Abstract
Background
We explore severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody lateral flow immunoassay (LFIA) performance under field conditions compared to laboratory-based electrochemiluminescence immunoassay (ECLIA) and live virus neutralization.
Methods
In July 2021, 3758 participants performed, at home, a self-administered Fortress LFIA on finger-prick blood, reported and submitted a photograph of the result, and provided a self-collected capillary blood sample for assessment of immunoglobulin G (IgG) antibodies using the Roche Elecsys® Anti-SARS-CoV-2 ECLIA. We compared the self-reported LFIA result to the quantitative ECLIA and checked the reading of the LFIA result with an automated image analysis (ALFA). In a subsample of 250 participants, we compared the results to live virus neutralization.
Results
Almost all participants (3593/3758, 95.6%) had been vaccinated or reported prior infection. Overall, 2777/3758 (73.9%) were positive on self-reported LFIA, 2811/3457 (81.3%) positive by LFIA when ALFA-reported, and 3622/3758 (96.4%) positive on ECLIA (using the manufacturer reference standard threshold for positivity of 0.8 U mL–1). Live virus neutralization was detected in 169 of 250 randomly selected samples (67.6%); 133/169 were positive with self-reported LFIA (sensitivity 78.7%; 95% confidence interval [CI]: 71.8, 84.6), 142/155 (91.6%; 95% CI: 86.1, 95.5) with ALFA, and 169 (100%; 95% CI: 97.8, 100.0) with ECLIA. There were 81 samples with no detectable virus neutralization; 47/81 were negative with self-reported LFIA (specificity 58.0%; 95% CI: 46.5, 68.9), 34/75 (45.3%; 95% CI: 33.8, 57.3) with ALFA, and 0/81 (0%; 95% CI: 0, 4.5) with ECLIA.
Conclusions
Self-administered LFIA is less sensitive than a quantitative antibody test, but the positivity in LFIA correlates better than the quantitative ECLIA with virus neutralization.
Keywords: SARS-CoV-2, COVID-19, lateral flow immunoassay, home-testing, antibodies
We found positivity on lateral flow immunoassay (LFIA) correlates better than quantitative electrochemiluminescence immunoassay (ECLIA) with virus neutralization. Calibrated to the appropriate positivity threshold for protection from infection or disease, rapid antibody home-testing by LFIA may prove useful in ongoing population surveillance.
In April 2020 the REal-time Assessment of Community Transmission-2 (REACT-2) study of at-home severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody testing using self-administered finger prick lateral flow immunoassays (LFIAs) was initiated to provide community prevalence estimates of antibodies to SARS-CoV-2 in England, United Kingdom [1–4]. As coronavirus disease 2019 (COVID-19) vaccination programs are rolled out worldwide, large-scale LFIA antibody testing could have an important additional role in monitoring immune responses to vaccinations and informing policy regarding booster doses [5].
The REACT-2 program conducted extensive clinical and laboratory evaluation of SARS-CoV-2 antibody LFIA performance [6–10], summarized in Supplementary Table 1. The LFIA selected (Fortress, United Kingdom) was initially evaluated in a healthcare worker cohort known to have been infected with SARS-CoV-2, with a sensitivity 84.0% (95% confidence interval [CI]: 70.5, 93.5) and specificity 98.6% (95% CI: 97.1, 99.4) [6].
Prevalence studies based on self-administered LFIA have generally produced a lower estimate of population SARS-CoV-2 antibody positivity than those using quantitative laboratory assays, despite adjustment for test performance [11]. As a threshold test, it is likely that the LFIA is predominantly missing people with low antibody titers. To investigate the utility of the Fortress LFIA under field conditions, we compare results of self-reported qualitative LFIA results against a quantitative laboratory-based electrochemiluminescence immunoassay (ECLIA) performed on simultaneously self-collected capillary blood. We also explore the relationship between LFIA results and antibody titers with viral neutralization.
METHODS
Study Design and Sampling
The study was conducted between 1 July 2021 and 10 August 2021.
This study recruited participants from round 6 of the REACT-2 study of SARS-CoV-2 antibody prevalence in the community in England, United Kingdom. Methods for the REACT-2 study are published elsewhere [1, 12]. Briefly, REACT-2 is a series of cross-sectional population surveys. At each round, we contacted a random sample of the population by sending a letter to named individuals aged 18 or over from the National Health Service (NHS) patient list (covering almost the whole population) and respondents were sent an LFIA self-testing kit to perform at home. The LFIA used (Fortress, United Kingdom) detects antibody against the spike (“S”) protein of the virus (contained in, or coded by, all UK licensed vaccines).
For this follow-up study, purposeful random sampling was carried out by re-contacting 7000 participants who had participated in round 6 of REACT-2 in May 2021, aiming to achieve a sample size of 4000. We invited equal numbers in each of the following categories based on results from round 6 – unvaccinated and LFIA negative, double vaccinated (>20 days previously) and LFIA negative, unvaccinated and LFIA positive, and double vaccinated and LFIA positive. This sampling frame was chosen to recruit sufficient people with positive and negative self-test results post-infection and post-vaccination, recognizing that many people would have received further vaccination in the interim.
People were invited by post to register until approximately 4000 had signed up. Registration was undertaken online or by telephone. Those who registered were sent a further LFIA test kit to carry out at home, and asked to report the result online, upload a photograph of the result, and complete a short online questionnaire. In addition, participants were asked to take a 400–500 μL capillary blood sample at the same time-point using an at-home self-collection blood device (Tasso-SST [13]) and return the sample for serological assessment of antibodies.
ALFA (Automated Lateral Flow Analysis): Machine Learning Algorithm for Automated Analysis of LFIA Images
We have shown previously that participant reported LFIA interpretation is consistent with clinician interpreted results [9, 10]. However, we developed a computational pipeline (ALFA) that used machine learning algorithms to analyze participant-submitted images of the Fortress LFIA from REACT-2 rounds 1 to 5. Methods for development of ALFA are published elsewhere [14]. Automated analysis showed substantial agreement with human experts and performed consistently better than study participants, particularly for weak positive immunoglobulin G (IgG) results [14].
Laboratory Methods
Serological assessment was performed in a commercial laboratory on the Roche Elecsys® Anti-SARS-CoV-2 ECLIA, which reports a quantitative anti-Spike (anti-S) antibody titer. This assay has been previously validated by Public Health England who reported a specificity of 100% (95% CI: 99.1, 100), and a sensitivity of 98.5% (95% CI: 96.9, 99.4) in samples 21 days post-onset in people with polymerase chain reaction (PCR)-confirmed infection [15]. In addition, the Roche ECLIA demonstrates prolonged antibody detection compared to many other SARS-CoV-2 laboratory-based assays [16, 17]. The threshold value for antibody positivity for the Roche ECLIA is 0.8 U mL–1 based on manufacturer instructions [15]. The lower limit of quantification is 0.4 U mL–1 [18]. Measurements below this value were truncated at 0.4 U mL–1. The assay was analyzed in its original scale (U mL–1). WHO international standard units are BAU mL–1 for anti-spike IgG to allow comparison across studies and platforms [19]. The conversion factor for U mL–1 to BAU mL–1 for the Roche Elecsys® Anti-SARS-CoV-2 assay:
In addition, we selected 250 serum samples at random for assessment on a live virus neutralization assay. Serum samples were heat-inactivated and a 2-fold dilution series was performed in 96-well plates. Serum dilutions were incubated with 100 TCID50 SARS-CoV-2 (WT D614G) for 1 hour at 37°C. Vero E6 cells modified to overexpress ACE2 and TMPRSS2 (VAT cells) were then added to the wells and incubated at 37°C for 72 hours before assessing the cells for the presence or absence of virus-induced cytopathic effect (CPE). The neutralization titer of a serum sample was defined as the reciprocal of the highest serum dilution at which CPE was not observed, demonstrating antibody-mediated protection from virus, for example, protection of cells at a 1:20 dilution of serum gives a neutralization titer value of 20. Serum samples were titrated 2-fold in duplicate with a starting dilution of 1:10 meaning if 1 of the 2 replicate wells were protected at this first dilution, the titer was expressed as 7.1, halfway to the 1:10 dilution on a log2 scale. Serum samples for which CPE was observed in all wells were therefore defined as having neutralization titer of <7.1. Using a calculated conversion factor of 2.6 BAU per neutralization titer unit, the lower limit of detection of 7.1 equates to 18.5 BAU mL–1 [20] (Supplementary Figure 1).
Data Analysis
We report on positivity based on three results for each participant: self-administered and reported LFIA (hereafter self-LFIA), self-administered and machine-read LFIA (hereafter ALFA) and Roche Elecsys® platform (hereafter ECLIA) using the manufacturer recommended threshold ≥0.8 U mL–1. As the manufacturer’s threshold for antibody positivity for the ECLIA is likely too low to correlate with moderate-to-high levels of protection from infection based on recent studies in the UK population [21, 22], we also report positivity at different thresholds of ≥100 U mL–1, ≥350 U mL–1, and ≥1000 U mL–1 – equivalent to ≥103 BAU mL–1, ≥360 BAU mL–1, and ≥1029 BAU mL–1, respectively. In addition, we report the distribution of quantitative ECLIA results for self-LFIA and ALFA positive and negative results.
We assessed the association between self-LFIA, ALFA, ECLIA and live virus neutralization titers, with the threshold of neutralization detection defined as a titer of ≥7.1 (equivalent to 18.5 BAU mL–1). We then used this as a standard to determine sensitivity and specificity of self-LFIA, ALFA, and ECLIA at different thresholds as a measure of neutralization. The Mann-Whitney test was performed to compare neutralization titers according to whether positive or negative by self-LFIA, and to compare IgG antibody titers according to whether positive or negative by self-LFIA. The threshold for statistical significance was P < .05.
As a supplementary analysis, we used multiple linear regression to quantify associations between demographic characteristics, history of COVID-19, vaccination status and time since double vaccinated (2 doses) and log10-transformed antibody titers. Methods and results are described in Supplementary Table 3.
Data analyzed using statistical packages STATA version 15.0 and GraphPad Prism 9.0.0.
Ethics
Ethical approval from South Central–Berkshire B Research Ethics Committee (20/SC/0206; IRAS 283805).
RESULTS
Overall, 71.0% (4972/7000) of invited individuals agreed to take part in the study, of whom, 1214 (24.4%) were excluded from the analysis due to either a missing or invalid self-LFIA result (n = 327) or a missing or void ECLIA result (n = 887). The reasons for the large number of missing or void ECLIA results include insufficient and incorrectly labeled samples and laboratory error, but the distribution of these was not provided by the commercial laboratory performing the tests. A total of 3758 participants had paired self-LFIA and ECLIA results, 96.6% (3457/3578) of whom also uploaded a photograph of their self-LFIA test which enabled analysis using ALFA. Participant characteristics are shown in Table 1. Most participants had received 1 (862, 22.9%) or 2 (2430, 64.7%) COVID-19 vaccine doses, and 27.4% reported suspected or confirmed past COVID-19 (Table 1), meaning that almost all participants (3593/3758, 95.6%) reported either vaccine or prior infection.
Table 1.
Demographic and Clinical Characteristics of the Study Participants by Antibody Status for Self-LFIA, ALFA, and ECLIA at 0.8 U mL–1
| Characteristic | All Participants | Self-LFIA | ALFA | ECLIA (0.8 U mL–1) | |||
|---|---|---|---|---|---|---|---|
| N (% of total) | No. Positive/total | Positivity %b, (95% CI) | N. Positive/total | Positivity %b, (95% CI) | No. Positive/total | Positivity %a, (95% CI) | |
| All participants | 3758 | 2777/3758 | 73.9 (72.5, 75.3) | 2811/3457 | 81.3 (80.0, 82.6) | 3622/3758 | 96.4 (95.7, 97.0) |
| Sex | |||||||
| ȃFemale | 2275 (60.5) | 1760/2275 | 77.4 (75.6, 79.0) | 1766/2095 | 84.3 (82.7, 85.8) | 2192/2275 | 96.4 (95.5, 97.0) |
| ȃMale | 1483 (39.5) | 1017/1483 | 68.6 (66.2, 70.9) | 1045/1362 | 76.7 (74.4, 78.9) | 1430/1483 | 96.4 (95.3, 97.3) |
| Age group (years) | |||||||
| ȃ18–24 | 385 (10.2) | 343/385 | 89.1 (85.5, 91.8) | 341/372 | 91.7 (88.4, 94.1) | 369/385 | 95.8 (93.3, 97.4) |
| ȃ25–34 | 704 (18.7) | 624/704 | 88.6 (86.1, 90.8) | 625/688 | 90.8 (88.4, 92.8) | 669/704 | 95.0 (93.1, 96.4) |
| ȃ35–44 | 430 (11.4) | 386/430 | 89.8 (86.5, 92.3) | 381/481 | 91.2 (88.0, 93.5) | 416/430 | 96.7 (94.6, 98.1) |
| ȃ45–54 | 163 (4.3) | 128/163 | 78.5 (71.5, 84.2) | 126/157 | 80.3 (73.2, 85.8) | 152/163 | 93.3 (88.2, 96.2) |
| ȃ55–64 | 628 (16.7) | 449/628 | 71.5 (67.8, 74.9) | 459/574 | 80.0 (76.5, 83.0) | 592/628 | 94.3 (92.1, 95.8) |
| ȃ65–74 | 1292 (34.4) | 756/1292 | 58.5 (55.8, 61.2) | 795/1125 | 70.7 (67.9, 73.3) | 1270/1292 | 98.3 (97.4, 98.9) |
| ȃ≥75 | 156 (4.2) | 91/156 | 58.3 (50.4, 65.9) | 84/123 | 68.3 (59.4, 76.0) | 154/156 | 98.7 (94.9, 100.0) |
| Ethnicity | |||||||
| ȃWhite | 3420 (91.6) | 2502/3420 | 73.2 (71.6, 74.6) | 2533/3136 | 80.8 (79.4, 82.1) | 3298/3420 | 96.4 (95.8, 97.0) |
| ȃMixed | 59 (1.6) | 49/59 | 83.1 (70.9, 90.8) | 52/59 | 88.1 (76.7, 94.4) | 56/59 | 94.9 (84.9, 98.4) |
| ȃAsian | 152 (4.1) | 124/152 | 81.6 (74.5, 87.0) | 126/146 | 86.3 (79.6, 91.0) | 147/152 | 96.7 (92.3, 98.6) |
| ȃBlack | 69 (1.9) | 59/69 | 85.5 (74.8, 92.1) | 57/63 | 90.5 (80.0, 95.8) | 66/69 | 95.7 (87.0, 98.6) |
| ȃOther | 35 (0.9) | 25/35 | 71.4 (53.6, 84.4) | 24/31 | 77.4 (58.4, 89.3) | 32/35 | 91.4 (75.4, 97.4) |
| History of COVID-19 | |||||||
| ȃPositive PCR test | 489 (13.0) | 468/489 | 95.7 (93.5, 97.2) | 459/470 | 97.7 (95.8, 98.7) | 488/489 | 99.8 (98.6, 100.0) |
| ȃSuspected by doctor | 54 (1.4) | 48/54 | 88.9 (76.9, 95.1) | 49/53 | 92.5 (81.0, 97.2) | 52/54 | 96.3 (85.8, 99.1) |
| ȃSuspected by self | 487 (13.0) | 421/487 | 86.5 (83.1, 89.2) | 417/469 | 88.9 (85.7, 91.5) | 455/487 | 93.4 (90.8, 95.3) |
| ȃNo | 2728 (72.6) | 1840/2728 | 67.5 (65.7, 69.2) | 1886/2465 | 76.5 (74.8, 78.1) | 2627/2728 | 96.3 (95.5, 96.9) |
| No. of preexisting health conditionsb | |||||||
| ȃ>1 | 701 (18.7) | 433/701 | 61.8 (58.1, 65.3) | 443/621 | 71.3 (67.6, 74.8) | 668/701 | 95.3 (93.4, 96.6) |
| ȃ1 | 881 (23.4) | 606/881 | 68.8 (65.6, 71.8) | 626/800 | 78.3 (75.2, 81.0) | 855/881 | 97.0 (95.7, 98.0) |
| ȃ0 | 2176 (57.9) | 1738/2176 | 79.9 (78.1, 81.5) | 1742/2036 | 85.6 (84.0, 87.0) | 2099/2176 | 96.5 (95.6, 97.2) |
| Vaccine status | |||||||
| ȃ0 | 466 (12.4) | 335/466 | 71.9 (67.6, 75.8) | 329/444 | 74.1 (69.8, 78.0) | 363/466 | 77.9 (73.9, 81.4) |
| ȃ1 | 862 (22.9) | 793/862 | 92.0 (90.0, 93.6) | 789/837 | 94.3 (92.5, 95.7) | 856/862 | 99.3 (98.5, 99.7) |
| ȃ2 | 2430 (64.7) | 1649/2430 | 67.9 (66.0, 69.7) | 1693/2176 | 77.8 (76.0, 79.5) | 2403/2430 | 98.9 (98.4, 99.2) |
| Vaccine type | |||||||
| ȃPfizer-BioNTech | 1965 (59.8) | 1733/1965 | 88.2 (86.7, 89.5) | 1704/1836 | 92.8 (91.5, 93.9) | 1948/1965 | 99.1 (98.6, 99.5) |
| ȃAstraZeneca | 1210 (36.8) | 599/1210 | 49.5 (46.7, 52.3) | 671/1066 | 63.0 (60.0, 65.8) | 1195/1210 | 98.8 (98.0, 99.3) |
| ȃModerna | 110 (3.4) | 105/110 | 95.5 (89.4, 98.1) | 102/104 | 98.1 (92.5, 99.5) | 109/110 | 99.1 (93.7, 99.9) |
| Time since second vaccination (N = 2396) (weeks) | |||||||
| ȃ0–3 | 326 (13.6) | 312/326 | 95.7 (92.9, 97.4) | 306/317 | 96.5 (93.8, 98.1) | 326/326 | 100 (98.9, 100) |
| ȃ4–12 | 268 (11.2) | 175/268 | 65.5 (59.4, 70.8) | 178/232 | 76.7 (70.8, 81.8) | 268/268 | 100 (98.6, 100) |
| ȃ13–23 | 1766 (73.7) | 1122/1766 | 63.5 (61.3, 65.7) | 1171/1571 | 74.5 (72.3, 76.6) | 1739/1766 | 98.5 (97.8, 98.9) |
| ȃ≥24 | 36 (1.5) | 21/36 | 58.3 (41.1, 73.7) | 23/31 | 74.2 (55.1, 87.1) | 36/36 | 100 (90.3, 100) |
Abbreviations: ALFA, automated image analysis; CI, confidence interval; COVID-19, coronavirus disease 2019; ECLIA, electrochemiluminescence immunoassay; LFIA, lateral flow immunoassay; PCR, polymerase chain reaction.
Percentages are calculated from nonmissing values.
A preexisting health condition is any physical or mental illness or health condition that existed at the time of study.
IgG Anti-S Positivity and Antibody Titers
Self-LFIA positivity was 73.9% (2777/3758, 95% CI: 72.5, 75.3) (Table 1); ALFA positivity was 81.3% (2811/3457, 95% CI: 80.0, 82.6), and ECLIA positivity was 96.4% (3622/3758, 95% CI: 95.7, 97.0) using the manufacturer’s threshold of ≥0.8 U mL–1. ECLIA positivity decreased to 83.1% (95% CI: 81.9, 84.3), 62.7% (95% CI: 61.1, 64.2), and 47.0% (95% CI: 45.4, 48.6) by increasing the ECLIA threshold to ≥100 U mL–1, ≥350 U mL–1 and ≥1000 U mL–1, respectively.
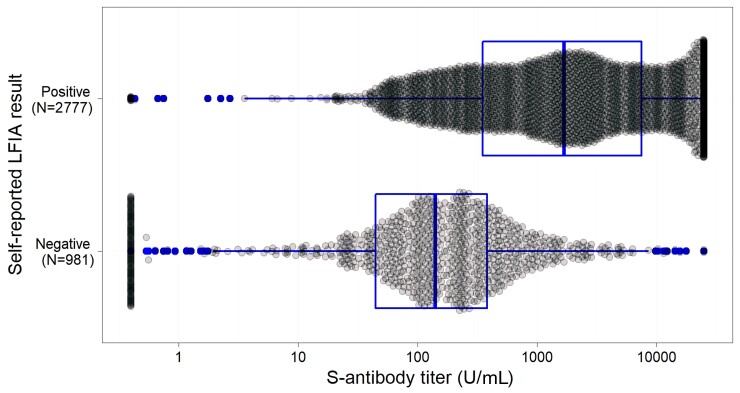
Figure 1 shows the distribution of ECLIA titers for samples that were positive and negative on self-reported LFIA. The self-LFIA positive samples had a median anti-S titer of 1702.0 U mL–1 (interquartile range [IQR] 357.9–7416.0) and a range of 0.40 U mL–1 to 25◦000.0 U mL–1. The self-LFIA negative samples had a median anti-S titer of 142.6 U mL–1 (IQR 46.6–384.0). There were 859 discrepant results with a negative self-LFIA and a positive ECLIA; for these samples the median anti-S titer was 197.6 U mL–1 (IQR 78.9–443.7) indicating that these were weaker positives on average. Of the self-LFIA positive samples with a negative ECLIA (n = 14), the median anti-S titer was 0.4 U mL–1; anti-S titer ranged from 0.4 U mL–1 to 0.75 U mL–1 indicating false positives (Table 2).
Figure 1.
Box plot (median and quartiles) illustrating the distribution of quantitative ECLIA antibody titers by self-LFIA result (N = 3758). Abbreviations: ECLIA, electrochemiluminescence immunoassay; LFIA, lateral flow immunoassay.
Table 2.
Comparison of Results From Paired Self-LFIA and ALFA, and ECLIA (using the Manufacturer’s Threshold of ≥0.8 U mL–1), N = 3758
| Self-LFIA | ECLIA positive N (Median (IQR) Titer) |
ECLIA negative N (Median (IQR) Titer) |
Total N (Median (IQR) Titer) |
|---|---|---|---|
| Positive | 2763 (1715.0; 368.9–7489.0) | 14 (0.4; 0.4–0.4) | 2777 (1702.0; 357.9–7416.0) |
| Negative | 859 (197.6; 78.9–443.7) | 122 (0.4; 0.4–0.4) | 981 (142.6; 46.6–384.0) |
| Total | 3622 (925.4; 207.5–4655.0) | 136 (0.4; 0.4–0.4) | 3758 (824.1; 168.5–4286.0) |
| ALFA | |||
| Positive | 2798 (1566.5; 313.0–7119.0) | 13 (0.4; 0.4–0.4) | 2811 (1541.0; 306.2–7079.0) |
| Negative | 531 (131.6; 63.3–267.3) | 115 (0.4; 0.4–0.4) | 646 (102.7; 24.7–235.7) |
| Total | 3329 (947.4; 201.4–4990.0) | 128 (0.4; 0.4–0.4) | 3457 (831.5; 165.1–4668.0) |
Abbreviations: ALFA, automated image analysis; ECLIA, electrochemiluminescence immunoassay; IQR, interquartile range; LFIA, lateral flow immunoassay.
Table 2 also shows the comparison using the machine-read (ALFA) LFIA results; for samples with a negative ALFA and positive ECLIA, the median anti-S titer was lower than self-LFIA at 131.67 (IQR 63.3–267.3) suggesting that ALFA was better at detecting weaker positives.
Supplementary Table 2 shows the same results calibrated with anti-S thresholds of ≥100 U mL–1, ≥350 U mL–1 and ≥1000 U mL–1.
Live Virus Neutralization
Neutralization assays were performed on 250 randomly selected serum samples, including 167 self-reported positive and 83 self-reported negative LFIA participants.
Live virus neutralization was detected in 169 of 250 samples. The self-LFIA had an estimated sensitivity of 78.7% (133/169; 95% CI: 71.8, 84.6) and specificity of 58.0% (47/81; 95% CI: 46.5, 68.9) using detectable neutralization (equivalent to at least 18.5 BAU mL–1) as the comparator (Table 3). The ALFA-LFIA had an estimated sensitivity of 92.3% (142/155; 95% CI: 86.9, 95.9) and specificity of 45.3% (34/75; 95% CI: 33.8, 57.3) (Table 3). The ECLIA had a sensitivity of 100% (95% CI 97.8, 100.0) and specificity of 0% (95% CI: .0, 4.5) as all neutralization titers <7.1 threshold were positive on the ECLIA (Table 3). All 250 samples remained positive by ECLIA when the anti-S titer threshold was increased to 1000 U mL–1.
Table 3.
Comparison of Results From Self-LFIA and ALFA, ECLIA, and SARS-CoV-2 Neutralization Titer (NT)
| Self- LFIA | NT Positive (Median (IQR) Titer) |
NT Negative (Median (IQR) Titer) |
Total (Median (IQR) Titer) |
Performance (95% CI) |
|---|---|---|---|---|
| Positive | 133 (20.0; 10.0–113.1) | 34 (0.1; 0.1–0.1) | 167 (14.1; 7.1–80.0) | Sensitivity: 78.7 (71.8–84.6) |
| Negative | 36 (10.0;7.1–14.1) | 47 (0.1; 0.1–0.1) | 83 (0.1; 0.1–10.0) | Specificity: 58.0 (46.5–68.9) |
| Total | 169 (20.0;10.0–80.0) | 81 (0.1; 0.1–0.1) | 250 (10.0; 0.1–28.3) | … |
| ALFA | ||||
| ȃPositive | 142 (20.0; 10.0–104.8) | 41 (0.1; 0.1–0.1) | 183 (14.1; 7.1–56.6) | Sensitivity: 91.6 (86.1–95.5) |
| ȃNegative | 13 (10.0;7 .1–14.1) | 34 (0.1; 0.1–0.1) | 47 (0.1; 0.1–7.1) | Specificity: 45.3 (33.8–57.3) |
| ȃTotal | 155 (20.0; 10.0–80.0) | 75 (0.1; 0.1–0.1) | 230 (10.0; 0.1–28.3) | … |
| ECLIA (≥0.8 U mL–1) | ||||
| ȃPositive | 169 (20; 10–80) | 81 (0.1; 0.1–0.1) | 250 (10; 0.1–28.3) | Sensitivity: 100% (97.8–100) |
| ȃNegative | 0 … | 0 … | 0 … | Specificity: 0% (0–4.5) |
| Total | 169 (20; 10–80) | 81 (0.1; 0.1–0.1) | 250 (10; 0.1–28.3) | … |
Neutralization titers of 7.1 have been assigned an arbitrary threshold of 0.1, N = 250 (Self-LFIA) and N = 230 (ALFA).
Abbreviations: ALFA, automated image analysis; CI, confidence interval; ECLIA, electrochemiluminescence immunoassay; IQR, interquartile range; LFIA, lateral flow immunoassay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
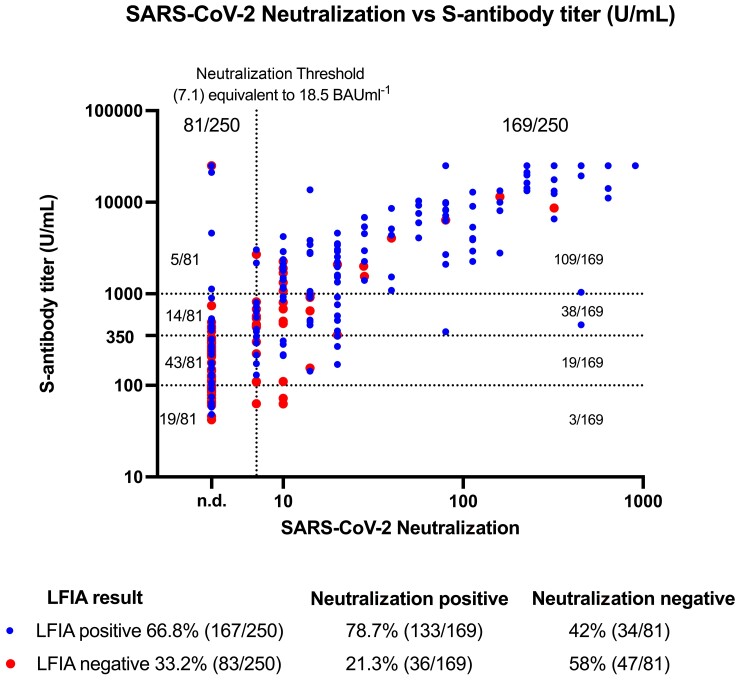
Figure 2 shows the distribution of live virus neutralization titers against anti-S titers, with points labelled for LFIA positive and negative. Neutralization titers were higher in participants with positive compared to negative LFIA results (P < .0001). A similar association was observed for anti-S titers and LFIA result (P < .0001).
Figure 2.
Relationship between SARS-CoV-2 live virus neutralization titer and ECLIA by self-LFIA. Positive self-LFIA results are represented in blue and negative LFIA results are represented in red. The threshold of SARS-CoV-2 neutralization detection is defined as ≥7.1, equivalent to 18.5 BAU mL–1, as denoted by the vertical black dotted line and samples below this are marked as not detected (n.d.) Both axes use a Log 10 scale. ECLIA anti-Spike antibody thresholds of ≥100 U mL–1, ≥350 U mL–1, and ≥1000 U mL–1 are denoted by horizontal dotted lines. Statistical significance is reported by performing a non-parametric Mann-Whitney test for neutralization titers by self-LFIA positive and negative results (P = .0001), and for ECLIA anti-Spike antibody titers by self-LFIA positive and negative results (P = .0001). Abbreviations: ECLIA, electrochemiluminescence immunoassay; LFIA, lateral flow immunoassay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The conversion of neutralization titers to BAU mL–1 following titration of a World Health Organization (WHO) antibody reference standard showed that 34.9% (59/169) of the neutralization positive samples had a titer of ≥100 BAU mL–1 (Supplementary Figure 1).
DISCUSSION
The self-administered LFIA offers a validated qualitative tool that provides a means for obtaining community-wide SARS-CoV-2 antibody positivity prevalence estimates rapidly and at scale, at reasonable cost by adjusting the results for known test performance. The threshold for positivity of the LFIA is higher than that of laboratory-based quantitative assays, producing lower estimates of population antibody prevalence.
Although the LFIA has a threshold that means it does not detect a proportion of positive anti-spike IgG registered on the ECLIA, that threshold is close to the level at which neutralizing antibody can be reliably measured. Indeed, we demonstrated that the estimated specificity of the self-administered self-reported Fortress LFIA against positive neutralization titers was substantially higher than that of the Roche ECLIA with manufacturer’s threshold of 0.8 U mL–1 (58.0% vs 0%). There is evidence that the presence of neutralizing antibodies in serum samples is highly predictive of protection from symptomatic disease following SARS-CoV-2 infection and that declining levels of neutralizing antibody titers correlate with increased risk of symptomatic infection and severe disease [23].
We question the clinical and epidemiological significance of detectable but low antibody titers (post-infection or post-vaccine) picked up by the low thresholds for positivity used for quantitative laboratory assays and suggest that these cutoffs may need to be recalibrated (upward) to be a useful marker of protection from infection and/or severe disease. The LFIA is predominantly missing people with low antibody titers. The implications of a higher threshold for IgG detection on LFIA testing are not yet well understood and may represent an important marker of protection. Wei at al recently explored the association between anti-spike IgG levels and protection from SARS-CoV-2 infection with majority Delta (B.1.617.2) variant in a large representative sample of households with longitudinal follow-up [22]. They showed that protection against infection rose sharply as antibody levels increased in unvaccinated participants with prior infection, with 67% protection at 33 BAU mL–1 using the OmniPATH 384 Combi SARS-CoV-2 IgG ELISA (Thermo Fisher Scientific) assay. Higher antibody levels were required to reach the same level of protection after vaccination, with 67% protection at 107 BAU mL–1 or 94 BAU mL–1 with ChAdOx1 (Oxford-AstraZeneca) or BNT162b2 (Pfizer), respectively [22]. The threshold for determining IgG positivity for the assay used was ≥23 BAU mL–1 [22]. Similarly, Fent et al showed a vaccine efficacy of 80% against symptomatic infection with majority Alpha (B.1.1.7) variant was achieved with 264 BAU mL–1 [21].
Although IgG detection on LFIA or quantitative laboratory-based assays is not designed to document the presence of neutralizing antibodies, these findings suggest that antibody positivity on the LFIA could be useful to measure waning of vaccine induced immunity in the population. This approach would indeed be more useful than quantitative assays with low thresholds for positivity: these could result in false reassurance, as the lower thresholds are not as well associated with positive neutralization titers. Given the strong evidence of a protective role for neutralizing serum antibodies [23, 24], and evidence for correlation between SARS-CoV-2 IgG antibody values and neutralization titers [21], calibrated to the appropriate positivity threshold for protection, rapid antibody testing by LFIA may prove a valuable tool for monitoring the distribution of protective serological antibody responses in the population to inform policy for subsequent vaccination programs, including the targeting of booster vaccines, and could be useful as a screening tool for identifying individuals in the community with below threshold antibody levels who may benefit from further vaccination or other prevention measures or treatment, including anti-viral therapy, as laboratory-based methods may cause a delay in initiating treatment. However, a cost-effectiveness analysis comparing the use of LFIAs to other options for targeting prevention and treatment programs would be required to inform future policy.
Strengths and Limitations
Unlike previous evaluations of the Fortress LFIA, this study replicates the “real-world” application of LFIAs in large-scale population antibody prevalence studies where users are self-administering the test in their own homes following detailed instructions. Therefore, the study authentically explores the accuracy of the Fortress LFIA under the field conditions in which it is most likely to be deployed for surveillance.
Our purposeful sampling strategy of selecting approximately equal numbers of unvaccinated and LFIA negative, double vaccinated and LFIA negative, unvaccinated and LFIA positive, and double vaccinated and LFIA positive may have introduced biases. By purposive selection of vaccinated LFIA negative individuals there is the possibility that we enriched our sample for low level antibody titers that might be less common at population level, thus overall figures on sensitivity cannot be extrapolated to real world use in a random population sample.
We used data from 1 July 2021 to 10 August 2021, that is, while the Delta (B.1.617.2) variant accounted for nearly all cases [25]. Our neutralization assays used a first wave isolate as target, with antigenicity the same as the Wuhan strain. In settings in which Delta is not the dominant variant causing disease, or where neutralization assays use different strains of the virus, the relationships between IgG antibody positivity by LFIA or quantitative anti-S assays and neutralization titers shown here may not apply. Indeed, Wall et al demonstrated neutralizing antibody titers were 5.8-fold lower against Delta relative to the Wuhan variant after 2 doses of BNT162b2 [26]. Neutralizing antibody titers against Omicron (B.1.1.529) have been shown to be 8-fold lower than with Delta after 2 BNT162b2 vaccinations [27]. As such, emerging viral variants might need higher antibody levels for the same level of neutralizing activity [23]. In the case where relationships between antibody levels and levels of protection do not change with other variants and assuming that neutralization is a major mechanism of protection (or that the mechanism of protection remains correlated with neutralization over time), future LFIAs could be calibrated to the appropriate antibody positivity threshold for protection.
CONCLUSION
At-home self-testing and reporting with LFIAs provide a rapid and cost-effective means to assess population antibody prevalence of SARS-CoV-2. In the future, calibrating the threshold for antibody positivity of LFIAs to binding or neutralizing antibody levels correlated with protection from infection and/or severe disease, could provide a valuable role for home-testing by LFIA to inform vaccination and treatment strategies going forward. As a first step it would be important to understand the extent to which a positive LFIA result is predictive of protection against infection, illness, and hospitalization.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank key collaborators on this work— Imperial College London: Eric Johnson and Graham Blakoe. Ipsos: Stephen Finlay, John Kennedy, Duncan Peskett, Sam Clemens and Kelly Beaver; and the REACT Public Advisory Panel. They thank The Huo Family Foundation for their support of our work on COVID-19.
Author contributions. H. W., C. J. A., and G. S. C. conceptualized and designed the study and drafted the manuscript. C. J. A., H. W., M. W., M, M., J. C. B., N. C. K. W., A. A. B., and W. S. B. undertook data collection and data analysis. D. A. provided statistical advice. H. W., G. S. C., W. S. B., P. E., C. A, D., S. R., and A. d. provided study oversight. A. d. and P. E. obtained funding. S. R., R. A. M., A. A. B., D. A., W. S. B., C. A. D., A. d., and P. E. critically reviewed the manuscript. All authors read and approved the final version of the manuscript. H. W. is the guarantor for this paper. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Department of Health and Social Care in England. H. W. is a National Institute for Health Research (NIHR) Senior Investigator and acknowledges support from NIHR Biomedical Research Centre of Imperial College NHS Trust, NIHR School of Public Health Research, NIHR Applied Research Collaborative North West London, and Wellcome Trust (UNS32973). G. S. C. is supported by a National Institute for Health Research (NIHR) Professorship. C. A. D. acknowledges support from the MRC Centre for Global Infectious Disease Analysis (MR/R015600/1), from the UK National Institute for Health Research (NIHR) (grant number PR-OD-1017-20007) and from the UK NIHR Health Protection Research Unit (HPRU) on Emerging and Zoonotic Infections (NIHR200907). C. A. D. is also supported by the Abdul Latif Jameel Institute for Disease and Emergency Analytics. W. S. B. is the Action Medical Research Professor, A. d. is an NIHR senior investigator, and D. A. and P. E. are Emeritus NIHR Senior Investigators. P. E. is Director of the MRC Centre for Environment and Health (MR/L01341X/1, MR/S019669/1). P. E. acknowledges support from the NIHR Imperial Biomedical Research Centre and the NIHR HPRUs in Chemical and Radiation Threats and Hazards and in Environmental Exposures and Health, the British Heart Foundation Centre for Research Excellence at Imperial College London (RE/18/4/34215), Health Data Research UK (HDR UK) and the UK Dementia Research Institute at Imperial (MC_PC_17114).
Contributor Information
Christina J Atchison, School of Public Health, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom.
Maya Moshe, Department of Infectious Disease, Imperial College London, London, United Kingdom.
Jonathan C Brown, Department of Infectious Disease, Imperial College London, London, United Kingdom.
Matthew Whitaker, School of Public Health, Imperial College London, London, United Kingdom.
Nathan C K Wong, Department of Bioengineering, Imperial College London, London, United Kingdom.
Anil A Bharath, Department of Bioengineering, Imperial College London, London, United Kingdom.
Rachel A McKendry, London Centre for Nanotechnology & Division of Medicine, University College London, London, United Kingdom; Division of Medicine, University College London, London, United Kingdom.
Ara Darzi, Imperial College Healthcare NHS Trust, London, United Kingdom; Institute of Global Health Innovation at Imperial College London, London, United Kingdom.
Deborah Ashby, School of Public Health, Imperial College London, London, United Kingdom.
Christl A Donnelly, School of Public Health, Imperial College London, London, United Kingdom; Department of Statistics, University of Oxford, Oxford, United Kingdom; MRC Centre for Global infectious Disease Analysis and Abdul Latif Jameel Institute for Disease and Emergency Analytics, Imperial College London, London, United Kingdom.
Steven Riley, School of Public Health, Imperial College London, London, United Kingdom; MRC Centre for Global infectious Disease Analysis and Abdul Latif Jameel Institute for Disease and Emergency Analytics, Imperial College London, London, United Kingdom.
Paul Elliott, School of Public Health, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom; National Institute for Health Research Imperial Biomedical Research Centre, London, United Kingdom; MRC Centre for Environment and Health, School of Public Health, Imperial College London, London, United Kingdom; Health Data Research (HDR) UK London at Imperial College, London, United Kingdom; UK Dementia Research Institute at Imperial College, London, United Kingdom.
Wendy S Barclay, Department of Infectious Disease, Imperial College London, London, United Kingdom.
Graham S Cooke, Imperial College Healthcare NHS Trust, London, United Kingdom; Department of Infectious Disease, Imperial College London, London, United Kingdom; National Institute for Health Research Imperial Biomedical Research Centre, London, United Kingdom.
Helen Ward, School of Public Health, Imperial College London, London, United Kingdom; Imperial College Healthcare NHS Trust, London, United Kingdom; MRC Centre for Global infectious Disease Analysis and Abdul Latif Jameel Institute for Disease and Emergency Analytics, Imperial College London, London, United Kingdom; National Institute for Health Research Imperial Biomedical Research Centre, London, United Kingdom.
Data Availability
All data underlying the results are available as part of the article and no additional source data are required.
References
- 1. Riley S, Atchison C, Ashby D, et al. REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol. Wellcome Open Res 2020; 5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nature Commun 2021; 12:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward H, Cooke GS, Atchison C, et al. Prevalence of antibody positivity to SARS-CoV-2 following the first peak of infection in England: serial cross-sectional studies of 365 000 adults. Lancet Reg Health Eur 2021; 4:100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ward H, Cooke G, Whitaker M, et al. REACT-2 Round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv 2021:2021.02.26.21252512. [Google Scholar]
- 5. Maple PAC. Population (antibody) testing for COVID-19—technical challenges, application and relevance, an English perspective. Vaccines 2021; 9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flower B, Brown JC, Simmons B, et al. Clinical and laboratory evaluation of SARS-CoV-2 lateral flow assays for use in a national COVID-19 seroprevalence survey. Thorax 2020; 75:1082–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moshe M, Daunt A, Flower B, et al. SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (react 2): diagnostic accuracy study. BMJ 2021; 372:n423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cann A, Clarke C, Brown J, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody lateral flow assay for antibody prevalence studies following vaccination: a diagnostic accuracy study [version 1; peer review: awaiting peer review]. Wellcome Open Res 2022; 6:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies B, Araghi M, Moshe M, et al. Acceptability, usability, and performance of lateral flow immunoassay tests for severe acute respiratory syndrome coronavirus 2 antibodies: rEACT-2 study of self-testing in nonhealthcare key workers. Open Forum Inf Dis 2021; 8:ofab496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atchison C, Pristerà P, Cooper E, et al. Usability and acceptability of home-based self-testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies for population surveillance. Clin Inf Dis 2021; 72:e384–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UK_Government . Coronavirus (COVID-19) latest insights: Antibodies 2022 [Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/antibodies.
- 12. Ward H, Whitaker M, Tang SN, et al. Vaccine uptake and SARS-CoV-2 antibody prevalence among 207,337 adults during May 2021 in England: REACT-2 study. medRxiv 2021:2021.07.14.21260497. [Google Scholar]
- 13. Hendelman T, Chaudhary A, LeClair A, et al. Self-collection of capillary blood using Tasso-SST devices for anti-SARS-CoV-2 IgG antibody testing. PLoS ONE 2021; 16:e0255841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong NCK, Meshkinfamfard S, Turbé V, et al. Machine learning to support visual auditing of home-based lateral flow immunoassay self-test results for SARS-CoV-2 antibodies. Commun Med 2022; 2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Public_Health_England . Evaluation of Roche Elecsys Anti SARS-CoV-2 S serology assay for the detection of anti-SARS-CoV-2 S antibodies 2021 [Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/989460/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_S_assay_PHE.pdf.
- 16. Theel ES, Johnson PW, Kunze KL, et al. SARS-CoV-2 serologic assays dependent on dual-antigen binding demonstrate diverging kinetics relative to other antibody detection methods. J Clin Microbiol 2021; 59:e0123121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakagama Y, Nitahara Y, Kaku N, Tshibangu-Kabamba E, Kido Y. A dual-antigen SARS-CoV-2 serological assay reflects antibody avidity. J Clin Microbiol 2022; 60:e0226221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lukaszuk K, Kiewisz J, Rozanska K, et al. Is WHO international standard for anti-SARS-CoV-2 immunoglobulin clinically useful? medRxiv 2021:2021.04.29.21256246. [Google Scholar]
- 19. Infantino M, Pieri M, Nuccetelli M, et al. The WHO international standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharmacol 2021; 100:108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattiuzzo G, Bentley EM, Hassall M, et al. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody2020; (20 April 2022). Available at: https://www.nibsc.org/documents/ifu/20-268.pdf.
- 21. Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021; 27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei J, Pouwels KB, Stoesser N, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med 2022; 28:1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 24. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. UK_Health_Security_Agency . SARS-CoV-2 variants of concern and variants under investigation in England. 2021 [Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1025827/Technical_Briefing_25.pdf.
- 26. Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021; 397:2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. New Eng J Med 2021; 386:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.