Abstract
Objective
The current study aimed to investigate the temporal trend of in-hospital and intensive care unit (ICU) mortality of coronavirus disease 2019 (COVID-19) patients over 6 months in the spring and summer of 2021 in Iran.
Design
We performed an observational retrospective cohort study.
Setting
Qazvin Province- Iran during 6 month from April to September 2021.
Participants
All 14355 patients who were hospitalized with confirmed COVID-19 in hospitals of Qazvin Province.
Intervention
No intervention.
Main outcome measures
The trends of overall in-hospital mortality and ICU mortality were the main outcome of interest. We obtained crude and adjusted in-hospital and ICU mortality rates for each month of admission and over surge and lull periods of the disease.
Results
The overall in-hospital mortality, early mortality and ICU mortality were 8.8%, 3.2% and 67.6%, respectively. The trend for overall mortality was almost plateau ranging from 6.5% in July to 10.7% in April. The lowest ICU mortality was 60.0% observed in April, whereas it reached a peak in August (ICU mortality = 75.7%). Admission on surge days of COVID-19 was associated with an increased risk of overall mortality (Odds ratio = 1.3, 95% confidence interval = 1.1, 1.5). The comparison of surge and lull status showed that the odds of ICU mortality in the surge of COVID-19 was 1.7 higher than in the lull period (P-value < 0.001).
Conclusions
We found that the risk of both overall in-hospital and ICU mortality increased over the surge period and fourth and fifth waves of severe acute respiratory syndrome coronavirus 2 infection in Iran. The lack of hospital resources and particularly ICU capacities to respond to the crisis during the surge period is assumed to be the main culprit.
Keywords: patient outcomes, intensive care, infectious diseases, health-care system
Introduction
The coronavirus disease 2019 (COVID-19) pandemic started in the last days of 2019, and for the last 2 years, it has been the leading cause of death due to infection [1]. COVID-19 has led to more than 5 million deaths globally [2]. During the first emergence of the COVID-19 pandemic, the mortality rate among all hospitalized patients and particularly critically ill patients was pretty high [3–5]. Age, male gender, co-exciting disorders and lower socioeconomic status have already been identified as the factors associated with higher COVID-19 mortality in this period [6, 7]. Multiple studies have shown considerable improvement in the survival of these patients after the first disease surge [8–10]. Several factors such as better therapeutic strategies, an increase in health-care capacities and better hospital organization, a change in patient characteristics, an improvement in medical skills and fewer pathogenic viral variants have been highlighted as the primary drivers of this improvement [11]. After three waves of COVID-19 in most developed countries, the number of hospitalized patients and COVID-19-related deaths declined substantially mainly due to widespread public vaccination [12, 13]. In Iran, however, the four and fifth COVID waves occurred in the spring and summer of 2021; COVID-19 turned into an uncontrollable public health challenge across the country when the number of hospitalized patients and COVID-19 mortality reached a peak in August and September [14]. The health system’s inefficient performance in providing COVID-19-approved vaccines, delayed public vaccination, failed national vaccine projects in the country and international sanctions contributed to such circumstances. On the other hand, several studies have shown that surge periods are associated with an increased in-hospital and intensive care unit (ICU) mortality in COVID-19 patients due to a strained health-care system, limited resources and burnout of clinical staff [9, 15].
The current study aimed to investigate the in-hospital and ICU mortality of COVID-19 patients over 6 months in the spring and summer of 2021 in Iran. We examined the temporal trend of COVID-19 mortality adjusted for patients’ profiles to make sure that any changes in in-hospital mortality were not associated with the change in admitted patients’ characteristics.
Methods
Study design and patients
We performed an observational retrospective cohort study on 14 355 patients with COVID-19 hospitalized during April–September 2021 in Qazvin province, Iran. All patients had confirmed COVID-19 diagnosis. A diagnosis of COVID-19 was made based on real-time reverse transcription-polymerase chain reaction testing that was carried out on nasopharyngeal throat swab specimens or based on clinical diagnostic criteria provided by the Ministry of Health in Iran. According to the guidelines, the diagnosis of COVID-19 was made by the presence of any respiratory symptoms including cough, dyspnea, sore throat, congestion, loss of taste and/or smell, in addition to chest X-ray evidence indicating unilateral or bilateral interstitial infiltrations [16]. All recruited patients were over 18 and were hospitalized for at least 48 h in one of the hospitals in Qazvin province. The Qazvin University of Medical Sciences Ethics Committee and Review Board has reviewed and approved the current study. They have also waived the need for informed consent of patients due to the nature of the study.
Data collection and outcome
We collected data on demographic characteristics (age and sex), comorbidities (hypertension, diabetes mellitus, cardiovascular diseases (CVDs), chronic obstructive pulmonary diseases (COPDs), chronic liver diseases, chronic kidney disease and cancer), signs and symptoms (oxygen saturation, temperature, respiratory rate and symptom onset to hospitalization), risk factors (COVID-19 vaccination, tobacco smoking and opium use) and treatments (oxygen therapy, mechanical ventilation, admission in ICU and length of stay (LOS) at the hospital). In-hospital mortality defined as death at discharge time, early mortality (death within the first 5 days of admission) and ICU mortality (death in patients with ICU admission) were the main outcomes of interest in our study. The study duration was categorized into six separate periods based on the month of admission. The surge period of COVID-19 was categorized into the spring surge (April 2021) and the summer surge (August and September 2021), while May, June and July were regarded as lull periods in 2021. This classification was based on the official declaration of the Ministry of Health and Medical Education of Iran. All data were retrieved from the electronic databases of COVID-19 patients provided by the Qazvin University of Medical Sciences as described elsewhere [5].
Statistical analysis
Categorical variables are described using numbers and percentages. Continuous variables are shown as mean and standard deviations (SD) or median and interquartile ranges (IQR), as applicable. The mortality outcomes are presented as proportions and 95% confidence intervals (CI). The chi-squared test was used to examine the difference between dichotomous variables. To assess the difference in means and medians between the compared groups, the t-test and the analysis of variance were used, respectively. The Mann–Whitney U test was employed to compare groups when the data were skewed. Multiple logistic regression was used to examine the association between in-hospital mortality and ICU mortality as outcomes of interest and admission during the surge periods as the independent variable. Age, sex, comorbidities, respiratory rate, type of hospital and oxygen saturation upon admission were included in the model as possible confounders. For the model generation, each variable was entered into a simple regression model, and then the most influential variables (P < 0.1) were selected for multiple models. We generated two distinctive multinomial logistic regression models using the ‘mlogit’ command in Stata. The models were adjusted for age, sex, comorbidities, respiratory rate, type of hospital and oxygen saturation upon admission. The models compared the odds of death in the surge period to those during the lull period adjusted for the same confounding variables. Odds ratio (OR) and associated 95% CI for the surge period were adjusted for the possible confounding variables. We performed all statistical analyses using Stata (Ver. 14.1, StataCrop LLC, College Station, TX, USA).
Results
Changes in patients’ characteristics over the study time
We assessed data on 14 355 adult COVID-19 patients admitted to all hospitals in Qazvin province for 6 months, i.e. from April to September 2021. The average age of the study participants was 55.6 (17.1) years, and patients admitted during the surge period were significantly younger (P-value < 0.001). We observed a significant difference in sex distribution throughout the study (P-value < 0.001). We also observed a higher proportion of all comorbidities in the lull period, and the median score of the Charlson index for patients admitted in the surge period was significantly lower (P-value < 0.001). Regarding symptoms and signs, we found no significant difference in the patient’s temperature, respiratory rate and oxygen saturation. The overall proportion of oxygen saturation <93% was 75.3%. The median of symptom onset to hospitalization was 6 days in patients admitted, and it was significantly higher for patients admitted in the surge period (6.1 days) compared to the lull period (5.8 days) (P-value < 0.001). The overall proportion of patients with tachypnea (respiratory rate > 20) was 30.1%. The overall proportions of tobacco and opium smoking were 2.0% and 1.7%, respectively, and the observed difference between the lull and surge periods was statistically significant (P-value < 0.001). As depicted in Table 1, 10.9% of patients were admitted to ICU during the lull period, while the proportion of ICU admission decreased to 8.9% in the surge period of COVID-19 (P-value< 0.001). We also observed a similar pattern in the proportion of patients receiving mechanical ventilation. The proportion of patients who underwent mechanical ventilation was 7.1% in the lull period, which decreased to 5.5% during the surge period of the disease (P-value < 0.001). We also compared the LOS at the hospital and observed that LOS was significantly higher in the lull period (P-value < 0.001) (Table 1). We also categorized COVID-19 admission centers into three categories, including public educational hospitals, public non-educational hospitals and private hospitals, and provided patient distribution and outcomes in each center (Table 2).
Table 1.
Clinical characteristics of the hospitalized COVID-19 patients
| Lull period | Surge period | Overall | P-value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, median (IQR) | 57.1 (17.5) | 54.5 (16.8) | 55.6 (17.1) | <0.001 |
| Age > 65 years, n (%) | 1755 (35.6) | 2562 (27.1) | 4317 (30.0) | <0.001 |
| Sex, male (%) | 2270 (46.1) | 4211 (44.6) | 6481 (45.1) | <0.001 |
| Comorbidities | ||||
| Hypertension, n (%) | 1284 (26.0) | 1819 (19.2) | 3103 (21.6) | <0.001 |
| Diabetes mellitus, n (%) | 889 (18.0) | 1310 (13.9) | 2199 (15.3) | <0.001 |
| CVD, n (%) | 581 (11.8) | 867 (9.1) | 1448 (10.1) | <0.001 |
| COPD, n (%) | 199 (4.0) | 266 (2.8) | 465 (3.2) | <0.001 |
| Chronic liver disease, n (%) | 26 (0.5) | 28 (0.3) | 54 (0.4) | 0.032 |
| Chronic kidney disease, n (%) | 87 (1.8) | 91 (1.0) | 178 (1.2) | <0.001 |
| Cancer, n (%) | 69 (1.4) | 88 (0.9) | 157 (1.1) | 0.010 |
| Charlson index, mean (SD) | 1.9 (1.7) | 1.5 (1.5) | 1.6 (1.6) | <0.001 |
| Symptoms | ||||
| Symptom onset to hospitalization, mean (SD) | 5.8 (4.2) | 6.1 (3.7) | 6.0 (3.9) | <0.001 |
| Temperature, mean (SD) | 37.0 (1.3) | 37.0 (1.1) | 37.0 (1.2) | 0.354 |
| Respiratory rate > 20, n (%) (%) | 1495 (30.3) | 2831 (30.0) | 4326 (30.1) | 0.670 |
| Oxygen saturation, mean (SD) | 88.7 (7.2) | 88.9 (6.7) | 88.9 (6.9) | 0.123 |
| Oxygen saturation < 93%, n (%) (%) | 3689 (75.1) | 7113 (75.4) | 10 811 (75.3) | 0.673 |
| Risk factors | ||||
| Tobacco smoking, n (%) (%) | 125 (2.5) | 168 (1.7) | 293 (2.0) | <0.001 |
| Opium use, n (%) | 116 (2.3) | 133 (1.4) | 249 (1.7) | <0.001 |
| COVID-19 vaccination, n (%) | 377 (7.6) | 2006 (21.2) | 2383 (16.6) | <0.001 |
| Treatment | ||||
| ICU admission, n (%) | 538 (10.9) | 845 (8.9) | 1383 (9.6) | <0.001 |
| Oxygen therapy, n (%) | 3654 (74.5) | 6584 (70.7) | 10 238 (72.0) | <0.001 |
| Intubation, n (%) | 351 (7.1) | 520 (5.5) | 871 (6.0) | <0.001 |
| LOS, median (IQR) | 6.2 (5.1) | 5.8 (4.4) | 5.9 (4.6) | <0.001 |
| Outcome | ||||
| Early mortality, n (%) | 130 (2.6) | 334 (3.5) | 464 (3.2) | 0.004 |
| ICU mortality, n (%) | 354 (65.8) | 582 (68.8) | 936 (67.6) | 0.233 |
| Intubation mortality, n (%) | 268 (76.3) | 374 (71.9) | 642 (73.7) | 0.145 |
| Overall mortality, n (%) | 420 (8.5) | 845 (8.9) | 1265 (8.8) | 0.388 |
| Overall admission, n (%) | 4924 (100) | 9431 (100) | 14 355 (100) | |
Table 2.
COVID-19 patient’s characteristics and outcome by each type of admitting center
| Hospital types | |||
|---|---|---|---|
| Characteristics | Educational hospitals (N = 10 954) | Non-educational public hospital (N = 2249) | Private sector (N = 1152) |
| Age, median (IQR) | 56.0 (27.0) | 54.0 (25.0) | 57.0 (23.0) |
| Age > 65 years, n (%) | 3357 (30.6%) | 612 (27.2%) | 348 (30.2%) |
| Sex, male (%) | 5017 (45.8%) | 940 (41.8%) | 524 (45.5%) |
| Comorbidities | |||
| Hypertension, n (%) | 2297 (20.9%) | 547 (24.3%) | 259 (22.5%) |
| Diabetes mellitus, n (%) | 1612 (14.7%) | 395 (17.5%) | 192 (16.6%) |
| CVD, n (%) | 1048 (9.5%) | 259 (11.5) | 141 (12.2%) |
| COPD, n (%) | 336 (3.0%) | 90 (4.0%) | 39 (3.4%) |
| Chronic liver disease, n (%) | 35 (0.3%) | 9 (0.4%) | 10 (0.8%) |
| Chronic kidney disease, n (%) | 141 (1.3%) | 25 (1.1%) | 12 (1.0%) |
| Cancer, n (%) | 113 (1.0%) | 24 (1.0%) | 20 (1.7%) |
| Charlson index, mean (SD) | 1.6 (1.6) | 1.6 (1.6) | 1.7 (1.5) |
| Symptoms | |||
| Symptom onset to hospitalization, mean (SD) | 5.6 (3.8) | 7.2 (3.9) | 7.0 (3.8) |
| Temperature, mean (SD) | 37.0 (1.1) | 36.9 (1.3) | 37.1 (1.1) |
| Respiratory rate > 20, n (%) | 3392 (30.9%) | 617 (27.4%) | 317 (27.5) |
| Oxygen saturation, mean (SD) | 88.4 (7.1) | 90.5 (6.2) | 89.6 (5.7) |
| Oxygen saturation < 93%, n (%) | 8670 (79.1%) | 1272 (56.5%) | 869 (75.4%) |
| Risk factors | |||
| Tobacco smoking, n (%) | 201 (1.8%) | 67 (2.9%) | 25 (2.1%) |
| Opium use, n (%) | 183 (1.6%) | 47 (2.1%) | 19 (1.6%) |
| COVID-19 vaccination, n (%) | 1.6 (14.9%) | 379 (16.8%) | 367 (31.8%) |
| Treatment | |||
| ICU admission, n (%) | 1088 (9.9%) | 87 (3.8%) | 208 (18.0%) |
| Oxygen therapy, n (%) | 8895 (82.1%) | 1060 (47.4%) | 283 (24.8%) |
| Intubation, n (%) | 753 (6.8%) | 65 (2.9%) | 53 (4.6%) |
| LOS, median (IQR) | 5.0 (4.0) | 4.0 (3.0) | 5.0 (2.0) |
| Outcome | |||
| Early mortality, n (%) | 385 (3.5%) | 43 (1.9%) | 36 (3.1%) |
| ICU mortality, n (%) | 799 (73.4%) | 54 (62.0%) | 83 (39.9%) |
| Intubation mortality, n (%) | 568 (75.4%) | 36 (55.3%) | 38 (71.7%) |
| Overall mortality, n (%) | 1097 (10.0%) | 80 (3.5%) | 88 (7.6%) |
Mortality trend
The overall in-hospital mortality, early mortality and ICU mortality were 8.8%, 3.2% and 67.6%, respectively. This number was 73.7% for patients who underwent mechanical ventilation. The trend for overall mortality was almost plateau ranging from 6.5% in July to 10.7% in April. The trends for ICU mortality, overall mortality, mechanically ventilated and early mortality over the study period are depicted in Figure 1.
Figure 1.
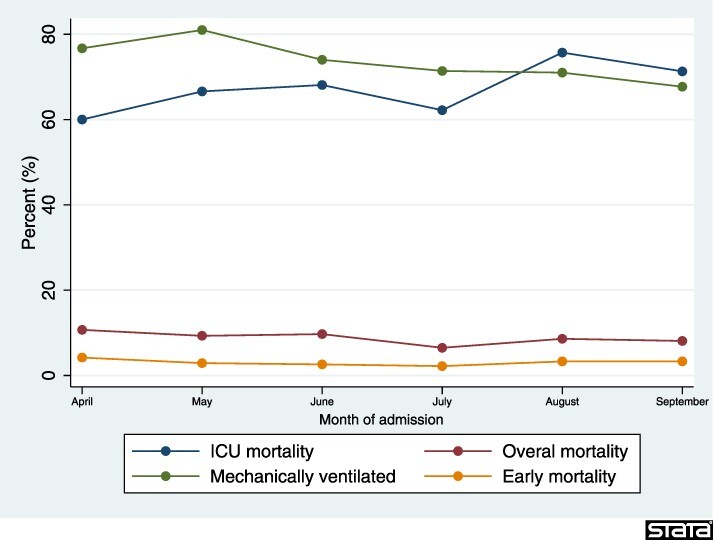
Crude mortality rates trends for patients admitted with COVID-19 in Qazvin province, Iran, stratified by month of admission.
Adjusted in-hospital and ICU mortality and their associated factors
The adjusted overall mortality for surge and lull periods was 9.5% and 7.6%, respectively. According to the multiple logistic regression model, the odds of death in the surge period were significantly higher than in the lull period as the reference category (OR= 1.3, 95% CI= 1.1, 1.5). We performed subgroup analyses and repeated the logistic regression for different age groups and observed that after adjustment for confounding variables, the odds of death in the surge of disease were significantly higher than the lull period in all age groups except in the patients younger than 40 (OR = 1.4, 95% CI= 0.8, 2.5) (Table 3).
Table 3.
Adjusted in-hospital and ICU mortality and prognostic factors associated with overall mortality and ICU mortality in patients admitted with COVID-19 in Qazvin province, Iran
| Overall mortality | ICU mortality | |||||
|---|---|---|---|---|---|---|
| Overall | Point estimate (95% CI) | OR (95% CI) | P-value | Point estimate (95% CI) | OR (95% CI) | P-value |
| Lull | 7.6 (7.5, 7.7) | Reference | 47.8 (47.5, 48.2) | Reference | ||
| Surge | 9.5 (9.4, 9.6) | 1.3 (1.1, 1.5) | <0.001 | 59.1 (58.7, 59.5) | 1.7 (1.3, 2.3) | <0.001 |
| Age < 40 | ||||||
| Lull | 1.8 (1.7, 1.9) | Reference | 28.1 (24.1, 32.2) | Reference | ||
| Surge | 2.6 (2.5, 2.7) | 1.4 (0.8, 2.5) | 0,179 | 48.7 (43.8, 53.6) | 3.8 (1.4, 10.0) | 0.004 |
| Age 40–59 | ||||||
| Lull | 4.8 (3.8, 4.2) | Reference | 53.5 (50.9, 56.0) | Reference | ||
| Surge | 6.1 (5.9, 6.3) | 1.5 (1.1, 2.0) | 0.001 | 66.6 (64.3, 68.9) | 2.0 (1.2, 3.5) | 0.003 |
| Age 60–79 | ||||||
| Lull | 11.1 (10.9, 11.4) | Reference | 67.4 (65.2, 69.6) | Reference | ||
| Surge | 13.6 (13.3, 13.9) | 1.2 (1.0, 1.4) | 0.054 | 76.7 (74.8, 78.5) | 1.9 (1.2, 3.1) | 0.004 |
| Age ≥ 80 | ||||||
| Lull | 20.8 (20.4, 21.2) | Reference | 77.3 (74.8, 79.9) | Reference | ||
| Surge | 25.2 (24.8, 25.7) | 1.3 (1.0, 1.7) | 0.050 | 76.6 (74.1, 79.2) | 0.9 (0.5, 1.8) | 0.564 |
The regression model was adjusted for age, sex, respiratory rate, oxygen saturation, comorbidity (Charlson index) and type of hospital.
The bold values denote statistical significance at 0.05 level, after adjustment for age, sex, oxygen saturation, comorbidity (Charlson index), and type of hospital.
Overall adjusted ICU mortality in the surge and lull periods of the current study was 59.1% and 47.8%, respectively. The comparison of surge and lull periods showed that the odds of ICU mortality in the surge of COVID-19 were 1.7 higher than in the lull period (P-value < 0.001). Subgroup analysis showed that the association was stronger in younger patients, and it was even not statistically significant in patients older than 80 (OR = 0.9, 95% CI= 0.5, 1.8) (Table 3).
Discussion
Statement of principal findings
The current study investigated the trend of overall in-hospital mortality and ICU mortality in patients hospitalized for more than 24 h in Qazvin province over the fourth and fifth waves of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Iran. Admission in the surge of disease was associated with increased odds of death. Adjusted overall mortality in the surge of disease was significantly higher than the lull period (9.5% versus 7.6%). Moreover, the odds of ICU mortality in the surge of COVID-19 was 1.7 times higher than the lull period.
Strength and limitations
This study was one of the few studies to figure out the trends of in-hospital and ICU mortality in COVID-19 patients in a low and middle-income country. We included a comprehensive cohort of all educational and non-educational hospitals assigned to admit COVID-19 patients in one of the provinces of Iran. However, our findings must be interpreted in light of our limitations. The current study was performed on data collected during daily care provided to the admitted patients; therefore, we did not collect variables like laboratory data and treatments. Second, we did not address viral variance since viral sequencing was not part of our study. However, as all the data were collected in 2021, we believe that the viral variant remained stable throughout the study period.
Interpretation within the context of the wider literature
According to the previous studies, in-hospital mortality in hospitalized COVID-19 patients downed to half over the time in countries like the USA, UK and Spain [8, 10, 11].
A couple of different scenarios including improvement in the expertise of health workers, better health care and improvement in the provided treatments, increase in hospital admission, different patient characteristics and the attenuated variant of the virus have been developed to explain the observed improvement in survival of hospitalized COVID-19 patients [8, 11]. According to our data, changes in the admitted patient characteristics were the major driving factor of the downward trend in COVID-19 mortality. Admitted patients in the lull period were significantly younger with a lower proportion of co-existing disorders. As shown in the adjusted analysis, in-hospital adjusted mortality exceeded 9.0% in the surge period, and the odds of death were 1.39 times higher in comparison to the lull period.
The increases in hospital census and ICU overload, resulting in impaired care, may have been the main factors leading to higher in-hospital mortality rates. A similar increase in in-hospital mortality was reported in other developing countries, such as South Africa [17], which contrasted with the earlier studies in developed countries [11]. Over the COVID-19 surges, the hospitals included in our study were unable to increase our ICU capacity as required, while more developed countries doubled theirs over the COVID-19 waves [11]. As shown in our data, the lowest proportion of ICU admissions, mechanical ventilation and oxygen therapy occurred in August and September when hospital admissions were at their highest. We found that the number of hospitalized patients in August and September 2021 was almost double that of the lull period in June and July 2021, whereas fewer than 8.0% of the hospitalized patients were admitted to the ICU in these 2 months compared to the prior period. The primary reason for such a difference seems to be the limited resources of the health system in developing countries. The observed increase in in-hospital mortality could be attributed to increased illness acuity upon ICU admission, i.e. patients with noninvasive respiratory support probably stayed out of ICU due to low ICU capacity and mostly intubated patients were admitted, and this has led to an increase in both in-ICU and out-ICU mortality, as well as an increase in overall in-hospital mortality over the COVID-19 surge.
Another outcome of interest was the crude rate of ICU mortalities, which varied from 60.0% in April to 75.7% in August. The reported ICU mortality in this study was substantially higher than the reported numbers from the developed countries [18–20]. This difference might be due to higher ICU capacities in such countries that let them admit less severe cases to their ICUs. The reported ICU morality by Ranzani et al. in Brazil was comparable with our findings, indicating that stretched health system resources and staffs in low and middle-income countries were the main reason for the observed gap [21].
The highest proportion of ICU admission in the current study was 13.1% in April, which was considerably lower than in high-income countries [11]. Our adjusted analysis showed a higher than 20% gap between summer surges and lull periods in ICU mortality. Our findings demonstrated that the odds of ICU mortality over the COVID-19 surge were almost 2-fold higher than during the lull period, which was supported by previous studies. A strong association between admission in the surge period and higher ICU mortality has already been proven [9]. A couple of health system-related factors like lack of hospital resources, higher strain on health-care systems and clinical staff, and personnel’s burnout due to long-term pandemics have already been discussed to explain the higher ICU mortality in the surge period [9].
Implication for policy, practice and research
Accelerating COVID-19 vaccination on a national scale might be a useful approach to increase the effectiveness of hospital care by reducing the risk of hospitalization and death in COVID-19 patients and avoiding patient overload in hospitals.
Conclusion
In conclusion, we found that the risk of both overall in-hospital and ICU mortality increased over the summer surge of 2021 and the fifth wave of SARS-CoV-2 infection in Iran. One possible explanation is that shortages in hospital resources and particularly in ICU capacities amid the imposed higher burden on the health system during the surge period have led to the increased risk of death in hospitalized COVID-19 patients.
Acknowledgements
We would like to extend our most profound gratitude to our colleagues at the Qazvin University of Medical Sciences and all associated hospitals who have made a big sacrifice and worked hard to provide excellent clinical care during the COVID-19 global pandemic.
The authors thank WE4H Writing and Editing, Vancouver, Canada, for the English editing of the manuscript.
Contributor Information
Sepideh Abdi, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran.
Saeed Nemati, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran.
Nader Nederi darbaghshahi, Emergency Medicine Management Research Center, Health Management Research Institute, Iran University of Medical Sciences, Hemat Highway, Tehran 14496-14535, Iran.
Mehdi Mohammadi, Emergency Medicine Management Research Center, Health Management Research Institute, Iran University of Medical Sciences, Hemat Highway, Tehran 14496-14535, Iran.
Elnaz Saeedi, Department of Health Sciences, University of Leicester, George Davies Centre, University Road, Leicester LE1 7RH, UK.
Parnian Naji, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran.
Negar Taheri, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran.
Ali Qandian, Communicable Disease Office, Deputy of Health, Qazvin University of Medical Sciences, Bahonar St, Qazvin 34197-59811, Iran.
Narges Joshang, Communicable Disease Office, Deputy of Health, Qazvin University of Medical Sciences, Bahonar St, Qazvin 34197-59811, Iran.
Pedram Fattahi, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran; Student Research Center, Qazvin University of Medical Sciences, Bahonar St, Qazvin 34197-59811, Iran.
Peyman Namdar, Social Determinants of Health Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Bahonar St, Qazvin 34197-59811, Iran.
Mojtaba Vand rajabpour, Cancer Research Center, Cancer Research Institute, Tehran University of Medical Sciences, Qarib St, Azadi St, Tehran 13145-158, Iran.
Funding
The authors disclose that they received no funds for the research.
Author contributions
S.A. and S.N. participated in the methodology, software, formal analysis and writing original drafts.
N.N.D. participated in the project administration and writing—review & editing.
M.M. participated in the project administration, validation and writing—review & editing.
E.S. participated in the statistical analysis and review & editing.
P.N. participated in the investigation, data collection and review & editing.
N.T. participated in the investigation, data collection and review & editing.
A.Q. participated in the resources, validation and data acquisition.
N.J. participated in the investigation.
P.F. participated in the writing—review & editing.
P.N. participated in the project administration, resources and validation.
M.V.R. participated in the conceptualization, methodology, validation and writing—review & editing.
Ethics and other permissions
The Qazvin University of Medical Sciences Ethics Committee and Review Board has reviewed and approved the current study. They have also waived the need for informed consent of patients due to the nature of the study.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Organization WH . Timeline: WHOs COVID-19 Response: World Health Organization. 2021. [cited 2021 November]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline.
- 2. Organization WH . WHO Coronavirus (COVID-19) Dashboard World Health Organization: World Health Organization. 2021. https://covid19.who.int/.
- 3. Emami A, Javanmardi F, Akbari A. et al. Characteristics of deceased patients with CoVID-19 after the first peak of the epidemic in Fars province, Iran. Infect Ecol Epidemiol 2020;10:1781330.doi: 10.1080/20008686.2020.1781330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joy M, Hobbs FR, Bernal JL. et al. Excess mortality in the first COVID pandemic peak: cross-sectional analyses of the impact of age, sex, ethnicity, household size, and long-term conditions in people of known SARS-CoV-2 status in England. Br J Gen Pract 2020;70:e890–e8.doi: 10.3399/bjgp20X713393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemati S, Najari HR, Eftekharzadeh A. et al. Association between rRT-PCR test results upon admission and outcome in hospitalized chest CT-positive COVID-19 patients: a provincial retrospective cohort with active follow-up. Arch Clin Infect Dis 2021;16:e111866.doi: 10.5812/archcid.111866. [DOI] [Google Scholar]
- 6. Nemati S, Saeedi E, Abdi S. et al. Decomposition of socioeconomic inequality in COVID‐19 mortality. In: Iran: A retrospective cohort study. Health Soc Care Community 2021;5.doi: 10.1111/hsc.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allameh SF, Nemati S, Ghalehtaki R. et al. Clinical characteristics and outcomes of 905 COVID-19 patients admitted to Imam Khomeini Hospital Complex in the capital city of Tehran, Iran. Arch Iran Med 2020;23:766–75.doi: 10.34172/aim.2020.102. [DOI] [PubMed] [Google Scholar]
- 8. Roth GA, Emmons-Bell S, Alger HM. et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open 2021;4:e218828–e.doi: 10.1001/jamanetworkopen.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auld SC, Harrington KRV, Adelman MW. et al. Trends in ICU mortality from coronavirus disease 2019. In: A Tale of Three Surges. Crit Care Med 2022;50:245–55.doi: 10.1097/CCM.0000000000005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray WK, Navaratnam AV, Day J. et al. Variability in COVID-19 in-hospital mortality rates between national health service trusts and regions in England: a national observational study for the Getting It Right First Time Programme. EClinicalMedicine 2021;35:100859.doi: 10.1016/j.eclinm.2021.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Vidal C, Cózar-Llistó A, Meira F. et al. Trends in mortality of hospitalised COVID-19 patients: a single centre observational cohort study from Spain. Lancet Regional Health – Eur 2021;3:100041.doi: 10.1016/j.lanepe.2021.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moghadas SM, Vilches TN, Zhang K. et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin Infect Dis 2021;73:2257–64.doi: 10.1101/2020.11.27.20240051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roghani A. The influence of COVID-19 vaccination on daily cases, hospitalization, and death rate in Tennessee, United States: case study. JMIRx Med 2021;2:e29324.doi: 10.2196/29324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heidari M, Jafari H. Challenges of COVID-19 vaccination in Iran: in the fourth wave of pandemic spread. Prehosp Disaster Med 2021;36:659–60.doi: 10.1017/S1049023X21000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soria A, Galimberti S, Lapadula G. et al. The high volume of patients admitted during the SARS-CoV-2 pandemic has an independent harmful impact on in-hospital mortality from COVID-19. PLoS One 2021;16:e0246170.doi: 10.1371/journal.pone.0246170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Health IMo . Iran National Interim Guidance on COVID-19 Diagnosis and Treatment 2020. Iran Ministry of Health, Deputy of Health, 2020. (3 April 2020, date last accessed). [Google Scholar]
- 17. Jassat W, Mudara C, Ozougwu L. et al. Increased mortality among individuals hospitalised with COVID-19 during the second wave in South Africa. medRxiv 2021.doi: 10.1101/2021.03.09.21253184. [DOI] [Google Scholar]
- 18. Auld SC, Caridi-Scheible M, Blum JM. et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020;48:e799–804.doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong R, Kane A, Cook T. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia 2020;75:1340–9.doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 20. Gupta S, Hayek SS, Wang W. et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020;180:1436–46.doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ranzani OT, Bastos LS, Gelli JGM. et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respirat Med 2021;9:407–18.doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


