Abstract
Electronic case reporting (eCR) is the automated generation and transmission of case reports from electronic health records to public health for review and action. These reports (electronic initial case reports: eICRs) adhere to recommended exchange and terminology standards. eCR is a partnership of the Centers for Disease Control and Prevention (CDC), Association of Public Health Laboratories (APHL) and Council of State and Territorial Epidemiologists (CSTE). The Minnesota Department of Health (MDH) received eICRs for COVID-19 from April 2020 (3 sites, manual process), automated eCR implementation in August 2020 (7 sites), and on-boarded ∼1780 clinical units in 460 sites across 6 integrated healthcare systems (through March 2022). Approximately 20 000 eICRs/month were reported to MDH during high-volume timeframes. With increasing provider/health system implementation, the proportion of COVID-19 cases with an eICR increased to 30% (March 2022). Evaluation of data quality for select demographic variables (gender, race, ethnicity, email, phone, language) across the 6 reporting health systems revealed a high proportion of completeness (>80%) for half of variables and less complete data for rest (ethnicity, email, language) along with low ethnicity data (<50%) for one health system. Presently eCR implementation at MDH includes only one EHR vendor. Next steps will focus on onboarding other EHRs, additional eICR data extraction/utilization, detailed analysis, outreach to address data quality issues, and expanding to other reportable conditions.
Keywords: public health informatics, public health reporting, COVID-19, electronic case reporting, standards, interoperability
INTRODUCTION
The COVID-19 pandemic highlighted the lack of a robust public health infrastructure resulting in deficit of timely and complete data. It also brought to light the provider burden of reporting to public health amplified by inefficient reporting mechanisms. Electronic case reporting (eCR), the automated generation and transmission of case reports from electronic health records (EHRs) to public health agencies1 (PHAs) was implemented to minimize this provider burden. eCR is a partnership of the Centers for Disease Control and Prevention (CDC),1 Association of Public Health Laboratories (APHL),2 and Council of State and Territorial Epidemiologists (CSTE).3 A national initiative, eCR Now for COVID-194 was launched to facilitate rapid deployment along with centralized infrastructure support and technical assistance. This eCR initiative was promoted nationally with increase in participation by provider sites and public health across the United States for COVID surveillance.5 The Minnesota Department of Health (MDH)6 is one of the state PHAs that implemented eCR and utilized data to bolster COVID-19 surveillance.
The potential of eCR is recognized by the recent health information technology (HIT) regulations. Starting January 2022, eCR is required by the Centers for Medicare and Medicaid Services (CMS) Promoting Interoperability Program (PIP)7 for eligible hospitals and critical access hospitals, and the Merit-Based Incentive Payment System (MIPS)8 Promoting Interoperability Performance Category for eligible clinicians. Current large-scale health policies with HIT implications (Promoting Interoperability,7,8 Cures Act,9 CARES Act10) and the National Academy of Medicine recommendations11 underscore the need for robust information systems that are interoperable and support the full breadth of today’s electronic healthcare ecosystem. A report by CSTE12 advocated for a “public health data superhighway” based on a core public health data infrastructure that supports efficient, standards-based, and electronic data exchange and eCR is one of the key components.
Prior studies on electronic data exchanges in public health have focused on electronic laboratory reporting (ELRs),13–15 immunization reporting,16,17 and interoperability across EHRs and immunization information systems.18–21 Studies on case reporting by Dixon et al and team22 have compared laboratory and provider reports submitted to a large county health department, evaluated the role of a health information exchange (HIE) in auto-populating provider reports,23 and examined notifiable condition reporting practices in clinical care settings.24,25 Reporting of public health notifiable conditions was piloted based on earlier version of national standards (HL7 v2.5) more than a decade back.26,27 Two recent studies28,29 examined the role of informatics and standardized codes in automated trigger and transfer of notifiable conditions (specifically sexually transmitted diseases) and demonstrated the benefits to both providers and public health in decreasing the burden while increasing timeliness and completeness. Common themes are the burden of reporting to public health by providers and the need for HIT solutions to increase timeliness and completeness of public health reporting.
None of the above mentioned case reporting studies utilized the national centralized technical infrastructure integral to the eCR Now Initiative. The foundation for eCR was laid many years ago with the efforts of Public Health Tiger Team by the Office of the National Coordinator for Health Information Technology (ONC) and the Public Health Community Platform initiatives by the Association of State and Territorial Health Officials (ASTHO).30 The Digital Bridge,31,32 a forum for experts across healthcare, public health, and HIT industry to advance standards-based information exchange across public health and health care laid the foundation for the eCR Now and helped incubate eCR developed from prior efforts. The potential of centralized eCR approach and insights on earlier process are outlined by Mackenzie et al33 and Staes et al.34 The implementation of HL7v3/CDA based standards,35 shared services infrastructure, national collaborative model of implementation including healthcare providers, EHR vendors, and PHAs and automation of case reporting are emerging topics which need to be studied. The implementation process, early results, and lessons learned from MDH eCR implementation are shared to assist other PHAs in their eCR journey and also to utilize lessons learned for future eCR enhancements and public health interoperability projects.
METHODS
The implementation of eCR is a multiorganizational endeavor with CDC, APHL, and CSTE leading the eCR implementation with healthcare organizations, EHR vendors, and PHAs. The lead PHA in MN (MDH)36 is the receiving entity, with reporting provider entity as sender and the AIMS platform (APHL Informatics Messaging Service)2 as the intermediary. The Minnesota Electronic Disease Surveillance System (MEDSS)37 is the information system for case management of all notifiable infectious diseases in Minnesota including COVID-19. This detailed schema of the players and processes is depicted in Figure 1.
Figure 1.
eCR infrastructure at MDH depicting a Public Health Agency Perspective.
eCR refers to the process of the automated generation and transmission of case reports from reporter (providers/EHRs) to PHAs (eg, MDH). The electronic initial case report (eICR) is a consensus-based Health Level Seven International (HL7) standard (HL7 CDA for exchange).35 The eICR includes standardized terminologies for representation of reportable disease codes, test orders, and test results (SNOMED, LOINC, ICD-10CM, CVX, RxNorm).2 The centralized decision logic engine (RCKMS: Reportable Conditions Knowledge Management System)3 provides an authoring interface for public health jurisdictions to tailor rules that meet their reportability criteria, while allowing other eICRs to be reported to other jurisdictions using different criteria. The eCR informatics team at MDH authored rules in RCKMS in collaboration with epidemiologists who are leading the surveillance efforts. The eICR processing at MDH is depicted on the right end of the schema in Figure 1. The standardized xml (HL7 CDA) received by MDH needs to be processed for use in public health surveillance.
Upon receipt of the eICR data, the xml is parsed for needed demographic information (first name, last name, date of birth, gender, address, phone number) to match it with a corresponding individual event/case in the disease surveillance system (MEDSS) or create a new record. The accompanying Reportability Response (RR) is processed to locate the reportability code which identifies the disease/diseases being reported. This code is mapped to relevant disease codes in MEDSS to assign the incoming eICR data to the appropriate disease program. The entire eICR xml to then converted to html using standard stylesheets and attached to the corresponding event/case for easy viewing by epidemiologists. Workflows were created in MEDSS to facilitate disease-specific screening of eICRs which were used by epidemiologists for review.
As the implementation progressed additional data elements were parsed out of eICR xml (additional phone numbers, race, ethnicity, language, reporting provider, reporting site/system). In addition, the eICR xmls and RR xmls are copied into a data lake external to MEDSS system with access to visualization tools to facilitate analysis and reporting. Analysis of completeness of select demographic data (gender, race, ethnicity, email, phone, language) was completed using tools (SQL, Tableau, Excel) and was parsed by type of encounter (ambulatory, emergency, inpatient) and by reporting health system to understand variability in data quality and to address completeness issues as needed.
RESULTS
The MDH received eICR for COVID-19 from April 2020 (3 sites; manual upload of files to new events/existing records in MEDSS) and implemented eICR automation (system matching of eICRs and attaching to existing records or creation of new records) in August 2020 (7 sites). Currently ∼1780 clinical units in 460 clinical sites across 7 integrated healthcare systems are on-boarded (as of March 2022) characterizing the quick adoption of COVID-19 eCR process. MDH has the capability to receive eICRs for Minnesota residents from Minnesota and other jurisdictions across the United States that have implemented eCRs based on reportability. More than 20 000 eICRs were submitted monthly to MDH during months with high reporting volume (Figure 2). The variation in volume is representative of additional provider sites implementing COVID-19 eCRs along with shifting pandemic case counts. This reporting is representative of only systems that use Epic EHRs due to streamlined on-boarding set up by CDC/APHL/CSTE in collaboration with the EHR vendor.
Figure 2.
Volume of eICRs received at the Minnesota Department of Health.
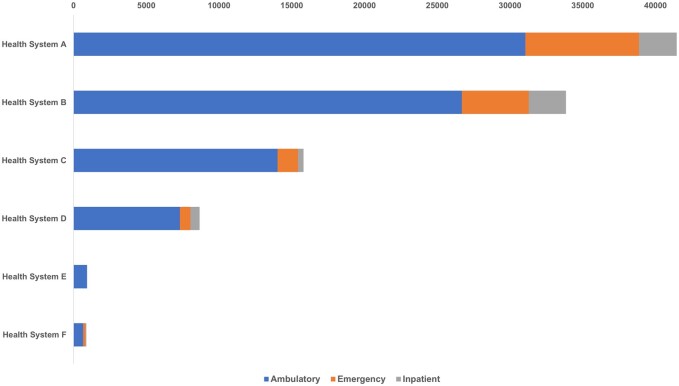
Figure 3 displays the details of the eICRs received from the health systems based on the encounter in which it was triggered (ambulatory, emergency, inpatient). Health system A, one of the largest healthcare system in the state, generated a total of 41 598 eICRs from ambulatory (31 056), emergency (7815), inpatient (2588), and other visits (139) for the time period January–March 2022.
Figure 3.
eICRs reported by health system and care setting for January–March 2022.
Table 1 presents results of evaluation of data quality (completeness) for select demographic variables (gender, race, ethnicity, email, phone, language) by types of encounter (ambulatory, emergency, and inpatient). It displays data by 6 eCR reporting health systems (Health system E is an ambulatory provider only) and color-coded (green >80%, yellow 50%–80%, and red <50%). This revealed a high proportion of completeness (>80%) for half of variables (gender, race, telephone) and rest (ethnicity, email, language) with less complete data (<80% for email) and low ethnicity completeness (<50%) for one health system.
Table 1.
Data completeness for select demographics by encounter across health systems

|
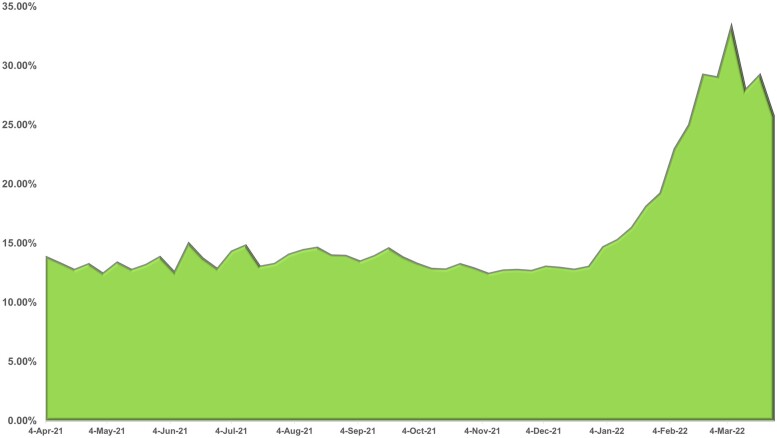
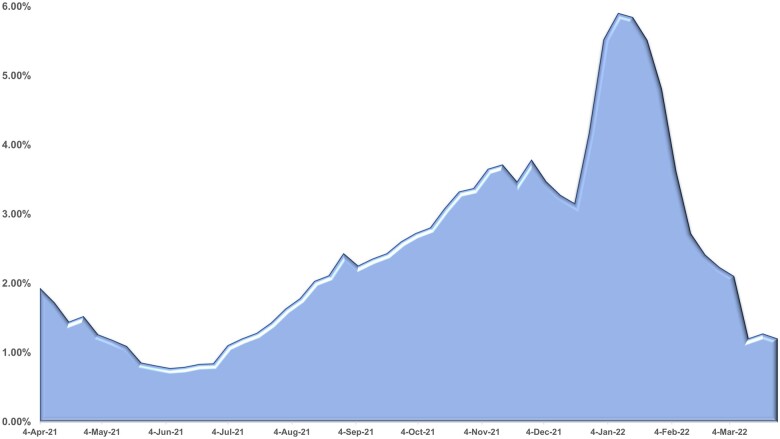
Figures 4 and 5 are based on weekly counts of eICRs received at MDH compared to weekly case count including only COVID-19 positive (Figure 4) and weekly case events (Figure 5) which includes both positive and negative results for COVID-19. Figure 4 portrays the contribution of eICRs to COVID surveillance. As more health systems implemented eCRs, the proportion of confirmed cases that had an eICR attached increased to almost one-third (30%) of cases during March 2022. Figure 5 depicts all COVID events in MEDSS with an eICR attached. The percentage is significantly lower due to the large number of COVID events created by negative lab reports. The peak of January 2022 (5.69%) aligns with the pandemic picture in that time period which led to increased volume of eICRs followed by review of all eICRs (positive and negative) by epidemiologists. Numerous criteria (eg, problem list, diagnosis, lab order, lab results both positive and negative) and their combinations are utilized to trigger eICRs in near real-time based on the Reportable Conditions Trigger Codes (RCTC) implemented in EHRs.38 This can potentially result in eICRs getting triggered if any of the criteria are met and result in high volumes of eICRs. Fortunately, the PHAs can constrain these based on rules that are authored (criteria that are chosen for reporting) in the RCKMS portal. The drop in February 2022 is attributed to editing of rules in RCKMS to constrain reporting of eICRs to include only those with COVID positive lab results.
Figure 4.
Proportion of COVID eICRs matched with positive COVID cases in MEDSS.
Figure 5.
Proportion of COVID eICRs matched to overall COVID events in MEDSS.
Review of time receipts revealed that eICRs were timely (near real-time reporting) when compared to current case reports received through REDCap/excel/faxes followed by manual processing. In addition, eICRs triggered from EHRs based on various criteria were reported earlier as it is done near real-time when compared to ELRs as majority of lab feeds were batch-reported on set times/day. For certain reporting sites with batch-reported ELRs, but had implemented eCR (n = 13), this was the timely source of COVID reporting. Preliminary qualitative assessment with epidemiologists pointed to the value of eICRs. Additional contact information (phone numbers, emails) and contextual information such as care team/care setting, pregnancy, and smoking history allowed for better data collection during case intake for COVID-19 surveillance. Over the course of the implementation, issues encountered were kept track by type of the responsible entity, along with resolution and status and these are presented in Table 2. As of April 2022, 18 key issues have been identified of which 8 (45%) have been resolved. This tracker is expected to be dynamic and be updated as new concerns arise with expansion of implementation with more facilities.
Table 2.
Review and resolution of implementation and data quality issues
| Entity | Issue/need | Resolution | Status |
|---|---|---|---|
| Receiver (MDH) | eICRs not assigned to appropriate disease programs | Map RR codes to MEDSS codes | Completed |
| Epidemiologists need to understand the reason for eICR trigger | Parse RR xml and include reportability criteria for epidemiologist review | Completed | |
| Incoming eICRs tagged as unknown | Fix logic to read RR codes | Completed | |
| Need for eICR data (select fields) for overall disease surveillance | Implement detailed logic to map eICR data to MEDSS along with rules to prevent overwrite of existing data | Completed | |
| Nonparsing and use of critical data in eICRs (eg, death date) | Extract key data extracted and develop rules to present as an alert in individual record | Completed | |
| Inability to track missing data feeds from reporting entities | Create criteria and implement alerts for missing feeds | Completed | |
| Need to identify trends in eICR over time and across health systems | Develop data dashboard with analytic and reporting tools | In progress | |
| Need to parse eICR xmls to include only disease-relevant data in MEDSS | Expand on existing xml parsing with eCR technical support | In progress | |
| Need for additional data elements (eg, occupation) for surveillance | Collaborate with stakeholders for adoption of next standards version | In progress | |
| Intermediary (APHL/AIMS) | Missing display of death date in html | Fix stylesheet to display data | Completed |
| Missing display of multirace in html | Fix stylesheet to display data | Completed | |
| Decision engine (RCKMS) | Need for reporting criteria to be constrained based on diagnosis and/or problem lists | Submit request to update rules | In progress |
| Reporter (providers/EHRs) | Missing Next of Kin info | Submit request to update EHR/eICR template for reporting | In progress |
| Multiple repeats of social history (eg, smoking over years instead of just current status) | Submit request to update EHR/eICR template for reporting | In progress | |
| Multirace not being reported regularly | Need to require data collection in EHRs and reporting | In progress | |
| Potential underreporting if RCTC codes are not updated as needed | Establish a process for on-going provider communication and regular reports on new RCTC codes | In progress | |
| Need for regular checking of RRs to monitor outgoing public health reporting | Establish a process for on-going provider check-ins and emphasize need prior to implementation of eCRs for all notifiable conditions | In progress | |
| Need for streamlined review of RRs to check for additional public health data requests to implement bidirectional loop of eCRs | Establish a process for on-going provider check-ins and emphasize need prior to implementation of eCRs for all notifiable conditions | In progress |
DISCUSSION
The successful implementation of eCR at MDH depended on multitude of factors ranging from on-boarding support and technical assistance provided by CDC, APHL, and CSTE, utilizing shared services across jurisdictions and a national collaborative model of implementation including healthcare providers, EHR vendors, and PHAs. An incremental approach to enhancement enabled MDH to begin receiving eICRs for COVID surveillance as system and staff bandwidth was expanded. The ability to tailor reporting rules based on jurisdictional criteria using the RCKMS authoring portal was critical in addressing the volume of negative reports received during pandemic peaks as shown by Figure 5. As noted in Table 1, the high completeness of data across many demographic variables across encounter types and health systems underscores the potential of eCR to support public health surveillance. This also displays opportunities for improvement (eg, low ethnicity data from a health system) and also points of encounter types with less completeness (eg, ambulatory). One of the limitations is that the eICR data quality is dependent on the EHR data quality and on-going collaboration with healthcare providers is needed to address this issue as noted in next steps for eCR at MDH. Further validation of data is needed to better understand quality (eg, codes such as LOINC and its mapping).
Once a health system initiated production of eICR data, the paper reporting was turned off within a few weeks after review and validation. This has decreased the burden of provider reporting, inefficiencies and inaccuracies from phone/fax/REDCap data entry. This value-add along with Promoting Interoperability Program requirements7 should support adoption of eCRs for public health reporting. The eCRs address the issues with lack of timeliness and completeness of public health data by providing faster case reports and provides additional data (eg, phone number, visit info) for case management/follow-up. In addition, eCRs provide the capability for public health to receive case reports from other states for persons in their jurisdictions due to centralized national infrastructure and customized rules authored in RCKMS by public health. As new modes of testing for infectious diseases (eg, home testing for COVID-19) are introduced and adopted, its implications on reporting and public health surveillance need to be addressed.
A limitation to note in this eCR implementation for COVID-19 at MDH is that all health systems on-boarded to date are organizations on Epic EHRs. Epic EHR is dominant vendor in Minnesota39 and so current experience will facilitate faster on-boarding of future Epic EHR provider sites. An eCR Now Fast Healthcare Interoperability Resources (FHIR) App40 has been made available to increase flexibility for adopters and this utilizes existing national eCR infrastructure. This app can be implemented in EHRs and builds on FHIR API and Argonaut work.40 An eCR Now FHIR App Challenge41 was conducted to encourage adoption and one of the EHR vendors (Cerner Corporation) was awarded in the hospital category. As various EHR vendors declare readiness, there is a need to bring those systems on board.
HIEs have been suggested as options for facilities to connect with the national eCR infrastructure, but limited information exists on this connectivity option. This has not been an issue to date in Minnesota due to the dominance of Epic EHR39 and lack of a centralized HIE entity in the state. Health systems have implemented eCR using the national centralized process which is also supported by Epic EHR and has been efficient. The public health reporting structure in Minnesota is centralized with MDH as the receiving entity42 and local public health departments have access to case data in MEDSS based on their roles and diseases under review. States with different public health reporting processes may face different set of challenges during implementation. Finally current eCR implementation experience is limited to COVID-19 only and future challenges may arise when eCRs are expanded to other infectious diseases and other public heath reportable conditions.
Future phases at MDH will focus on additional data extraction from eCRs (eg, additional contact info such as phone numbers, current/prior address, medications relevant to reportable condition, vaccinations, occupation, travel, social history) as these data are not available through ELRs and are a value-add to public health surveillance. Next steps will also comprise of onboarding other EHRs, detailed evaluation, and expanding eCRs beyond COVID-19 to include all reportable conditions to public health. Some data elements (eg, race, ethnicity) in eCR need to be prioritized and assessed for their utility in contributing to health equity efforts in the agency. Continued progress will require ongoing collaboration between reporters (healthcare providers, EHR vendors), intermediaries (APHL, RCKMS teams), and receivers (PHAs) to address current issues (Table 2) including data quality challenges and future concerns. The alignment of eCR with CDC’s Data Modernization Initiative (DMI)43 is critical for ongoing allocation of resources to sustain success and expand eCR efforts. An informatics-savvy workforce is another vital component and there is a need to establish partnership with the Public Health Informatics and Technology (PHIT) workforce training programs funded and supported by the ONC.44,45
The eCR Now initiative has been implemented nationally with more than 13 300 facilities (as of June 24, 2022) sending COVID-19 eICRs to PHAs.5 The centralized framework and infrastructure along with scalability and a collaborative approach, combined with technical assistance to reporters and receivers have proven to be vital in meeting both provider and public health needs. Lessons learned from implementing eCR for COVID-19 can be applied to eCRs for all reportable conditions and to future public health informatics projects. Collaboration amongst PHAs is needed to share best practices, challenges encountered, and potential solutions. Additional technical assistance will be needed in certain jurisdictions for eCR implementation. The eCR journey at MDH is being shared to assist other PHAs as they plan and implement eCRs, and to utilize lessons learned for future eCR enhancements. Additional details on current eCR implementation (costs, technology, staff expertise) and future eCR enhancements (more EHR vendors, reportable conditions beyond COVID-19, detailed data quality analysis) need to be disseminated and focused research on identified issues is required. Recent reports12 and commentaries46–48 along with strategies for achieving Public Health 3.049,50 have underscored the importance of a robust public health information infrastructure and eCR holds promise as a solution. eCR is a good complement to ELRs for public health reporting and presents a great opportunity to strengthen state-based public health surveillance which in turn sets the stage for a strong national surveillance.
FUNDING
This work was supported by funding from CDC through the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC) cooperative agreement. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
AUTHOR CONTRIBUTIONS
SR led the drafting of the manuscript and all authors read and approved the final version. All authors (SR, AK, AlR, JC, MP, TH, AnR, MH, EE, ADS, and SS) are involved with eCR implementation at the Minnesota Department of Health and collaborated in the writing process.
ACKNOWLEDGMENTS
The authors express gratitude to the eCR onboarding team at CDC, eCR technical experts at APHL, eCR subject matter experts at CSTE, and to the RCKMS content/technical team for their assistance. The authors also thank Sam Martin, contractor from Lyniate assigned to support eCR implementation at MDH and the Minnesota IT (MN.IT) services team across messaging services and system administration at MDH.
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Sripriya Rajamani, Informatics Program, School of Nursing, University of Minnesota, Minneapolis, Minnesota, USA; Institute for Health Informatics, University of Minnesota, Minneapolis, Minnesota, USA; Minnesota Department of Health, Saint Paul, Minnesota, USA.
Ann Kayser, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Ali Ruprecht, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Jacqueline Cassman, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Megan Polzer, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Teri Homan, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Ann Reid, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Melinda Hanson, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Emily Emerson, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Aasa Dahlberg Schmit, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Sarah Solarz, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Data Availability
Data (eCRs reported to public health and analysis by cases/events and by health systems) cannot be shared for ethical/privacy reasons.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC). Electronic Case Reporting (eCR). 2020. https://www.cdc.gov/ecr/index.html. Accessed April 4, 2022.
- 2. APHL Informatics Messaging Services (AIMS). Electronic case Reporting (eCR). 2019. https://ecr.aimsplatform.org/. Accessed April 4, 2022.
- 3. Council of State and Territorial Epidemiologists (CSTE). Reportable Condition Knowledge Management System (RCKMS), 2020. https://www.rckms.org/. Accessed April 3, 2022.
- 4. Centers for Disease Control and Prevention (CDC). eCR Now: COVID-19 Electronic Case Reporting for Healthcare Providers 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/hcp/eCR-Now-Electronic-Case-Reporting-for-healthcare-providers.pdf. Accessed April 16, 2022.
- 5. Centers for Disease Control and Prevention (CDC). Healthcare Facilities in Production for COVID-19 Electronic Case Reporting. 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/electronic-case-reporting/hcfacilities-map.html. Accessed April 4, 2022.
- 6. Minnesota Department of Health (MDH). MDH Electronic Case Reporting (eCR) 2020. https://www.health.state.mn.us/diseases/reportable/medss/ecr.html. Accessed April 6, 2022.
- 7. Centers for Medicare & Medicaid Services. Promoting Interoperability. 2022. https://www.federalregister.gov/documents/2021/08/13/2021-16519/medicare-program-hospital-inpatient-prospective-payment-systems-for-acute-care-hospitals. Accessed July 23, 2022.
- 8. Centers for Medicare & Medicaid Services. Merit-Based Incentive Payment System. 2022. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part. Accessed July 23, 2022. [DOI] [PMC free article] [PubMed]
- 9. Office of the National Coordinator for Health Information Technology. 21st Century Cures Act 2020. https://www.healthit.gov/curesrule/. Accessed April 9, 2022.
- 10. Healthcare Information and Management Systems Society (HIMSS). CARES Act Provisions for Healthcare and Health IT 2020. https://www.himss.org/news/cares-act-provisions-healthcare-and-health-it. Accessed April 6, 2022.
- 11. National Academy of Medicine. Information Technology Interoperability and Use for Better Care and Evidence: A Vital Direction for Health and Health Care 2016. https://nam.edu/information-technology-interoperability-and-use-for-better-care-and-evidence-a-vital-direction-for-health-and-health-care/. Accessed April 2, 2022.
- 12. Council of State and Territorial Epidemiologists (CSTE). Driving Public Health in the Fast Lane: The Urgent Need for a 21st Century Data Superhighway. 2019. https://resources.cste.org/data-superhighway/mobile/index.html. Accessed January 23, 2021.
- 13. Dixon BE, McGowan JJ, Grannis SJ.. Electronic laboratory data quality and the value of a health information exchange to support public health reporting processes. AMIA Annu Symp Proc 2011; 2011: 322–30. [PMC free article] [PubMed] [Google Scholar]
- 14. Dixon BE, Siegel JA, Oemig TV, Grannis SJ.. Electronic health information quality challenges and interventions to improve public health surveillance data and practice. Public Health Rep 2013; 128 (6): 546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajamani S, Kayser A, Emerson E, Solarz S.. Evaluation of data exchange process for interoperability and impact on electronic laboratory reporting quality to a state public health agency. Online J Public Health Inform 2018; 10 (2): e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merrill J, Phillips A, Keeling J, Kaushal R, Senathirajah Y.. Effects of automated immunization registry reporting via an electronic health record deployed in community practice settings. Appl Clin Inform 2013; 4 (2): 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajamani S, Roche E, Soderberg K, Bieringer A.. Technological and organizational context around immunization reporting and interoperability in Minnesota. Online J Public Health Inform 2014; 6 (3): e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajamani S, Bieringer A, Wallerius S, Jensen D, Winden T, Muscoplat MH.. Direct and electronic health record access to the clinical decision support for immunizations in the Minnesota Immunization Information System. Biomed Inform Insights 2016; 8 (Suppl 2): 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajamani S, Bieringer A, Sowunmi S, Muscoplat M.. Stakeholder use and feedback on vaccination history and clinical decision support for immunizations offered by public health. AMIA Annu Symp Proc 2017; 2017: 1450–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Stockwell MS, Catallozzi M, Camargo S, et al. Registry-linked electronic influenza vaccine provider reminders: a cluster-crossover trial. Pediatrics 2015; 135 (1): e75–82. [DOI] [PubMed] [Google Scholar]
- 21. Stockwell MS, Natarajan K, Ramakrishnan R, et al. Immunization data exchange with electronic health records. Pediatrics 2016; 137 (6): e20154335. [DOI] [PubMed] [Google Scholar]
- 22. Dixon BE, Zhang Z, Lai PTS, et al. Completeness and timeliness of notifiable disease reporting: a comparison of laboratory and provider reports submitted to a large county health department. BMC Med Inform Decis Mak 2017; 17 (1): 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dixon BE, Grannis SJ, Revere D.. Measuring the impact of a health information exchange intervention on provider-based notifiable disease reporting using mixed methods: a study protocol. BMC Med Inform Decis Mak 2013; 13: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Revere D, Hills RH, Dixon BE, Gibson PJ, Grannis SJ.. Notifiable condition reporting practices: implications for public health agency participation in a health information exchange. BMC Public Health 2017; 17 (1): 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dixon BE, Jones JF, Grannis SJ.. Infection preventionists’ awareness of and engagement in health information exchange to improve public health surveillance. Am J Infect Control 2013; 41 (9): 787–92. [DOI] [PubMed] [Google Scholar]
- 26. Rajeev D, Staes CJ, Evans RS, et al. Development of an electronic public health case report using HL7 v2.5 to meet public health needs. J Am Med Inform Assoc 2010; 17 (1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajeev D, Staes C, Evans RS, et al. Evaluation of HL7 v2.5.1 electronic case reports transmitted from a healthcare enterprise to public health. Proc AMIA Annu Symp 2011; 2011: 1144–52. [PMC free article] [PubMed] [Google Scholar]
- 28. Mishra NK, Jellison JB, Hamilton A, Carr JB, Padilla RM, Viator NA.. Leveraging informatics to identify reportable cases: pilot findings on electronic case reporting of Chlamydia and Gonorrhea. J Public Health Manag Pract 2019; 25 (6): 595–7. [DOI] [PubMed] [Google Scholar]
- 29. Whipple A, Jackson J, Ridderhoff J, Nakashima AK.. Piloting electronic case reporting for improved surveillance of sexually transmitted diseases in Utah. Online J Public Health Inform 2019; 11 (2): e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cooney MA, Iademarco MF, Huang M, MacKenzie WR, Davidson AJ.. The public health community platform, electronic case reporting, and the digital bridge. J Public Health Manag Pract 2018; 24 (2): 185–9. [DOI] [PubMed] [Google Scholar]
- 31. Black J, Hulkower R, Suarez W, Patel S, Elliott B.. Public health surveillance: electronic reporting as a point of reference. J Law Med Ethics 2019; 47 (2_Suppl): 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Digital Bridge. Multisite Evaluation Plan: Digital Bridge eCR Implementations. 2018. https://digitalbridge.us/wp-content/uploads/2018/10/Digital-Bridge-eCR-Implementation-Evaluation-Plan-final-report.pdf. Accessed April 20, 2022.
- 33. Mac Kenzie WR, Davidson AJ, Wiesenthal A, et al. The promise of electronic case reporting. Public Health Rep 2016; 131 (6): 742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Staes C, Loonsk J, Turner K, Arzt N, Zarcone-Gagne P. Advancing Electronic Case Reporting (eCR) to Enable Public Health Disease Control and Emergency Response: Getting into the Technical Weeds. AMIA Annu Symp Proc2017; 2017: 336–8.
- 35. HL7 International. HL7 CDA® R2 Implementation Guide: Public Health Case Report, Release 2: The Electronic Initial Case Report (eICR), Release 1, STU Release 1.1—US Realm. 2020. http://www.hl7.org/implement/standards/product_brief.cfm?product_id=436. Accessed March 9, 2022.
- 36. Minnesota Department of Health (MDH). MDH Electronic Case Reporting (eCR) 2021. https://www.health.state.mn.us/diseases/reportable/medss/ecr.html. Accessed April 26, 2022.
- 37. Minnesota Department of Health (MDH). Minnesota Electronic Disease Surveillance System (MEDSS) 2010. https://www.health.state.mn.us/diseases/reportable/medss/index.html. Accessed April 26, 2022.
- 38. APHL Informatics Messaging Services (AIMS). eCR: EHR Implementers—EHR Triggering 2019. https://ecr.aimsplatform.org/ehr-implementers/triggering/. Accessed July 2, 2022.
- 39. Minnesota Department of Health. Minnesota eHealth Initiative, 2019 Report to the Legislature 2019. https://www.health.state.mn.us/facilities/ehealth/legrpt/docs/legrpt2019.pdf. Accessed April 26, 2022.
- 40. Association of Public Health Laboratories (APHL). eCR Now FHIR App. 2022. https://ecr.aimsplatform.org/ecr-now-fhir-app. Accessed April 4, 2022.
- 41. Association of Public Health Laboratories (APHL). eCR Now FHIR App Challenge. 2022. https://ecr.aimsplatform.org/general/ecr-now-covid-19-fhir-app-challenge.php. Accessed July 2, 2022.
- 42. Minnesota Department of Health. Infectious Disease Reporting 2000. https://www.health.state.mn.us/diseases/reportable/. Accessed July 2, 2022.
- 43. Centers for Disease Control and Prevention (CDC). Data Modernization Initiative (DMI) 2022. https://www.cdc.gov/surveillance/surveillance-data-strategies/dmi-investments.html. Accessed April 17, 2022.
- 44. Office of the National Coordinator for Health Information Technology (ONC). Public Health Informatics & Technology (PHIT) Workforce Development Program 2021. https://www.healthit.gov/topic/onc-funding-opportunities/public-health-informatics-technology-phit-workforce-development. Accessed April 17, 2022.
- 45. School of Nursing UoM. University Leading Consortium to Train Underrepresented in Using Data to Improve Public Health 2022. https://nursing.umn.edu/news-events/university-leading-consortium-train-underrepresented-using-data-improve-public-health. Accessed April 17, 2022.
- 46. Firestone MJ, Rajamani S, Hedberg CW.. A public health informatics solution to improving food safety in restaurants: putting the missing piece in the puzzle. Online J Public Health Inform 2021; 13 (1): e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dixon BE, Caine VA, Halverson PK.. Deficient response to COVID-19 makes the case for evolving the public health system. Am J Prev Med 2020; 59 (6): 887–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singletary V, Richards CL Jr, Ross DA, O’Carroll P, Baker EL.. Modernizing our nation’s public health information system: toward an integrated approach. J Public Health Manag Pract 2021; 27 (5): 521–5. [DOI] [PubMed] [Google Scholar]
- 49. DeSalvo KB, Wang YC, Harris A, Auerbach J, Koo D, O’Carroll P.. Public health 3.0: a call to action for public health to meet the challenges of the 21st century. Prev Chronic Dis 2017; 14: E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeSalvo K, Hughes B, Bassett M, et al. Public health COVID-19 impact assessment: lessons learned and compelling needs. NAM Perspect 2021; doi:10.31478/202104c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data (eCRs reported to public health and analysis by cases/events and by health systems) cannot be shared for ethical/privacy reasons.