Abstract
Objective
After mild COVID-19, a subgroup of patients reported post–acute-phase sequelae of COVID-19 (PASC) in which exertional dyspnea and perceived exercise intolerance were common. Underlying pathophysiological mechanisms remain incompletely understood. The purpose of this study was to examine outcomes from cardiopulmonary exercise testing (CPET) in these patients.
Methods
In this observational study, participants were patients who were referred for the analysis of PASC after mild COVID-19 and in whom CPET was performed after standard clinical workup turned out unremarkable. Cardiocirculatory, ventilatory, and metabolic responses to and breathing patterns during exercise at physiological limits were analyzed.
Results
Twenty-one patients (76% women; mean age = 40 years) who reported severe disability in physical functioning underwent CPET at 32 weeks (interquartile range = 22–52) after COVID-19. Mean peak O2 uptake was 99% of predicted with normal anaerobic thresholds. No cardiovascular or gas exchange abnormalities were detected. Twenty of the 21 patients (95%) demonstrated breathing dysregulation (ventilatory inefficiency [29%], abnormal course of breathing frequency and tidal volume [57%], absent increase of end-tidal Pco2 [57%], and abnormal resting blood gases [67%]).
Conclusion
Breathing dysregulation may explain exertional dyspnea and perceived exercise intolerance in patients with PASC after mild COVID-19 and can be present in the absence of deconditioning. This finding warrants further study on the levels of neural control of breathing and muscle function, and simultaneously provides a potential treatment opportunity.
Impact
This study contributes to the understanding of persistent exertional dyspnea and perceived exercise intolerance following mild COVID-19, which is vital for the development of effective rehabilitation strategies.
Keywords: Breathing Dysregulation, Cardiopulmonary Exercise Test, COVID-19, Dyspnea, Hyperventilation, Ventilatory Inefficiency
Introduction
Since the outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), coronavirus disease (COVID-19) has been registered in 481 million people, and numbers are still rising.1 Although COVID-19 has been associated with many deaths around the globe, the vast majority of patients recover from the acute phase of the infection.2 It has become clear, however, that a significant proportion is suffering from persisting symptoms (long) after acute illness, imposing new challenges. Long-lasting symptoms after COVID-19, also referred to as long COVID, post–COVID-19 syndrome, or post–acute-phase sequelae of COVID-19 (PASC), are being observed across severity grades of acute COVID-19. In a recent World Health Organization Delphi consensus meeting,3 the post–COVID-19 condition has been defined as symptoms lasting at least 3 months after infection and impacting everyday functioning. Symptoms frequently involve (exertional) dyspnea, exercise intolerance, and fatigue.4
An intriguing subgroup of patients who have PASC and report major symptom burden consists of individuals who had previous relatively mild COVID-19 for which no hospitalization was required. Upon clinical assessment for persisting symptoms, these patients generally have unremarkable findings, including normal lung function and chest imaging, and usually do not have significant comorbidities explaining the clinical picture.5 Much is still unknown about underlying pathophysiological mechanisms of exertional dyspnea and perceived exercise intolerance in these patients. Dyspnea is a complex and often multifactorial symptom that is defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity.”6 The sensation of dyspnea often originates from an increase of respiratory drive and can be caused by increase of respiratory load, decrease in respiratory capacity, or both.7 Interacting systems involved in balanced ventilatory control comprise the cardiovascular system, gas exchange, respiratory muscles, peripheral muscles, and central nervous system.
Cardiopulmonary exercise testing (CPET) is a diagnostic tool that can help to clarify the limiting factor in exercise and may reveal causes of exertional dyspnea. To date, CPET studies in cohorts after COVID-19 primarily focused on patients after hospitalization, including those who required intensive care unit treatment. These studies generally have demonstrated reduced aerobic exercise capacity, possibly attributable to deconditioning or loss of muscle function,8–10 as well as ventilatory inefficiency as factors involved in exercise-related symptoms.8,11,12 Cardiovascular or gas exchange problems appear less frequently involved,8–10 even in those with significant residual pulmonary parenchymal abnormalities.10,13 Inherent to these specific cohorts, hospitalization/intensive care unit–associated factors as well as COVID-19 severity–associated factors are probably involved in explaining part of long-lasting symptomatology and CPET outcomes, which indeed may be different in patients with PASC after mild COVID-19.
CPET data in cohorts with exclusively mild COVID-19 and persisting symptoms are still scarcely reported. In the apparent absence of evidence for cardiopulmonary sequelae, we hypothesized that dysfunctional breathing in this group might explain some of the persistent symptoms. Dysfunctional breathing is a term used to describe an abnormal breathing pattern resulting in dyspnea and other symptoms without a known underlying disease explaining this breathing pattern.14 Its most recognized pattern is hyperventilation syndrome. Other forms, like irregular breathing and thoracic dominant breathing, are recognized, but there is no gold standard for diagnosing dysfunctional breathing.15 Different patterns may coexist, with some patients experiencing symptoms at rest and others only on exertion. CPET is an attractive tool to objectify breathing patterns during exercise and simultaneously allows for ruling out cardiovascular, respiratory, and metabolic causes of exercise intolerance. In the current study, we evaluated CPET outcomes in patients who were post–COVID-19, had not been hospitalized, and had unexplained (exertional) dyspnea and perceived exercise intolerance.
Methods
In this retrospective observational study performed with data from the outpatient post–COVID-19 facility at Radboud University Medical Center,5 Nijmegen, the Netherlands, we analyzed available CPET data from patients who were referred for the analysis of persisting symptoms after having recovered from mild COVID-19 at home. The standard workup consisted of history taking, physical examination, laboratory tests (including hemoglobin, d-dimer, and thyroid-stimulating hormone), pulmonary function tests, chest radiograph, and 6-Minute Walk Test (6MWT). In addition, dyspnea (by the modified Medical Research Council Questionnaire),16 fatigue (by the Checklist for Individual Strength fatigue subscale),17 health status (by the Medical Outcomes Study Short Form-36), smoking status, and comorbidities were recorded. In addition to this standard workup, further evaluation of still unexplained dyspnea or exercise intolerance was conducted with CPET. The CPET was performed on a cycle ergometer (Ergoselect 5P; Ergoline GmbH, Bitz, Germany) according to international standards.18 After 3 minutes of unloaded pedaling at 60 rotations per minute, a ramp protocol increasing load with 15 to 25 W/min was introduced with the aim of achieving maximal workload after 8 to 12 minutes of loaded pedaling. During exercise, gas exchange was measured every 30 seconds breath-by-breath (Vyntus CPX; Vyaire Medical, Chicago, IL, USA) along with arterial blood gas sampling every 3 minutes from a cannula in the radial or brachial artery. Heart rate, O2 saturation, and electrocardiography were continuously measured and combined with measuring blood pressure every 3 minutes. Interpretation strategy for the CPET was based on international standard18 and principles.19 Reference values for maximal O2 uptake ( o2max) according to Gläser20 were chosen. Ventilation was considered inefficient when the relationship between minute ventilation (
o2max) according to Gläser20 were chosen. Ventilation was considered inefficient when the relationship between minute ventilation (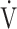 E) and CO2 production until the respiratory compensation point (
E) and CO2 production until the respiratory compensation point (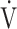 E/
E/ co2 slope) or the ventilatory equivalent of CO2 at the anaerobic threshold (AT) (
co2 slope) or the ventilatory equivalent of CO2 at the anaerobic threshold (AT) ( E/
E/ co2 at AT) was above age- and sex-adjusted upper limits of normal.21
co2 at AT) was above age- and sex-adjusted upper limits of normal.21
We included 21 patients who performed a CPET, reaching physiological limits (maximal heart rate or lactate increase) of exercise. All of these patients had laboratory proof of previous COVID-19 (either polymerase chain reaction at diagnosis or positive SARS-CoV-2 Immunoglobulin G), and none of them were hospitalized for COVID-19. Data collection was part of the POSTCOVERY study5 that was approved by the local medical ethics committee of Arnhem-Nijmegen, the Netherlands (ref. 2020-0660) and was not subject to the Medical Research Involving Human Subjects Act. Data were de-identified and analyzed using SPPS (version 25; IBM SPSS, Chicago, IL, USA). Data are presented as mean (SD) or median (interquartile range [IQR]) as appropriate.
Results
Baseline characteristics of the 21 patients are shown in Table 1. Patients were first consulted at a median of 21 (IQR = 15–44) weeks since onset of COVID-19 symptoms. Severe dyspnea (modified Medical Research Council Questionnaire grade ≥2) was present in 52% of patients. All patients reported Checklist for Individual Strength fatigue subscale scores ≥35, indicating severe fatigue. According to the Medical Outcomes Study Short Form-36, patients experienced poor health status particularly in the domains of physical role functioning, vitality, general health perception, physical functioning, and social functioning. Also, 13 patients reported much worse, 5 reported somewhat worse, 1 reported similar, and 2 reported better health status compared with 1 year ago. The latter 2 had previous COVID-19 > 1 year ago. Four patients (19%) had a diagnosis of mild asthma, 3 (14%) had a history of cardiovascular disease, and 57% had no comorbidities. Hemoglobin and thyroid stimulating hormone were normal in all patients, and d-dimer levels were not indicative of pulmonary embolism (data not shown). Chest radiographs were unremarkable in 19 patients, and the remaining 2 patients had only very minor and clinically nonrelevant abnormalities with uncertain relation to COVID-19. Spirometric indices and lung diffusion capacity were above the lower limits of normal in all but 1 patient with asthma who had very mild airflow obstruction. The mean 6-minute walk distance was >80% of that predicted, with 30% of patients having a 6-minute walk distance <80% of that predicted. Two patients desaturated during the 6MWT, starting at 95% and 98% and reaching 89% and 92% after 2 minutes, after which saturation increased to a stable 92% and 94% throughout the remainder of the test, respectively. Both of these patients were obese, and neither desaturated during subsequent CPET.
Table 1.
Patient Characteristicsa
| Characteristic | Value |
|---|---|
| No. of patients | 21 |
| Women, no. (%) | 16 (76) |
| Age, mean (SD), y | 40 (16) |
| Body mass index, mean (SD), kg/m2 | 28 (6) |
| Smoking history, % | |
| Never/past/current | 71/24/5 |
| Symptoms and quality of life | |
| Dyspnea, mMRC grade | 2 (1, 2) |
| Fatigue, CIS-fatigue total score | 52 (41, 55) |
| SF-36 domain score | |
| Physical functioning | 50 (40, 72) |
| Social functioning | 50 (31, 75) |
| Physical role functioning | 0 (0, 0) |
| Emotional role functioning | 67 (33, 100) |
| Mental health | 76 (62, 80) |
| Vitality | 25 (18, 43) |
| Bodily pain | 69 (44, 94) |
| General health perception | 40 (32, 60) |
| SF-36 Index | 0.59 (0.58, 0.69) |
| SF-36 health transition | |
| Much worse/somewhat worse/about the same/somewhat better/much better (% of patients) | 62/23/5/5/5 |
| Comorbidity, no. (%) of patients | |
| Hypertension | 4 (19) |
| Cardiovascular disease | 3 (14) |
| Type 2 diabetes mellitus | 1 (5) |
| Asthma | 4 (19) |
| Immunocompromised | 2 (10) |
| None | 12 (57) |
| Pulmonary function | |
| VCmax, % of predicted | 99 (10) |
| FEV1, % of predicted | 100 (11) |
| FEV1/VCmax, % | 83 (6) |
| DLCO, % of predicted | 96 (14) |
| KCO, % of predicted | 102 (11) |
| 6-Min walking testb | |
| Distance in m | 534 (63) |
| Distance as % of predicted | 87 (15) |
Data are presented as median (25th percentile, 75th percentile) unless otherwise indicated. CIS-fatigue = Checklist for Individual Strength fatigue subscale; DLCO = diffusion of carbon monoxide in the lung; FEV1 = forced expiratory volume in 1 second; KCO = carbon monoxide transfer coefficient; mMRC = modified Medical Research Council; SF-36 = Medical Outcomes Study Short Form-36; VCmax = maximum vital capacity.
Data for 1 patient were missing.
Cardiopulmonary function at peak exercise is shown in Table 2. The median time between the onset of COVID-19 symptoms and CPET was 32 (IQR = 22–52) weeks. Resting arterial blood gases were abnormal in 14 patients (67%), of whom 9 had acute respiratory alkalosis and 5 had metabolically compensated respiratory alkalosis consistent with acute and chronic hyperventilation, respectively. Mean resting arterial Pco2 was 4.3 (SD = 0.7) kPa. All patients performed a maximal volitional effort up to a maximal heart rate or lactate increase. The mean 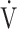 o2 peak was normal, and in only 3 patients was a
o2 peak was normal, and in only 3 patients was a 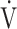 o2 peak marginally below 85% of that predicted observed. Normal aerobic capacity together with a normal AT indicated preserved exercise capacity. Despite a normal
o2 peak marginally below 85% of that predicted observed. Normal aerobic capacity together with a normal AT indicated preserved exercise capacity. Despite a normal  o2 peak, aerobic work efficiency (
o2 peak, aerobic work efficiency (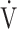 o2/work slope) was on the lower edge of normal, with 8 patients (38%) demonstrating slopes of <8.3 mL/min/W. However, no abnormalities in O2 pulse and blood pressure were observed, and only 1 patient showed electrocardiographic abnormalities during CPET; the latter had no clinical relevance, as concluded after subsequent cardiac analysis. So, cardiac diseases and/or pulmonary vascular disease seemed very unlikely in these patients. Ventilatory limitations, expressed as low breathing reserve, hypercapnia, or increased tidal volume (VT)/vital capacity, were not seen. Gas exchange variables demonstrated no O2 uptake problems (normal Pao2 and gradient of the difference between alveolar and arterial O2 tensions). Mean
o2/work slope) was on the lower edge of normal, with 8 patients (38%) demonstrating slopes of <8.3 mL/min/W. However, no abnormalities in O2 pulse and blood pressure were observed, and only 1 patient showed electrocardiographic abnormalities during CPET; the latter had no clinical relevance, as concluded after subsequent cardiac analysis. So, cardiac diseases and/or pulmonary vascular disease seemed very unlikely in these patients. Ventilatory limitations, expressed as low breathing reserve, hypercapnia, or increased tidal volume (VT)/vital capacity, were not seen. Gas exchange variables demonstrated no O2 uptake problems (normal Pao2 and gradient of the difference between alveolar and arterial O2 tensions). Mean 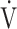 E/
E/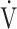 co2 slope and
co2 slope and 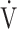 E/
E/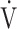 co2 at AT were normal, whereas 6 patients (29%) demonstrated ventilatory inefficiency (Fig. 1A). Without signs of dead-space ventilation, based on a normal physiological dead space/VT and difference between arterial and end-tidal CO2 tensions, inefficient ventilation observed in these patients was most likely related to dysfunctional breathing. Abnormal courses of breathing frequency and VT were seen in 12 patients (57%). Of these, 7 demonstrated irregular patterns, of which 2 were highly erratic (Figs. 1B and C). Another 5 simultaneously increased their VT and breathing frequency (Fig. 1D) instead of the normal pattern of increasing VT prior to increasing breathing frequency. A normal rise in end-tidal Pco2 during the first phase of exercise was absent in 12 patients (57%) (Fig. 2). Overall, 20 of the 21 patients studied (95%) demonstrated 1 or several abnormalities in resting arterial blood gases, ventilatory efficiency, end-tidal Pco2, or breathing patterns consistent with different forms of breathing dysregulation.
co2 at AT were normal, whereas 6 patients (29%) demonstrated ventilatory inefficiency (Fig. 1A). Without signs of dead-space ventilation, based on a normal physiological dead space/VT and difference between arterial and end-tidal CO2 tensions, inefficient ventilation observed in these patients was most likely related to dysfunctional breathing. Abnormal courses of breathing frequency and VT were seen in 12 patients (57%). Of these, 7 demonstrated irregular patterns, of which 2 were highly erratic (Figs. 1B and C). Another 5 simultaneously increased their VT and breathing frequency (Fig. 1D) instead of the normal pattern of increasing VT prior to increasing breathing frequency. A normal rise in end-tidal Pco2 during the first phase of exercise was absent in 12 patients (57%) (Fig. 2). Overall, 20 of the 21 patients studied (95%) demonstrated 1 or several abnormalities in resting arterial blood gases, ventilatory efficiency, end-tidal Pco2, or breathing patterns consistent with different forms of breathing dysregulation.
Table 2.
Cardiopulmonary Exercise Test Variablesa
| Variable | Mean (SD) |
|---|---|
| Performance | |
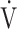 o2 peak, mL/min o2 peak, mL/min |
2065 (523) |
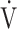 o2 peak, % predicted o2 peak, % predicted |
99 (13)b |
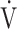 o2 peak/kg, mL/min/kg o2 peak/kg, mL/min/kg |
26.1 (8.3) |
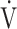 o2/work slope, mL/min/W o2/work slope, mL/min/W |
8.3 (1.0) |
| BORG Scale (Borg CR10) of dyspnea at peak, score | 7 (2)c |
| BORG Scale (Borg CR10) of perceived exertion at peak, score | 7 (2)c |
| Circulation | |
| Peak HR, % predicted | 96 (6) |
| Systolic BP at peak, mm Hg | 178 (21) |
| Diastolic BP at peak, mm Hg | 86 (11) |
| O2 pulse at peak, % predicted | 96 (12)b |
| Ventilation | |
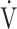 E at peak, L/min E at peak, L/min |
86 (18) |
| Breathing reserve, % | 32 (13) |
| Respiratory frequency at peak, min−1 | 43 (21) |
| VT/VC, % | 51 (6) |
| Gas exchange | |
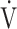 E / E / co2 slope co2 slope |
28 (6) |
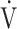 E/ E/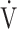 co2 at AT co2 at AT |
28 (4) |
| Pao2 at peak, kPa | 13.2 (0.8) |
| Paco2 at peak, kPa | 4.2 (0.6) |
| P(A − a)o2 at peak, kPa | 2.5 (1.3) |
| VD/VT at peak, % | 13 (6) |
| P(a − ET)co2 at peak, kPa | −0.3 (0.4) |
| Metabolism | |
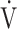 o2 at AT, % predicted o2 at AT, % predicted 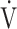 o2max o2max |
62 (11)b |
| RER at peak | 1.20 (0.08) |
| Lactate, mmol/L | |
| At peak | 9.1 (2.3) |
| At 3 min of recovery | 10.1 (2.4) |
AT = anaerobic threshold; BP = blood pressure; HR = heart rate; Paco2 = arterial CO2 tension; Pao2 = arterial O2 tension; P(A − a)o2 = difference between alveolar and arterial O2 tensions; P(a − ET)co2 = arterial–end-tidal CO2 tensions; RER = respiratory exchange ratio; VC = vital capacity; 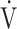 co2 = CO2 production; VD = physiological dead space;
co2 = CO2 production; VD = physiological dead space; 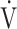 E = minute ventilation;
E = minute ventilation; 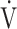 o2 = O2 uptake; VT = tidal volume.
o2 = O2 uptake; VT = tidal volume.
For patients younger than 25 years old, the reference age was set at 25 years (n = 3).
One value was missing.
Figure 1.
Examples of course of minute ventilation (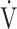 E),
E), 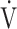 E as a function of CO2 production (
E as a function of CO2 production (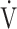 co2), breathing frequency (BF), and inspiratory tidal volume (VTin) as functions of time in patients with increased
co2), breathing frequency (BF), and inspiratory tidal volume (VTin) as functions of time in patients with increased 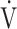 E/
E/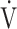 co2 slope (A), irregular breathing pattern (B), erratic breathing pattern (C), and simultaneous increases in BF and VTin (D).
co2 slope (A), irregular breathing pattern (B), erratic breathing pattern (C), and simultaneous increases in BF and VTin (D).
Figure 2.
Three representative examples of course of end-tidal Pco2 (PETCO2) as function of time, illustrating the absence of a normal rise of PETCO2 during exercise in 3 patients with dyspnea and perceived exercise intolerance after mild COVID-19. PETO2 = end-tidal Po2.
Discussion
In our cohort of patients who developed PASC after mild COVID-19 without hospitalization, and without apparent cardiopulmonary sequelae upon regular clinical workup, CPET uncovered different forms of dysregulated breathing as likely explanations for exertional dyspnea and perceived exercise intolerance. Indeed, 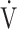 o2max and the AT were normal, arguing against deconditioning as an explanatory factor, and no cardiovascular or gas exchange abnormalities were detected. In order to diagnose dysfunctional breathing, other causes of hyperventilation must be considered. In our cohort, we could rule out exertional hypoxemia, anemia, thyroid disorder, and increased dead-space ventilation. Indeed, the latter argued against pulmonary hypertension, pulmonary embolism, or heart failure.
o2max and the AT were normal, arguing against deconditioning as an explanatory factor, and no cardiovascular or gas exchange abnormalities were detected. In order to diagnose dysfunctional breathing, other causes of hyperventilation must be considered. In our cohort, we could rule out exertional hypoxemia, anemia, thyroid disorder, and increased dead-space ventilation. Indeed, the latter argued against pulmonary hypertension, pulmonary embolism, or heart failure.
Whereas dysfunctional breathing has been described as a condition that can coexist in other respiratory diseases, mainly in obstructive lung diseases,14 we are, to the best of our knowledge, unaware of controlled studies examining breathing dysregulation after other respiratory infections. Our observational data do not allow for a definitive conclusion on Covid-19 specificity, and our finding of breathing dysregulation may very well be one of the shared elements with comparable multisystem postinfectious syndromes. Given the worldwide high prevalence of PASC, this is an opportunity to study this phenomenon in more detail, which could provide insights that may prove relevant for other patient groups as well.
The prevalence of dysfunctional breathing in our cohort is higher than in the general population, which is estimated to be approximately 8%22 and can thus only partially be influenced by female predominance and inclusion of patients with asthma who are more prone to dysfunctional breathing.22,23 Although we lack a control group, our finding is in line with a recent study by Mancini et al,24 who observed dysfunctional breathing in 88% of their studied patients with PASC after mild COVID-19. In contrast to our data, however, they observed overall lower 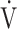 o2max and
o2max and 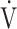 o2 at AT, consistent with deconditioning. It thus appears that dysfunctional breathing in patients with PASC after mild COVID-19 may occur regardless of overall physical condition.
o2 at AT, consistent with deconditioning. It thus appears that dysfunctional breathing in patients with PASC after mild COVID-19 may occur regardless of overall physical condition.
Although it has been well-described that stressful events can elicit a habitual change in breathing pattern,15 which might indeed be part of the explanation why this is observed in some patients after COVID-19, pathophysiological mechanisms underlying dysfunctional breathing in PASC warrant further study on the levels of neural ventilatory control and skeletal muscle metabolic function. Two recent pioneer studies in comparable cohorts after COVID-19 have suggested that altered skeletal muscle mitochondrial function25 and impaired systemic O2 extraction26 are factors to consider. In our cohort, we observed that more than one-third of the patients demonstrated decreased aerobic work efficiency; thus, we cannot completely exclude skeletal muscle abnormalities. Albeit our study lacks invasive measurements to assess systemic O2 extraction or other mitochondrial abnormalities, normal  o2, AT, lactate increase, and the absence of typical hyperdynamic circulatory responses do not point directly to involvement of the peripheral muscles in the main group of patients with signs of dysfunctional breathing. Because we did not systematically assess respiratory muscle strength in our cohort, we also cannot definitively rule out respiratory muscle weakness as a factor involved in dyspnea in PASC. However, considering that clinically relevant respiratory muscle weakness would significantly decrease vital capacity27 and
o2, AT, lactate increase, and the absence of typical hyperdynamic circulatory responses do not point directly to involvement of the peripheral muscles in the main group of patients with signs of dysfunctional breathing. Because we did not systematically assess respiratory muscle strength in our cohort, we also cannot definitively rule out respiratory muscle weakness as a factor involved in dyspnea in PASC. However, considering that clinically relevant respiratory muscle weakness would significantly decrease vital capacity27 and 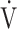 o228 and that neither were observed in our cohort, we argue that respiratory muscle weakness may not be the primary underlying factor.
o228 and that neither were observed in our cohort, we argue that respiratory muscle weakness may not be the primary underlying factor.
Regarding the neural ventilatory control, earlier research identified an increased ventilatory response possibly linked to increased chemosensitivity.26 The CO2 receptor response combined with CO2 production and breathing frequency determine the end-tidal CO2, which physiologically increases during exercise until the first ventilatory threshold.29 In our study, more than one-half of the patients displayed a constant, nonincreasing end-tidal Pco2 during exercise. This pattern, previously reported to be specific for hyperventilation syndrome,30 supports the presence of an increased ventilatory drive in these patients. Distinct assessments such as hypoxic, hyperoxic, and hypercapnic challenge tests and CO2 rebreathing techniques should be explored to conclude in more detail if this ventilatory drive can be attributed to increased chemosensitivity in PASC.
Although our data are observational and were produced from a small sample, our consistent finding of breathing dysregulation corroborates previous studies and collectively provides a physiological base for further studies into this phenomenon in patients with PASC. Also, it provides a potential treatment opportunity. Breathing relaxation strategies might be helpful for some, whereas others may require rebreathing therapy, which has, for example, resulted in decline of symptoms in patients with asthma and idiopathic hyperventilation.31,32 Whether such treatment modalities are also beneficial in PASC, and whether clinically relevant outcomes can be reached when administered as a stand-alone intervention or require integration into multisystem rehabilitation, should be further studied. Although CPET has proven a useful tool in the assessment of breathing dysregulation, one should consider its laborious setup and costs in the context of the high prevalence of PASC as well as the observation that exercising at physiological limits may exacerbate symptoms in these patients, also referred to as postexercise malaise.33,34 Alternatively, although less sensitive, questionnaires or tools such as the Nijmegen questionnaire,35 the breathing pattern assessment tool,36 or manual assessment of respiratory motion37 may prove useful for assessment and evaluation of treatment progress in daily post–COVID-19 practice.
In conclusion, breathing dysregulation may explain exertional dyspnea and perceived exercise intolerance in patients with PASC after mild COVID-19 and can be present in the absence of deconditioning. Underlying pathophysiological mechanisms warrant further exploration on the levels of neural ventilatory control and muscle function.
Contributor Information
Esther L van Voorthuizen, Department of Pulmonary Diseases, Radboud University Medical Center Nijmegen, the Netherlands.
Hanneke A C van Helvoort, Department of Pulmonary Diseases, Radboud University Medical Center Nijmegen, the Netherlands.
Jeanette B Peters, Department of Pulmonary Diseases, Radboud University Medical Center Nijmegen, the Netherlands.
Michel M van den Heuvel, Department of Pulmonary Diseases, Radboud University Medical Center Nijmegen, the Netherlands.
Bram van den Borst, Department of Pulmonary Diseases, Radboud University Medical Center Nijmegen, the Netherlands.
Author Contributions
Concept/idea/research design: E.L. van Voorthuizen, H.A.C. van Helvoort, B. van den Borst
Writing: E.L. van Voorthuizen, H.A.C. van Helvoort, J.B. Peters, M.M. van den Heuvel, B. van den Borst
Data collection: E.L. van Voorthuizen, H.A.C. van Helvoort, J.B. Peters, B. van den Borst
Data analysis: E.L. van Voorthuizen, H.A.C. van Helvoort, B. van den Borst
Project management: J.B. Peters, M.M. van den Heuvel, B. van den Borst
Providing participants: E.L. van Voorthuizen, B. van den Borst
Providing facilities/equipment: M.M. van den Heuvel
Consultation (including review of manuscript before submitting): H.A.C. van Helvoort, J.B. Peters, M.M. van den Heuvel
Acknowledgments
The authors are grateful to all clinical and scientific team members of the COVID-19 aftercare facility and pulmonary function laboratory at Radboud University Medical Center.
Funding
There are no funders to report for this study.
Ethics Approval
Data collection was part of the POSTCOVERY study that was approved by the local medical ethics committee of Arnhem-Nijmegen, the Netherlands (ref. 2020-0660), and was not subject to the Medical Research Involving Human Subjects Act.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. WHO . WHO Coronavirus Disease (COVID-19) Dashboard: World Health Organization; 2022. Accessed March 30, 2022. https://covid19.who.int/. [Google Scholar]
- 2. Borst B. Recovery after Covid-19. Lancet Reg Health West Pac. 2021;12:100208. 10.1016/j.lanwpc.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO . A Clinical Case Definition of Post Covid-19 Condition by a Delphi Consensus. Geneva: World Health Organization; 2021. [Google Scholar]
- 4. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6:e005427. 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020;73:e1089–e1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mioxham J, Jolley C. Breathlessness, fatigue and the respiratory muscles. Clin Med (Lond). 2009;9:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skjørten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58:2100996. 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clavario P, De Marzo V, Lotti R, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. 2021;340:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francesco Rinaldo R, Mondoni M, Maria Parazzini E, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58:2100870. 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorelli G, Braggio M, Gabbiani D, et al. Importance of cardiopulmonary exercise testing amongst subjects recovering from COVID-19. Diagnostics (Basel). 2021;11:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Debeaumont D, Boujibar F, Ferrand-Devouge E, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther. 2021;101:pzab099. 10.1093/ptj/pzab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blokland IJ, Ilbrink S, Houdijk H, et al. Exercise capacity after mechanical ventilation because of COVID-19: cardiopulmonary exercise tests in clinical rehabilitation. Ned Tijdschr Geneeskd. 2020;164:D5253. [PubMed] [Google Scholar]
- 14. Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev. 2016;25:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ionescu MF, Mani-Babu S, Degani-Costa LH, et al. Cardiopulmonary exercise testing in the assessment of dysfunctional breathing. Front Physiol. 2020;11:620955. 10.3389/fphys.2020.620955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. [DOI] [PubMed] [Google Scholar]
- 17. Vercoulen JH, Swanink CM, Fennis JF, Galama JM, Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–392. [DOI] [PubMed] [Google Scholar]
- 18. American Thoracic Society . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 19. Wasserman K, Hansen J, Sue D, Whipp B, Casaburi R. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 4th ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 20. Gläser S, Ittermann T, Schäper C, et al. The Study of Health in Pomerania (SHIP) reference values for cardiopulmonary exercise testing. Pneumologie. 2013;67:58–63. [DOI] [PubMed] [Google Scholar]
- 21. Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. [DOI] [PubMed] [Google Scholar]
- 22. Thomas M, McKinley RK, Freeman E, Foy C, Price D. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J. 2005;14:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lum LC. Hyperventilation: the tip and the iceberg. J Psychosom Res. 1975;19:375–383. [DOI] [PubMed] [Google Scholar]
- 24. Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC: Heart Failure. 2021;9:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boer E, Petrache I, Goldstein NM, et al. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am J Respir Crit Care Med. 2022;205:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laveneziana P, Albuquerque A, Aliverti A, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53:1801214. 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 28. Berton DC, Gass R, Feldmann B, et al. Responses to progressive exercise in subjects with chronic dyspnea and inspiratory muscle weakness. Clin Respir J. 2021;15:26–35. [DOI] [PubMed] [Google Scholar]
- 29. Bussotti M, Magrì D, Previtali E, et al. End-tidal pressure of CO2 and exercise performance in healthy subjects. Eur J Appl Physiol. 2008;103:727–732. [DOI] [PubMed] [Google Scholar]
- 30. Brat K, Stastna N, Merta Z, Olson LJ, Johnson BD, Cundrle I Jr. Cardiopulmonary exercise testing for identification of patients with hyperventilation syndrome. PLoS One. 2019;14:e0215997. 10.1371/journal.pone.0215997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Research. 2015;1:00001–02015. 10.1183/23120541.00001-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas M, McKinley RK, Freeman E, Foy C, Prodger P, Price D. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax. 2003;58:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Physiotherapy . World Physiotherapy response to COVID-19 Briefing Paper 9. Safe rehabilitation approaches for people living with long COVID: physical activity and exercise. World Physiotherapy, London, UK; 2021. Accessed January 6, 2022. https://world.physio/covid-19-information-hub/long-covid. [Google Scholar]
- 34. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact med. EClinical Medicine. 2021:38:101019. 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29:199–206. [DOI] [PubMed] [Google Scholar]
- 36. Todd S, Walsted ES, Grillo L, Livingston R, Menzies-Gow A, Hull JH. Novel assessment tool to detect breathing pattern disorder in patients with refractory asthma. Respirology. 2018;23:284–290. [DOI] [PubMed] [Google Scholar]
- 37. Dixhoorn J, ed. A method for assessment of one dimension of dysfunctional breathing: distribution of breathing movement. In: Biological Psychology. Netherlands: Elsevier Science; 2004; 415–416. [Google Scholar]




