (22) |
| ȃImmunosuppression containing MMF | 51 (56) | 18 (78) |
| Positive polymerase chain reaction ≥ 48 hours before hospitalization, n (%) | 0 (0.0) | 15 (65) |
| Coronavirus disease 2019 treatment, n (%) | 0 (0.0) | |
| ȃNo oxygen requirement | 8 (35) | |
| ȃ1–5 L/min oxygen | 6 (26) | |
| ȃ5–15 L/min oxygen | 2 (9) | |
| ȃHigh-flow nasal cannula therapy | 7 (30) | |
| ȃMechanical ventilation | 0 | |
| ȃSotrovimab treatment | 17 (74) | |
| ȃDexamethasone treatment | 17 (74) | |
| ȃAnti-interleukin 6 treatment | 8 (35) | |
| ȃMethylprednisolone pulseb | 3 (13) | |
| Time to hospitalization, median (IQR), days | 0 (0.0) | 10 (1–34) |
| Duration of hospitalization, median (IQR), days | 0 (0.0) | 11 (2–25) |
| Duration of hospitalization, n (%), days | 0 (0.0) | |
| ȃ2–5 | 6 (26) | |
| ȃ6–10 | 6 (26) | |
| ȃ>10 | 11 (48) | |
| Clinical outcome at the end of follow-up, n (%) | ||
| ȃNot recovered | 16 (18) | 12 (52) |
| ȃRecovered | 75 (82) | 10 (44) |
| ȃDeath | 0 | 1 (4) |
| Time to recovery (if recovered), median (IQR), days | 10 (2–49) | 22 (4–44) |
| SARS-CoV-2 spike IgG (BAU/mL), mean titer (SD)c | 3771 (8466) | 46 (96) |
| SARS-CoV-2 spike IgG category (BAU/mL), n (%) | ||
| ȃSeronegative | 19 (21) | 16 (70) |
| ȃSeropositive (enzyme-linked immunosorbent assay)d | 6 (7) | 0 (0.0) |
| ȃ33.8–299e | 1 (1) | 5 (22) |
| ȃ300–1000 | 5 (5) | 1 (4) |
| ȃ>1000 | 12 (13) | 0 |
| ȃUnknown | 48 (53) | 1 (4) |
| Vaccination status, n (%) | ||
| ȃNonvaccinated | 8 (9) | 5 (22) |
| ȃFully vaccinated | 83 (91) | 18 (78) |
| ȃBoosted | 9 (10) | 5 (22) |
Abbreviations: ADM, adalimumab; AZA, azathioprine; Bela, belatacept; CNI, calcineurin inhibitor; CsA, ciclosporin A; ETN, etanercept; EVE, everolimus; IgG, immunoglobulin G; IQR, interquartile range; MMF, mycophenolate mofetil; Pred, prednisolone; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SRL, sirolimus; TCZ, tocilizumab.
Other immunosuppressive regimens include: Pred + CNI + EVE (n = 3); Pred + CNI + ADM (n = 1), CNI + ADM (n = 1), MMF + SRL (n = 1), CNI + EVE (n = 1), MMF mono (n = 1), Pred + SRL (n = 1), Pred + MMF (n = 1), CsA mono (n = 1), an oncolytic (n = 1), Pred mono (n = 1), Pred + CNI + AZA (n = 1), Pred + AZA (n = 1), CNI + AZA (n = 1), Pred + MMF + CsA (n = 2), Pred + CsA (n = 2), Pred + CNI + MMF + TCZ (n = 1), Pred + MMF + Bela (n = 2), CNI + MMF + ETN (n = 1).
On top of or after dexamethasone.
If SARS-CoV-2 spike IgG is known.
In some patients, only an enzyme-linked immunosorbent assay was performed to detect SARS-CoV-2 seroconversion, which only gives a positive or negative result and no IgG titer.
33.8 BAU/mL is the detection limit of the LIAISON test.
Figure 1.
Time to symptom resolution in 114 immunocompromised patients. One minus the Kaplan-Meier estimator (multiplies by 100%) is shown as a solid line, the 95% confidence interval is shown as a dotted line, and the censored observations are shown as tick marks.
Based on evolving observation that the hospital admission rate of the lung transplant recipients was very high compared with all other immunocompromised patients, outpatient sotrovimab therapy was implemented for all lung transplant recipients on 13 February 2022. Before this policy was implemented, 11 of 16 (69%) lung transplant recipients diagnosed with COVID-19 required hospital admission, 7 of 11 required supplemental oxygen (64%) of whom 4 (36%) required at least 5 L/min, 4 (36%) required at least 15 L/min or high-flow nasal oxygen therapy, and 1 died. After the implementation of sotrovimab as outpatient therapy, 1 of 14 patients (7%) was hospitalized (P < .001). Of the other 14 immunocompromised patients (on anti-CD20 therapy or alloHSCT), 2 required hospital admission and 3 eventually received outpatient treatment with sotrovimab for prolonged symptoms and ongoing viral replication (with low cycle threshold PCR values). One of these 3 patients was hospitalized during follow-up, and all 3 cleared their virus after sotrovimab therapy.
Associations With Hospitalization
We identified baseline predictors of hospitalization for these patients in an exploratory way using a univariate logistic regression analysis. This showed that being older, having a lower IgG titer <300 BAU/mL, being a lung transplant recipient, having more comorbidities, and having a higher CFS were significantly associated with hospitalization (Table 3). The number of hospitalizations was too low to allow identification of predictors in a multivariate regression analysis.
Table 3.
Univariate Logistic Regression Analysis
| Independent Variable | P Value | Odds Ratio (95% Confidence Interval) |
|---|---|---|
| Sex (male/female) | .43 | 0.69 (.27–1.7) |
| Age (year) | .00036 | 1.1 (1.0–1.1) |
| Ethnicity | .12 | 2.1 (.81–5.6) |
| Chronic kidney diseasea (yes vs no) | .090 | 2.3 (.88–5.9) |
| Use of mycophenolate mofetil (yes vs no) | .058 | 2.8 (.96–8.3) |
| Fully vaccinated (yes vs no) | .091 | 0.35 (.10–1.2) |
| Boosted (yes vs no) | .11 | 2.7 (.79–9.0) |
| Immunoglobulin G titer (BAU/mL) | .091 | 0.99 (.99–1.0) |
| Being an adequate responder (≥300 BAU/mL) | .0064 | 0.053 (.0060–.44) |
| Being a kidney transplant recipient (yes vs no) | .88 | 1.1 (.42–2.8) |
| Being a lung transplant recipient (yes vs no) | <.000010 | 16 (4.7–53) |
| Obesityb (yes vs no) | .22 | 2.6 (.57–12) |
| Number of comorbidities (0–5) | .00065 | 2.1 (1.4–3.2) |
| Frailty score (1–9) | .00092 | 1.8 (1.3–2.6) |
Dependent variable is hospitalization vs no hospitalization.
Estimated glomerular filtration rate, <60 mL/min/1.73 m2.
Body mass index, >35 kg/m2.
DISCUSSION
Compared with previous SARS-CoV-2 variants, infection with Omicron results in fewer hospital admissions and a lower mortality rate [12]. However, little is known about the course of disease with Omicron in immunocompromised patients. Here, we describe the clinical characteristics, morbidity, and mortality in 114 severely immunocompromised patients with COVID-19 when Omicron BA.1 was dominant in the Netherlands. Of all patients, 89% had previously received 3 mRNA vaccinations and 12% had received a fourth vaccination. State-of-the-art COVID-19 therapy was available to all. In this specific context, the overall mortality of 1% is in sharp contrast to reported mortality rates ranging from 14% to 20% in hospitalized SOTRs after infection with previous VOCs [20]. This also contrasts with the admission rate and mortality we observed at a time when the ancestral virus was dominant in the Netherlands. Indeed, during the very first wave of COVID-19 in the Netherlands, 5 of the 23 (22%) SOTRs who were infected died and 83% were hospitalized [2]. However, the observed morbidity in our study remains substantial with 20% still requiring hospital admission. Also, 25% continued to have symptoms more than 30 days after infection onset. This contrasts sharply with data from a large community cohort of 29 000 Omicron-infected inhabitants in the United Kingdom. With 87% having had 3 vaccinations and a median age of 55 years, this cohort was comparable to our study population regarding age and vaccination status. However, only 1.9% of them required hospital admission [21].
Several variables were associated with an increased risk of hospitalization, such as having a SARS-CoV-2 IgG spike antibody titer of <300 BAU/mL around the date of diagnosis (despite 89% of all patients having previously received 3 mRNA vaccinations) and being a lung transplant recipient. The high hospital admission rate in lung transplant recipients (69%) confirms observations during the circulation of previous variants and can have multiple explanations, including the use of higher doses of immunosuppressive therapy, the respiratory nature of their condition, and transplanted lungs suffering more from the recipient’s immune response [22–25]. The observations in lung and kidney transplant recipients were in sharp contrast to the disease course observed in 17 heart and 18 liver transplant recipients, as none of them required hospitalization and none received sotrovimab therapy as outpatients.
In the pre-Omicron era, treatment of patients who were (SARS-CoV-2 IgG spike) seronegative at the time of hospital admission with casirivimab/imdevimab was associated with a 20% lower overall mortality [26]. Although a similar study was not performed with other monoclonal antibodies in seronegative patients, we considered it sufficiently likely that sotrovimab would have a similar beneficial effect in immunocompromised patients with a low or negative SARS-CoV-2 IgG spike antibody titer. Therefore, the hospital COVID-19 treatment guideline recommended the off-label use of sotrovimab for these patients as soon as the drug became available. Strikingly, despite the fact that 78% of hospitalized patients had been fully vaccinated, the majority (70%) were still seronegative and 92% had a titer of <300 BAU/mL. Of the 23 hospitalized patients, 17 received sotrovimab. While 16 patients could be discharged without the need for mechanical ventilation, 1 patient with multiple comorbidities, a frailty score of 7, and for whom mechanical ventilation was deliberately not initiated died. The decision to be restrictive with sotrovimab as outpatient therapy was due to the uncertain benefit of sotrovimab for Omicron in a setting where the vaccination coverage was 90%. However, the high hospital admission rate observed in lung transplant recipients resulted in a policy change, where sotrovimab outpatient therapy was implemented for all lung transplant recipients immediately after COVID-19 diagnosis. Before this policy change, 11 of 16 (69%) lung transplant recipients diagnosed with Omicron required hospital admission. After this policy change, 14 lung transplant recipients received sotrovimab in an outpatient setting, of whom only 1 (7%) required hospital admission. This patient was hospitalized with both diverticulitis and COVID-19.
Our study has several limitations. First, associations with hospital admission should be interpreted with caution as the number of end points was small and the number of potential risk factors was high and therefore a comprehensive multivariate analysis was not possible. Second, it is possible that the observed morbidity is overestimated since we cannot exclude that mild COVID-19 cases remained undiagnosed. However, test locations are easily accessible across the Netherlands, self-tests are available at very low cost, and the importance of testing in these vulnerable patients is constantly emphasized by their healthcare workers. In addition, our cohort is relatively small, with the majority being a SOTR, and therefore represents a rather small part of the diverse immunocompromised patient population. Studies on the burden of infection with Omicron in other prevalent patient groups, including those treated for chronic lymphocytic leukemia, multiple myeloma, or other T- or B-cell diseases, are therefore needed. Finally, some hospital admissions could have been prevented by the use of sotrovimab in the outpatient setting. Unfortunately, with BA.2 now being the dominant variant in the Netherlands, sotrovimab is unlikely to be of benefit as its in vitro activity to this variant is limited [9, 11].
In conclusion, in a highly vaccinated population of immunocompromised patients, we observed very low mortality from COVID-19 caused by Omicron. The morbidity, however, continues to be very substantial despite Omicron BA.1 being the dominant variant. In particular, lung and kidney transplant recipients are likely to benefit from early treatment for COVID-19. However, the recently reported reduced or complete absence of activity of sotrovimab against the BA.2 variant will require the use of other monoclonal antibodies or direct-acting antiviral drugs such as nirmatrelvir/ritonavir once they become broadly available.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The corresponding author had full access to all data and the final responsibility to submit for publication.
Supplementary Material
Contributor Information
S Reshwan K Malahe, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands; Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands.
Rogier A S Hoek, Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
Virgil A S H Dalm, Department of Internal Medicine, Division of Allergy and Clinical Immunology, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Immunology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Annoek E C Broers, Department of Hematology, Erasmus Cancer Institute, Rotterdam, The Netherlands.
Caroline M den Hoed, Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Olivier C Manintveld, Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Cardiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Carla C Baan, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands; Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands.
Charlotte M van Deuzen, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, The Netherlands.
Grigorios Papageorgiou, Department of Biostatistics and Department of Epidemiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Hannelore I Bax, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, The Netherlands.
Jeroen J Van Kampen, Department of Viroscience, Erasmus University Medical Center, Rotterdam, The Netherlands.
Merel E Hellemons, Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands; Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
Marcia M L Kho, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands; Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands.
Rory D de Vries, Department of Viroscience, Erasmus University Medical Center, Rotterdam, The Netherlands.
Richard Molenkamp, Department of Viroscience, Erasmus University Medical Center, Rotterdam, The Netherlands.
Marlies E J Reinders, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands; Erasmus MC Transplant Institute, Erasmus University Medical Center, Rotterdam, The Netherlands.
Bart J A Rijnders, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, Rotterdam, The Netherlands.
References
- 1. Williamson EJ, Walker AJ, Bhaskaran K, et al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoek RAS, Manintveld OC, Betjes MGH, et al. . COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int 2020; 33:1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vinson AJ, Agarwal G, Dai R, et al. . COVID-19 in solid organ transplantation: results of the National COVID Cohort Collaborative. Transplant Direct 2021; 7:e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feikin DR, Higdon MM, Abu-Raddad LJ, et al. . Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022; 399:924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanders JF, Bemelman FJ, Messchendorp AL, et al. . The RECOVAC Immune-Response Study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 2022; 106:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022; 62:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao Y, Wang J, Jian F, et al. . Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022; 602:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takashita E, Kinoshita N, Yamayoshi S, et al. . Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med 2022; 386:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonager J, Bennedbaek M, Bager P, et al. . Molecular epidemiology of the SARS-CoV-2 variant Omicron BA.2 sub-lineage in Denmark, 29 November 2021 to 2 January 2022. Euro Surveill 2022; 27:2200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iketani S, Liu L, Guo Y, et al. . Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022; 604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nyberg T, Ferguson NM, Nash SG, et al. . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keeton R, Tincho MB, Ngomti A, et al. . T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GeurtsvanKessel CH, Geers D, Schmitz KS, et al. . Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol 2022; 7:eabo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMahan K, Giffin V, Tostanoski LH, et al. . Reduced pathogenicity of the SARS-CoV-2 Omicron variant in hamsters. Med (N Y) 2022; 3:262–268.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rockwood K, Song X, MacKnight C, et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coronavirus Dashboard. [cited 24 March 2022]. Available at: https://coronadashboard.government.nl/landelijk/varianten. Accessed 21 July 2022.
- 18. Medicamenteuze behandeling voor patiënten met COVID-19 (infectie met SARS–CoV-2). [cited 24 March 2022]. Available at: https://swab.nl/nl/covid-19. Accessed 21 July 2022.
- 19. COVID-19-vaccinatie van immuungecompromitteerde patiënten (handleiding). [cited 24 March 2022]. Available at: https://lci.rivm.nl/handleiding-covid-19-vaccinatie-van-immuungecompromitteerde-patienten#samenvatting. Accessed 21 July 2022.
- 20. Heldman MR, Kates OS, Safa K, et al. . Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant 2022; 22:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menni C, Valdes A, Polidori L, et al. . Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet 2022; 399:1618–1624.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pereira MR, Mohan S, Cohen DJ, et al. . COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; 20:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Softeland JM, Friman G, von Zur-Muhlen B, et al. . COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am J Transplant 2021; 21:2762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. An W, Wang Q, Kim TE, Kang JS. Clinical characteristics and outcome of coronavirus disease 2019 infection in patients with solid organ transplants: a systematic review and meta-analysis. J Infect Public Health 2022; 15:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnusson JM, Larsson H, Alsaleh A, et al. . COVID-19 in lung transplant recipients: an overview of the Swedish national experience. Transpl Int 2021; 34:2597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RECOVERY Collaborative Group . Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022; 399:665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.