Abstract
Objectives
The objective of this review was to describe the COVID-19 complications after recovery.
Methods
The researchers systematically reviewed studies that reported post-COVID-19 complications from three databases: PubMed, Google Scholar and the World Health Organization (WHO) COVID-19 database. The search was conducted between 21 November 2020 and 14 January 2021. Inclusion criteria were articles written in English, with primary data, reporting complications of COVID-19 after full recovery. The review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) 2020 statement.
Key findings
This review included 69 studies with 146 725 patients from 22 countries related to post-COVID-19 complications. Thirty-six studies reported post-cure respiratory complications, ranging from dyspnoea to residual pulmonary fibrosis. Cardiac symptoms were reported in nine studies, including palpitation, chest pain and diastolic dysfunction. Neurological complications included post-traumatic stress syndrome, anxiety, depression, memory issues, insomnia and sleeping disturbance, cognitive impairments and stigma. Gastrointestinal symptoms included nausea, vomiting, diarrhoea and acute liver injury. The physical decline was the most common symptom reported in the musculoskeletal complications.
Conclusion
COVID-19 may cause several types of complications after recovery (testing negative PCR). The identified complications include respiratory, neurological/mental, cardiovascular, gastrointestinal tract, urinary tract, musculoskeletal and miscellaneous complications. However, the key impairments were pulmonary consequences, psychological problems and exercise intolerance. Thus, COVID-19 patients may need long-term follow-up.
Keywords: post COVID-19 discharge, post COVID-19 follow-up, COVID-19 long-term complications, long COVID
Introduction
At the end of 2019, a series of pneumonia cases emerged in Wuhan, China, due to severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). After that, coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization (WHO) on 11 March 2020.[1] As of 12 January 2022, the number of the confirmed COVID-19 cases has reached 312 million, including around 5.501 million deaths worldwide.[2] The main clinical characteristics of COVID-19 range from minor symptoms, which include headache or dizziness, diarrhoea, nausea and vomiting, to moderate symptoms that include fever, cough, myalgia or fatigue and/or dyspnoea. Sore throat, congestion, runny nose or loss of taste/smell were reported in some cases recently.[3] However, some cases developed complications such as RNAemia (SARS-COV-2 viral load), acute respiratory distress syndrome, acute cardiac injury, secondary infection, sepsis, septic shock and multiple organs failure.[4–7]
The potential long-term complications due to COVID-19 infection have become a critical concern among physicians and COVID-19 survivors. It is paramount to assess the long-term consequences of COVID–19, as some survivors have been complaining of limitations in physical function that involved general weakness and/or shortness of breath (SOB).[8] In contrast, others suffer from psychological complications such as post-traumatic stress disorder (PTSD).[9] In addition, while screening the psychiatric symptoms of 402 survivors, the high rates of PTSD (28%), anxiety (42%), depression (31%) and insomnia (40%) were self-rated.[10] Although the score is significantly higher in the patients with a previous positive history of psychiatric disorders, follow-up after the recovery from COVID-19 is needed.[10]
This review aimed to describe the complications of COVID-19 after recovery from the infection.
Methods
The review was conducted and is reported following the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) 2020 statement.
Data sources and search strategy
The authors searched studies available in three databases: PubMed, Google Scholar and WHO COVID-19 databases [from Global research on coronavirus disease (COVID-19)].
Inclusion and exclusion criteria
Studies were deemed eligible if they included descriptions of post-COVID-19 complications in adult patients, that is, after one or more negative COVID-19 PCR tests among previously confirmed cases. In addition, the review included studies dealing with COVID-19-related complications among both hospitalized and non-hospitalized patients. Only research studies with primary data, written in English and published between 1 January 2020 and 14 January 2021 were included.
Articles without empirical data or articles including secondary data, such as reviews, commentaries, editorial reports, case studies and case reports, were excluded.
Search strategy and data selection
The search for studies including post-COVID-19 complications was conducted on 14 January 2021. The keywords included (‘Post COVID-19 discharge’ OR ‘post COVID-19 follow-up’ OR ‘COVID-19 long-term complications’ OR ‘post COVID-19 complications’ OR ‘COVID-19 survivors’ OR ‘Long COVID’) (see Supplementary Material 1).
Initially, two authors (R.E. and M.J.Y.) screened all studies independently using the titles and the abstracts. The third (A.A.A.-J., senior) author intervened to resolve any disagreement between the two screening authors. Thereafter, articles that reported post-COVID-19 follow-up were retained. Then, three authors (A.A.A.-J., R.E. and M.J.Y.) evaluated the full texts of the selected articles to ensure that they met the review’s inclusion criteria. Ultimately, after excluding the irrelevant articles (according to the exclusion criteria), eligible studies were included. There was no quality assessment for the included studies because our focus was on the detected post-COVID-19 complications.
Data extraction
The initial data extraction of this review was conducted by two specialist pharmacists (R.E. and M.J.Y). Microsoft Excel was used for data extraction. The following information was collected: study title, authors’ names, country, study design, follow-up onset, follow-up duration, objective and primary outcomes (see Supplementary Material 2 for data extraction form).
Results
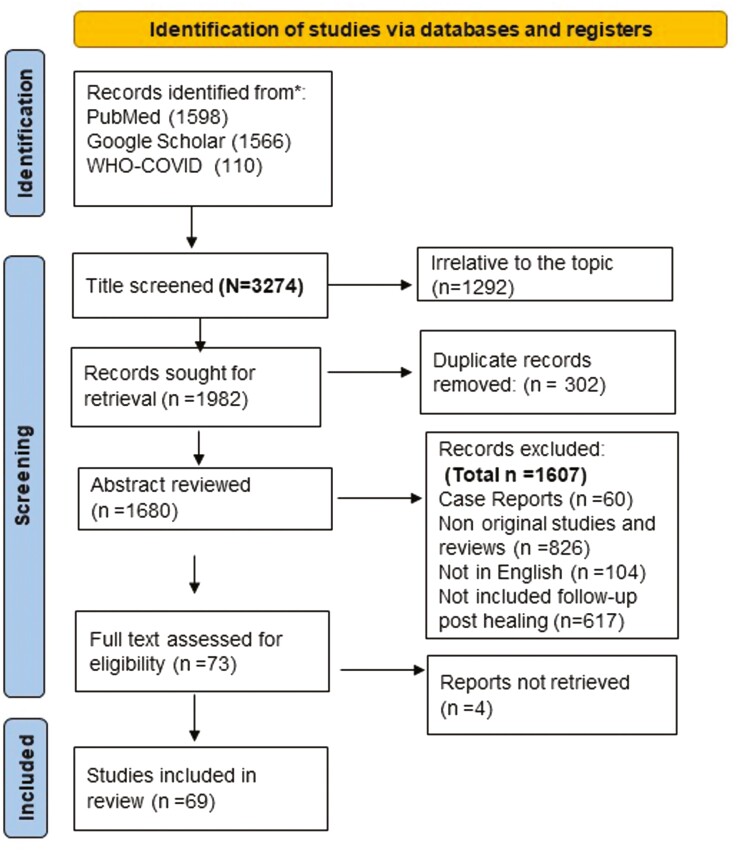
The search identified 3274 article titles [PubMed (n = 1598), Google Scholar (n = 1566) and the WHO COVID-19 database (n = 110)]. Ultimately, after excluding 302 duplicated and 2903 ineligible articles according to the exclusion criteria, a total of 69 studies were included (Figure 1, Prisma flowchart). The included studies were from 22 countries with 146 725 patients across five continents [Europe (32), Asia (23), North America (11), Australia (2) and Africa (1)]. The majority of the included studies (45) calculated the follow-up period after hospital discharge,[10–55] 14 studies identified the follow-up period after home recovery (negative PCR test),[56–68] 8 studies counted the follow-up period from the onset of the symptoms and 2 qualitative studies interviewed patients living with Long COVID.[55, 59, 66–73] Long COVID-19 is the term used to describe the persistent symptoms of COVID-19 post recovery, and it is commonly used in the identified UK studies.[50, 67, 73] Long COVID-19 can result from prolonged neurological, respiratory, cardiovascular, musculoskeletal and gastrointestinal symptoms.[67, 73] The longest follow-up duration was 140 (interquartile range [IQR] 105–160) days from the initial diagnosis. The information of the included studies dealing with post-COVID complications was summarized and presented in Table S1.
Figure 1.
PRISMA flowchart of identification and selection studies regarding post-COVID-19 complications. We followed the PRISMA 2020 statement: an updated guideline for reporting systematic reviews.
Out of 69 studies, 35 studies reported the severity of the cases according to the WHO classification during the course of the infection, 27 studies included patients with severe symptoms (n = 1384), 17 studies included patients with moderate symptoms (n = 1086), 16 studies included patients with mild symptoms (n = 396) and 3 studies included asymptomatic patients (n = 44).
The design of the included studies was prospective cohort studies (33), retrospective cohort studies (11), cross-sectional (17), retrospective and perspective (1), unspecified observational (2), case–control (1), qualitative (semi-structured interviews) (2) and case series (2).
Respiratory sequelae
Thirty-six studies from 15 different countries reported respiratory complications among COVID-19 survivors.[11–14, 16, 19, 20, 22, 24, 26–28, 31–33, 36–38, 40–42, 44, 45, 47–49, 51, 54, 55, 58, 59, 67, 69, 72, 74, 75] Around 1778 patients suffered from respiratory complications after their recovery, which ranged from breathlessness to changes in pulmonary function. Out of this number, almost 581 patients had required oxygen supply and 237 had invasive or non-invasive ventilation during their infection. The duration of the follow-up ranged from 6 weeks to 4 months post hospital discharge[31, 32, 40, 47] (Table 1). Worsened breathlessness/dyspnoea (when compared with pre-COVID-19 infection) is one of the most common reported symptoms post-COVID-19 infection.[20, 26, 32, 44, 58, 59, 67] According to the results reported in a British study, 29 (42.6%) and 21 (65.6%) patients who were discharged from intensive care unit (ICU) and warded, respectively, suffered from moderate to severe breathlessness for several weeks post-discharge,[11] while a prospective study reported significant breathlessness among 64% of the survivors up to 3 months post-discharge.[37] An Italian study revealed that 54 (29.2%) survivors experienced SOB and tachypnea.[16] Similarly, Chinese and Saudi studies, respectively, revealed that 115 (21.4%) of the survivors had tachypnoea even after mild activity, and 48.8% had difficulty breathing that impedes them from completing a 6-min walk test.[20, 32] These symptoms are common among obese patients, ICU survivors, senior survivors and patients with previous respiratory diseases.[11] In addition, cough is a persisting symptom in a plethora of survivors. Besides excessive sputum, the cough may be accompanied by throat pain.[12, 48]
Table 1.
Post-COVID-19 complications reported in the included studies
| Complications | Symptoms | Number of reported studies | Countries of the reported studies | Follow-up duration |
|---|---|---|---|---|
| Respiratory complications | (1) Breathlessness/dyspnoea/ tachypnea (2) Cough (3) Lung function abnormalities (↓FEV1, ↓FEV1/FVC) (4) Pulmonary fibrosis Interstitial thickening Crazy paving (5) Residual ground-glass opacity (6) Abnormal diffusion (7) Pulmonary embolism (8) Pneumonia |
36 studies | 10 Chinese, 1 Egyptian, 1 German, 6 British, 3 Italian 3 Netherlanders, 1 Saudi, 1 Belgian, 3 USA, 1 Australian, 1 French, 1 Belgian & Netherlanders 1 Canadian, 1 Iranian, 1 Spanish and 1 Norwegian |
6 weeks post-hospital discharge[31, 40, 47] 4 months post-hospital discharge[32] |
| Nervous system complications | (1) Post-traumatic stress disorder (2) Depression (3) Anxiety (4) Memory problems: Immediate verbal memory, delayed memory, semantic verbal fluency (5) Insomnia (6) Sleeping disturbance (7) Cognitive impairment & concentration problem (8) Stigma |
28 studies | 5 Chinese, 1 Egyptian, 7 British, 1 German, 3 Italian, 2 Netherlanders, 1 Irish, 1 Spanish, 2 Korean, 1 Bangladesh, 3 USA and 1 Indian |
14 days post-hospital discharge[56] 4 months post-hospital discharge[34] |
| Musculoskeletal complications | (1) Generalized pain (2) Joint pain (3) Muscles pain (4) Fatigue/physical decline |
23 Studies | 1 Egyptian, 1 German, 8 British, 2 Chinese, 1 Italian, 1 Saudi, 1 Belgium, 3 U.S., 1 Bangladesh,1 Indian, 2 Netherlanders & Belgian and 1 Faroe Island |
35 days post-hospital discharge.[45] 3 months post-discharge [39] |
| Miscellaneous complications | Headache, weight loss, alopecia, loss of taste, loss of smell, sore throat, voice and swallow abnormalities and malnutrition. | 17 studies | 1 Italian, 2 British, 5 Chinese, 1 Australian, 1 Mexican, 1 Helsinki, 1 Egyptian, 1 Bangladesh, 1 Netherlanders+ Belgian, 1 Faroe Island, 1 French and 1 Indian |
7.5 days (IQR, 6–13)[12] 125 (17, 45–153) days post the onset of symptoms[68] |
| Cardiovascular complications | (1) ↑ resting rates (2) Palpitation (3) Elevation in the blood pressure (4)Pericardial chest pain (5) Chest tightness (6) T2 signal and positive late gadolinium enhancement (LGE) (7) Myocardial oedema (8) Pericardial effusion (9) Diastolic dysfunction (10) Pulmonary hypertension (11) Non-specific patterns of capillaries abnormalities (12) Hemosiderin deposits (13) Cardiac arrhythmias |
9 studies | 3 Chinese, 2 USA, 2 Italian, 1 Australian and 1 German | 47 (36–58) days[21] 3 months post-hospital discharge[36] |
| Gastrointestinal complications | (1) Nausea, vomiting and diarrhoea (2) Acute liver injury |
4 studies | 1 UK, 1 German and 2 Chinese | 1 month post-hospital discharge[74] 3 month post-hospital discharge[37] |
| Readmission | Pneumonia, pulmonary embolism, Lumbar puncture, and cough without positive COVID-19 reinfection. | 3 studies | 2 Chinese and 1 USA | 22 days (20–30 days)[54] 80 days (IQR, 68–84 days) [13] |
| Urinary system complications | Residual renal impairment | 1 study | 1 British | 3 months post-hospital discharge [37] |
Lung function abnormalities were measured in several studies by measuring forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), the maximal expiratory flow at 50% of the forced vital capacity (MEF50), MEF25 and maximal mid-expiratory flow (MMEF).[24, 37] In a retrospective multi-center study, out of 55 patients, 14 (25.45%) survivors had abnormalities in total lung capacity (TLC), FEV1, and FVC.[24] In a British study, 60% of the survivors reported some persistent lung parenchyma abnormalities through lung magnetic resonance imaging (MRI) at 2 to 3 months post-discharge.[37] Furthermore, abnormal FEV1 (<80%) or FEV1/FVC < 70% , MEF 50, MEF 25, MMEF 75/25 (< 70% ) were presented in 52.17%, 72.73% , and 45.45% of three groups of the survivors of moderate/severe COVID-19, respectively.[75]
Lung fibrosis presented as interstitial thickening (27.3%) and crazy paving (ground-glass opacities with superimposed interlobular septal thickening and intralobular septal thickening) (5.5%) as reported in some 55 survivors after 3 months of discharge.[24] Residual ground-glass opacity (GGO) was common in some survivors. According to an Iranian study, 54.5% of patients had GGO, and 31.8% had mixed GGO and sub-pleural parenchymal bands.[41] Additionally, two studies reported that 86% and 48% of the survivors had residual GGO, respectively.[33, 59] In addition, a fifth of the participants had parenchymal bands that could indicate fibrosis progression.[47] Lung high-resolution computed tomography (HRCT) was performed for 21 survivors post 3 months of discharge; 5 of them revealed abnormalities, 2 patients with local GGO and 3 patients with local GGO and fibrosis.[48] CT chest scans of recovered patients (asymptomatic, moderate or severe) revealed a residual lesion in the lung or sub-pleural lesions after 3 months post hospital discharge. Furthermore, fibrous strips and thickening of the adjacent pleura were presented in patients who recovered from severe infection.[42] This study also revealed a correlation between the presence of differential metabolites such as homotyrosine, lactoylglutathione and indolelactate in the recovered patients and the residual lesions in the lung.[42] Other studies reported complications including abnormal diffusion/pleural diffusion, pulmonary embolism and pneumonia.[13, 32, 44, 64, 75]
Cardiovascular sequelae
Cardiac symptoms were reported among COVID-19 survivors. In a longitudinal study, out of 538 survivors, 70 (13%) survivors underwent some cardiovascular-related symptoms post-discharge that involved high resting heart rates (n = 15), palpitations (n = 26) and elevation in the blood pressure level (n = 7), in which some patients started taking antihypertensive agent.[20] Similarly, out of 26 survivors, pericardial chest pain 3 (12%), chest tightness 6 (23%) and palpitation 23 (88%) were reported.[21] These cardiopulmonary symptoms were reported in three other studies among patients after COVID-19 infection.[14, 36, 48]
Fifty-eight percent of COVID-19 survivors in one hospital in Wuhan, China experienced abnormal cardiac magnetic resonance (CMR). More specifically, 54% of the COVID-19 survivors had myocardial oedema and 31% had positive late gadolinium enhancement (LGE).[21] Furthermore, high-sensitivity Troponin T values (>3 pg/ml) were reported in 71% of survivors in a German study; most of 78 survivors had presented with abnormal CMR findings: elevated myocardial native T1 (n = 73), elevated myocardial native T2 (n = 60), myocardial LGE (n = 32), or pericardial enhancement (n = 22). Additionally, ischaemic-type patterns of myocardial LGE presented in 12 patients, and 3 patients suffered from severe abnormalities such as elevated levels of high sensitive Troponin T (hsTnT), native T1, native T2 measures, LEG and left ventricular ejection fraction less than 50%.[62] In an Australian study, trans-thoracic echocardiography during two follow-up visits revealed a high rate of diastolic dysfunction, 60%, and 55%, besides pulmonary hypertension and pericardial effusion.[55]
According to an Italian study of nailfold videocapillaroscopy (NVC) examination in recovered patients, approximately 1 month after hospital discharge, 46.3% of 54 survivors presented with some non-specific patterns of capillary abnormalities in two or more fingers, which involved enlarged capillaries (85.2%), meandering capillaries (81.4%) and pericapillary oedema (70.4%). Furthermore, hemosiderin deposits resulting from micro-haemorrhage and micro-thrombosis were revealed in 11.1% of survivors.[30] The duration of follow-up ranged from 47 days to 3 months post-hospital discharge.[21, 36]
Nervous system sequelae
Twenty-eight studies from 12 countries reported psychological and neurological complications among COVID-19 survivors.[10, 11, 15, 16, 20, 23, 25, 27–29, 31, 33–35, 38–40, 50, 52–54, 56, 61, 66, 73, 76, 77] The duration of follow-up ranged from 2 weeks to 4 months post-hospital discharge[34, 56] (Table 1). PTSD is deemed one of the common psychological complications among COVID-19 survivors.[10, 11, 23] Two studies have evaluated PTSD according to the post-traumatic stress disorder checklist-5 (PCL-5).[10, 23] A Korean study reported that 20.3% of the patients have PTSD (PCL-5 score of ≥33), and an Italian study reported that 12.9% have PTSD after COVID-19. There is an inverse correlation between the duration of hospitalization with PCL-5.[10] In addition, based on a cross-sectional study result, 46.9% of ICU survivors complained of PTSD, and 23.5% of hospital admitted survivors had the same mental sequelae.[11] The main feature of PTSD is that it is more prevalent among women than men, among the young population of COVID-19 survivors and among people with a history of psychological diseases.[10, 16, 56]
Depression and anxiety are other common symptoms among COVID-19 survivors. In a Chinese study, out of 126 survivors, 22.2% reported anxiety, and 38.1 % reported depression.[56] Furthermore, while the severity of anxiety reported by old survivors was lower than the young survivors, there was no difference in the severity of depression symptoms according to the patient’s age.[56] In a Chinese study, out of 538 COVID-19 survivors, 23(4.3%) reported depression and loss of interest in things around them.[20] An Irish study reported that 35.5% of patients who were discharged from ICU had moderate to severe anxiety and depression during the follow-up.[52] The severity of depression and anxiety in COVID-19 survivors was evaluated using the Patient Health Questionnaire-9 (PHQ-9) and self-reported by the Anxiety Screening Scale (GAD-7), and their scores were 4.20 ± 3.98 and 4.28 ± 4.05, respectively.[15] Using the same screening scales, in another study, 50 recovered patients reported anxiety with intensity varied between mild (30 cases), moderate (12 cases), moderately severe (5 cases) and severe anxiety (3 cases).[54]
Memory problems were reported in two studies.[11, 35] In a British study, new or worsened memory problems were reported in 17.6% of ICU survivors versus 18.8% of the ward survivors.[11] A Spanish study reported that 12.3% of patients had memory complaints ranging from moderate (38%) to severe impairment for immediate verbal memory. In contrast, for delayed memory, 11.8% of COVID-19 survivors had moderate and 2.8% had severe impairment.[35] In addition, moderate (34.6%) and severe (8.4%) deficits in semantic verbal fluency were reported in the survivors.[35] Insomnia, sleeping disorder, stigma and concentration problems are also reported among survivors.[11, 16, 20, 25, 28]
Musculoskeletal sequelae
Musculoskeletal symptoms including myalgia and joint pain were reported among COVID-19 survivors in 23 studies.[11, 12, 14, 19, 22, 27, 28, 31, 32, 37–39, 45, 50, 57–60, 65–68, 70, 73] The duration of follow-up ranged from 35 days to 3 months post-hospital discharge.[39, 45] Fatigue was reported in the 12 studies among COVID-19 survivors. In a Chinese study that followed 337 patients, fatigue or myalgia was reported in 6 patients out of 44 patients.[12] Fatigue and physical decline were reported among COVID-19 survivors in different countries: China (48%), Italy (51%) and the Netherlands (87%).[19, 22, 58] According to another Chinese study that followed 538 patients, although 152 (28.3%) of 267 survivors presented with physical decline, only 35 of them reported no improvement.[20] Fatigue was reported in 30% of the survivors for 125 days post symptoms onset, and the intensity of fatigue ranged from mild to very severe (100% versus 94.5%) when measured using Utrecht Symptom Diary.[70]
There were also reports that recovered patients presenting with myalgia in Germany (5 of 33), the USA (39 of 77) and China (24 of 267).[20, 31, 45] The pain was presented in 49.6% out of 115 survivors, which was usually in the shoulder (29%), chest (23%), lower limb (9%) and the back (13%) and joint pain (7.9%).[38] In two studies in the USA, generalized pain was reported in 64% of survivors, 50.6% of the survivors had muscular pain and 54.7% reported joint pain at day 35 post-hospital discharge.[39, 45]
Gastrointestinal tract sequelae
Persistent gastrointestinal symptoms post COVID-19 recovery were reported in four studies.[24, 31, 37, 74] Nausea may remain until the fourth week after hospital discharge.[74]
According to a British study, persistent liver injury was identified by blood tests at 2 to 3 months among 11% of COVID-19 patients. However, another study reported that 10% of 52 survivors had signs of liver injury on MRI, which was demonstrated by the increase in the iron-corrected liver T1.[37]
Urinary system sequelae
Residual renal impairment was reported in a prospective study – 3% (2 of 58) of patients after 2–3 months post-hospital discharge, although they had no kidney injury before COVID-19.[37]
Miscellaneous symptoms
Headache, weight loss, alopecia, sore throat, loss of taste/smell, voice/swallow abnormalities, fever and malnutrition were also reported among COVID-19 survivors. The duration of follow-up ranged from 7 to 125 days from the onset of symptoms.[12, 68]
Headache was the most commonly reported symptom; the reported intensity of headache was mild-moderate among the respondents.[24, 68, 74] Weight loss was reported in an Italian study, in which 29% of survivors lost >5% of their initial body weight.[17] In addition, it was reported that ≥5% of weight loss persisted for 2 months in some survivors.[72] Alopecia was presented in two studies, 28.6% and 8.8% of COVID-19 survivors, and it was common in women more than in men. However, hair loss was improved in 30 recovered patients after 3 months of discharge.[20, 57] The sore throat was reported in three studies.[12, 57, 74] Voice and swallow abnormalities were reported in 61% of survivors, according to a British study.[43] Mild to moderate loss of taste and mild loss of smell persisted till Day 30 and Day 60 after the initial symptoms in 15% and 25% of survivors, respectively.[43, 68] Transient fever was reported among 8.4% of survivors at the first and second week post-hospital discharge.[74] Malnutrition was reported in 62.7% of survivors, according to an Italian study.[16]
Hospital readmission
According to a case series of 370 patients, five survivors were readmitted to the hospital because of the cough, pneumonia and lumbar disease without positive SARS-COVID-19 infection.[54] Furthermore, 22 recovered patients were re-hospitalized because of persistent COVID-19 symptoms, pneumonia and pulmonary embolism.[13]
Discussion
This review assembled data that involved the reported complications following COVID-19 infection among survivors. The identified complications involved respiratory, neurological/mental, cardiovascular, gastrointestinal tract (GIT), urinary tract, musculoskeletal and miscellaneous complications. However, the key impairments were pulmonary consequences, psychological problems and exercise intolerance.
The review has some limitations including neither assessing the risk of bias nor the quality of the included studies. Seven of the included studies were preprint (not peer-reviewed)[39, 43, 44, 52, 60, 75, 77]. The review did not use the quality checklist. Additionally, the protocol for the review was not registered with one of the standard registries. Another potential limitation was that our search could have missed a few studies because the authors only included papers written in English – this could bias results towards a subset of the population (i.e. excluded those from particular ethnicities).
Respiratory problems are one of the frequently reported issues among COVID-19 survivors. They include breathlessness, dyspnoea, tachypnoea and cough. Abnormalities in diffusion capacity for carbon monoxide (DLCO) and TLC were detected among COVID-19 survivors. The abnormal parenchymal diffusion has persisted in some recovered patients for up to 6 months post-infection.[75] In addition, reduction in lung function based on FEV1 measurements may prove the restrictive lung disease that persisted in COVID-19 survivors.[37] HRCT findings in some studies revealed the lesions of GGO and interstitial thickening in survivors 3 months post-hospital discharge.[24, 33] These observations are reminiscent of the previous SARS that cause residual GGO, intralobular and interlobular septal thickening after 84 months post recovery.[78] However, the recovery and stability of pulmonary lesions were observed among recovered patients on an annual follow-up that had lasted for 15 years after SARS infection.[79] Therefore, the respiratory consequences of COVID-19 may need a longer time to be revealed.
Neurological complications of COVID-19 included some psychological problems such as PTSD, depression, anxiety, sleep disturbance, insomnia and memory problems. These complications ranged from 12.9% to 46.9% among COVID-19 survivors. These mental issues were also reported among 79.64% (±22.34) of Middle East Respiratory Syndrome (MERS) survivors after 14 months of hospital discharge.[80] Another study also reported that 70% of patients with MERS infection experienced psychological symptoms such as insomnia, depression, impaired memory, disorientation and hallucination during hospitalization.[81] Anxiety, depressive mood and suicidal behaviour among SARS patients were demonstrated due to hospital isolation, quarantine and the severity of symptoms.[82] Furthermore, depressive disorders and PTSD had persisted for 1 year in patients after 7 days or more of mechanical ventilation in medical or surgical ICU.[83] Thus, neurological/mental complications may develop after the most severe respiratory viral infection: COVID-19, MERS and SARS.
Reduction in exercise tolerance, muscle pain and joint pain are the musculoskeletal consequences among COVID-19 survivors. Fatigue and exercise intolerance may be due to the pulmonary complications and the SOB that prevent some survivors from completing the 6-min walk test.[32] Reduced exercise capacity and myalgia were reported among acute respiratory distress syndrome (ARDS) survivors within 6 to 24 months after ICU discharge because of mono-neuropathies with focal changes, myopathies and chronic neurological changes.[84] In addition, hospitalization for an extended period and pulmonary disease have been deemed to reduce the functional capacity among young ARDS survivors after 6 months of ICU discharge.[85]
Nausea, vomiting and diarrhoea are among the gastrointestinal symptoms of post-COVID-19 infection. In addition, acute liver injury was reported. Previous studies on SARS had shown an active viral replication in both the small and large intestines among SARS patients; thus, based on the high culture yielded from the intestinal tract, it was suggested that the intestinal tract was the primarily targeted organ.[86] According to Pan et al., the higher the severity of COVID-19, the more pronounced GIT symptoms; however, some patients had GIT symptoms without respiratory symptoms during infection.[87] Acute liver injury is demonstrated as a result of direct viral hepatitis or as a result of taking anti-viral medicine through treatment, besides the inflammatory reaction in the body.[88] GIT symptoms and acute liver injury were reported among MERS and SARS patients during the disease. Residual renal impairment was one of the complications of COVID-19. Kidney injury was reported due to the inflammatory response (inflammatory cytokine release) against the viral infection, hypercoagulation state and angiotensin pathway activation.[89] Renal injury was reported in 42.9% of MERS patients.[90]
We cannot conclude the long-term complications and residual dysfunctions at this stage, especially in the pulmonary and neurological systems. We need to follow up on the lung abnormalities for at least 1 year after the cure and the psychological symptoms.
Conclusion
The unique contribution of this review was to systematically identify and categorize the post-COVID-19 complications from the published literature according to the affected body systems. The reviewed 69 studies with 146 725 patients from 22 countries included hospitalized or non-hospitalized patients who developed post-COVID-19 complications after their healing. COVID-19 may cause several types of complications after recovery (testing negative PCR). The identified complications involved respiratory, neurological/mental, cardiovascular, gastrointestinal tract, urinary tract, musculoskeletal and miscellaneous complications. However, the key impairments were pulmonary consequences, psychological problems and exercise intolerance. Thus, COVID-19 patients may need long-term follow-up.
Supplementary Material
Acknowledgement
The authors would like to thank Dr. Bernard Sorofman, PhD, Professor Emeritus, the University of Iowa College of Pharmacy, Iowa City, IA, USA, for his feedback.
Contributor Information
Rehab Elhiny, Clinical Pharmacy Department, Faculty of Pharmacy, Minia University, Minia, Egypt.
Ali Azeez Al-Jumaili, Clinical Pharmacy Department, College of Pharmacy, University of Baghdad, Baghdad, Iraq; Pharmacy Practice and Science Department, College of Pharmacy, The University of Iowa, Iowa City, IA, USA; Public Health Department, University of California Davis School of Medicine, Davis, CA, USA.
Mohammed Jamal Yawuz, Clinical Pharmacy Department, College of Pharmacy, University of Baghdad, Baghdad, Iraq.
Author Contributions
R.E. participated in the acquisition of data, analysis, and interpretation of data, drafting the article and revising it critically for important intellectual content. A.A.A.-J. participated in the conception and design of the study, analysis, and interpretation of data, drafting the article, revising it critically for important intellectual content. M.J.Y. participated in the acquisition of data and drafting the article. All three authors participated in the final approval of the version to be submitted.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Data availability
The study data is available in supplementary Table S1.
References
- 1. Di Gennaro F, Pizzol D, Marotta C et al. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health 2020; 17: 2690. 10.3390/ijerph17082690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (12 January 2022, date last accessed). [Google Scholar]
- 3. Elhiny R, Al-Jumaili AA, Yawuz MJ. An overview of post-COVID-19 complications. Int J Clin Pract 2021; 75: e14614. 10.1111/ijcp.14614 [DOI] [PubMed] [Google Scholar]
- 4. Li L, Huang T, Wang Y et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020; 92: 577–83. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osman AA, Al Daajani MM, Alsahafi AJ. Re-positive COVID-19 PCR test: could it be a reinfection? New Microbes New Infect 2020; 37: 100748 (1–6). 10.1016/j.nmni.2020.100748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abbas HM, Al-Jumaili AA, Nassir KF et al. Assessment of COVID-19 treatment containing both hydroxychloroquine and azithromycin: a natural clinical trial. Int J Clin Pract 2021; 75: e13856. 10.1111/ijcp.13856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan K, Zheng J, Mok Y et al. SARS: prognosis, outcome and sequelae. Respirology 2003; 8: S36–40. 10.1046/j.1440-1843.2003.00522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaseda ET, Levine AJ. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol 2020; 34: 1498–514. 10.1080/13854046.2020.1811894 [DOI] [PubMed] [Google Scholar]
- 10. Mazza MG, De Lorenzo R, Conte C et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89: 594–600. 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halpin SJ, McIvor C, Whyatt G et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93: 1013–22. 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 12. Yan N, Wang W, Gao Y et al. Medium term follow-up of 337 patients with coronavirus disease 2019 (COVID-19) in a Fangcang shelter hospital in Wuhan, China. Front Med 2020; 7: 373. https://doi.org/10.3389%2Ffmed.2020.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarthy CP, Murphy S, Jones-OʹConnor M et al. Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine 2020; 26: 100504 (1–9). 10.1016/j.eclinm.2020.100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Somani SS, Richter F, Fuster V et al. Characterization of patients who return to hospital following discharge from hospitalization for COVID-19. J Gen Intern Med 2020; 35: 2838–44. 10.1007/s11606-020-06120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou H, Lu S, Chen J et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res 2020; 129: 98–102. 10.1016/j.jpsychires.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Lorenzo R, Conte C, Lanzani C et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One 2020; 15: e0239570. 10.1371/journal.pone.0239570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Filippo L, De Lorenzo R, DʹAmico M et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr 2020; 40: 2420–2426. https://doi.org/10.1016%2Fj.clnu.2020.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. An J, Liao X, Xiao T et al. Clinical characteristics of recovered COVID-19 patients with re-detectable positive RNA test. Ann Transl Med 2020; 8: 1084. 10.21037/atm-20-5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He S, Zhou K, Hu M et al. Clinical characteristics of “re-positive” discharged COVID-19 pneumonia patients in Wuhan, China. Sci Rep 2020; 10: 1–9. 10.1038/s41598-020-74284-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong Q, Xu M, Li J et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27: 89–95. 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang L, Zhao P, Tang D et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. Cardiovas Imaging 2020; 13: 2330–9. 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landi F, Carfì, A, Benvenuto F et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from Covid-19. Am J Prev Med 2021; 60: 13–9. 10.1016/j.amepre.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang MC, Park D. Incidence of post-traumatic stress disorder after coronavirus disease. Healthcare, 2020; 8: 373. 10.3390/healthcare8040373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Y-M, Shang Y-M, Song W-B et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dar SA, Khurshid SQ, Wani ZA et al. Stigma in coronavirus disease-19 survivors in Kashmir, India: a cross-sectional exploratory study. PLoS One 2020; 15: e0240152. 10.1371/journal.pone.0240152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mandal S, Barnett J, Brill SE et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2020; 76: 396–398. 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamal M, Abo Omirah M, Hussein A et al. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 2021; 75: e13746. 10.1111/ijcp.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold DT, Hamilton FW, Milne A et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021; 76: 399–401. 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weerahandi H, Hochman KA, Simon E et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med 2021; 36: 738–745. 10.1007/s11606-020-06338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Natalello G, De Luca G, Gigante L et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement. Microvasc Res 2021; 133: 104071. 10.1016/j.mvr.2020.104071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daher A, Balfanz P, Cornelissen C et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med 2020; 174: 106197. 10.1016/j.rmed.2020.106197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alharthy A, Abuhamdah M, Balhamar A et al. Residual lung injury in patients recovering from COVID-19 critical illness: a prospective longitudinal point-of-care lung ultrasound study. J Ultrasound Med 2020; 40; 1823–1838. 10.1002/jum.15563 [DOI] [PubMed] [Google Scholar]
- 33. van den Borst B, Peters JB, Brink M et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis 2020; 73: e1089–e1098. 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chieffo D, Delle Donne V, Massaroni V et al. Psychopathological profile in COVID-19 patients including healthcare workers: the implications. Eur Rev Med Pharmacol Sci 2020; 24: 11964–11970. 10.26355/eurrev_202011_23858 [DOI] [PubMed] [Google Scholar]
- 35. Mendez R, Balanza-Martinez V, Luperdi SC et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med 2021; 290: 621–631. 10.1111/joim.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clavario P, De Marzo V, Lotti R et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months. Int J Cardiol 2021; 340: 113–118. 10.1016/j.ijcard.2021.07.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raman B, Cassar MP, Tunnicliffe EM et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021; 31: 100683. 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DʹCruz RF, Waller MD, Perrin F et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res 2021; 7: 00655–2020. 10.1183/23120541.00655-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savarraj JP, Burkett AB, Hinds SN et al. Three-month outcomes in hospitalized COVID-19 patients. medRxiv, 10.1101/2020.10.16.20211029, 18 October 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 40. Talman S, Boonman-de Winter L, de Mol M et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med 2021; 176: 106272. 10.1016/j.rmed.2020.106272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabatabaei SMH, Rajebi H, Moghaddas F et al. Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol 2020; 27: 711–9. 10.1007/s10140-020-01869-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sufei W, Xu J, Luo P et al. Plasma metabolomic profiles and clinical features in recovered COVID-19 patients without previous underlying diseases 3 months after discharge. 2021; 14: 4485–4501. 10.2147/JIR.S325853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller B, Tornari C, Miu K et al. Airway, voice and swallow outcomes following endotracheal intubation and mechanical ventilation for COVID-19 pneumonitis: preliminary results of a prospective cohort study. 2020. Preprint: not peer reviewed. 10.21203/rs.3.rs-65826/v1 [DOI]
- 44. Valiente-De Santis L, Perez-Camacho I, Sobrino B et al. Clinical and immunoserological status 12 weeks after infection with COVID-19: prospective observational study. medRxiv, 10.1101/2020.10.06.20206060, 8 October 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 45. Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. 10.1371/journal.pone.0243882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li G, Du L, Cao X et al. Follow-up study on serum cholesterol profiles in recovered COVID-19 patients. BMC Infect Dis 2021; 21: 299. 10.1186/s12879-021-05984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lerum TV, Aaløkken TM, Brønstad E et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang L, Yang B, Jiang N et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. 10.3346/jkms.2020.35.e418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Gassel RJ, Bels JL, Raafs A et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med 2021; 203: 371–4. 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ladds E, Rushforth A, Wieringa S et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res 2020; 20: 1–13. 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liao X, Wang Y, He Z et al. Three-month pulmonary function and radiological outcomes in COVID-19 survivors: a longitudinal patient cohort study. Open Forum Infect Dis 2020; 8: ofaa540. 10.1093/ofid/ofaa540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neil C, Lakey S, McMahon S et al. Clinical characteristics and post-intensive care outcomes of COVID-19 pneumonia. 2020. Preprint: not peer reviewed. 10.21203/rs.3.rs-58685/v1 [DOI]
- 53. Park HY, Jung J, Park HY et al. Psychological consequences of survivors of COVID-19 pneumonia 1 month after discharge. J Korean Med Sci 2020; 35: e409. 10.3346/jkms.2020.35.e409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu C, Hu X, Song J et al. Mental health status of survivors following COVID-19 in Wuhan, China: a descriptive study. Clin Transl Med 2020; 10: e52. 10.1002/ctm2.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sonnweber T, Sahanic S, Pizzini A et al. Cardiopulmonary recovery after COVID-19—an observational prospective multi-center trial. Eur Respir J 2021; 57: 2003481. 10.1183/13993003.03481-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cai X, Hu X, Ekumi IO et al. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am J Geriatr Psychiatry 2020; 28: 1030–9. 10.1016/j.jagp.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akter F, Mannan A, Mehedi HH et al. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr 2020; 14: 2031–8. 10.1016/j.dsx.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goërtz YM, Van Herck M, Delbressine JM et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6: 00542–2020. 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smet J, Stylemans D, Hanon S et al. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir Med 2021; 176: 106276. 10.1016/j.rmed.2020.106276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shah SB, Chawla R, Pahade A et al. Immunity status of Health Care Workers post recovery from COVID-19: an online longitudinal panel survey. medRxiv, 10.1101/2020.11.27.20239426, 30 November 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 61. Hampshire A, Trender W, Chamberlain S et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021; 39: 101044. 10.1016/j.eclinm.2021.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Puntmann VO, Carerj ML, Wieters I et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1265–73. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Galván-Tejada CE, Herrera-García CF, Godina-González S et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int J Environ Res Public Health 2020; 17: 9367. 10.3390/ijerph17249367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pawlowski C, Venkatakrishnan A, Ramudu E et al. Pre-existing conditions are associated with COVID patients’ hospitalization, despite confirmed clearance of SARS-CoV-2 virus. EClinicalMedicine 2021; 34: 100793. 10.1016/j.eclinm.2021.100793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walsh-Messinger J, Manis H, Vrabec A et al. The kids are not alright: a preliminary report of post-COVID syndrome in university students. J Am Coll Health. 2021: 1–7. 10.1080/07448481.2021.1927053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kingstone T, Taylor AK, OʹDonnell CA et al. Finding the ‘right’ GP: a qualitative study of the experiences of people with long-COVID. BJGP Open 2020; 4: bjgpopen20X101143. 10.3399/bjgpopen20X101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dennis A, Wamil M, Alberts J et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021; 11: e048391. 10.1136/bmjopen-2020-048391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Petersen MS, Kristiansen MF, Hanusson KD et al. Long COVID in the Faroe Islands—a longitudinal study among non-hospitalized patients. Clin Infect Dis 2021; 73: e4058–e4063. 10.1093/cid/ciaa1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shah AS, Wong AW, Hague CJ et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021; 76: 402–404. 10.1136/thoraxjnl-2020-216308 [DOI] [PubMed] [Google Scholar]
- 70. Vaes AW, Machado FV, Meys R et al. Care dependency in non-hospitalized patients with COVID-19. J Clin Med 2020; 9: 2946. 10.3390/jcm9092946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Horvath L, Lim JWJ, Taylor JW et al. Smell and taste loss in COVID-19 patients: assessment outcomes in a Victorian population. Acta Otolaryngol 2021; 141: 299–302. 10.1080/00016489.2020.1855366 [DOI] [PubMed] [Google Scholar]
- 72. Carvalho-Schneider C, Laurent E, Lemaignen A et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2020; 27: 258–263. 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Humphreys H, Kilby L, Kudiersky N et al. Long Covid and the role of physical activity: a qualitative study. BMJ Open 2021; 11: e047632. 10.1136/bmjopen-2020-047632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang X, Xu H, Jiang H et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM 2020; 113: 657–65. 10.1093/qjmed/hcaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meng X, Kang K, Gao Y et al. Pulmonary dysfunction in patients recovered from COVID-19 pneumonia: a 6-month follow-up study. 2020. Preprint: not peer reviewed. 10.21203/rs.3.rs-111265/v1 [DOI]
- 76. Taquet M, Luciano S, Geddes JR et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiat 2021; 8: 130–40. 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen B, Wang Y, Yang T et al. Mental health among COVID-19 survivors and healthcare workers exposed to COVID-19 in Wuhan, China: a cross-sectional study. Authorea, 10.22541/au.158879055.59290774, 6 May 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 78. Wu X, Dong D, Ma D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS). Med Sci Monit 2016; 22: 2793–2799. 10.12659/msm.896985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang P, Li J, Liu H et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8: 1–8. 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Batawi S, Tarazan N, Al-Raddadi R et al. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS). Health Qual Life Outcomes 2019; 17: 1–7. 10.1186/s12955-019-1165-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim H-C, Yoo S-Y, Lee B-H et al. Psychiatric findings in suspected and confirmed middle east respiratory syndrome patients quarantined in hospital: a retrospective chart analysis. Psychiatry Investig 2018; 15: 355. 10.30773/pi.2017.10.25.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cheng SK-W, Tsang JS-K, Ku K-H et al. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: a series of 10 cases. Br J Psychiatry 2004; 184: 359–60. 10.1192/bjp.184.4.359 [DOI] [PubMed] [Google Scholar]
- 83. Herridge MS, Chu LM, Matte A et al. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med 2016; 194: 831–44. 10.1164/rccm.201512-2343OC [DOI] [PubMed] [Google Scholar]
- 84. Angel MJ, Bril V, Shannon P et al. Neuromuscular function in survivors of the acute respiratory distress syndrome. Can J Neurol Sci 2007; 34: 427–32. 10.1017/s0317167100007307 [DOI] [PubMed] [Google Scholar]
- 85. Jadhav AR, Shinde SB. Functional exercise capacity in young survivors of acute respiratory distresssyndrome. Indian J Tuberc 2020; 67: 163–6. 10.1016/j.ijtb.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 86. Leung WK, To K-F, Chan PK et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 2003; 125: 1011–7. 10.1016/s0016-5085(03)01215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pan L, Mu M, Yang P et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020; 115: 766–773. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kukla M, Skonieczna-Żydecka K, Kotfis K et al. COVID-19, MERS and SARS with concomitant liver injury—systematic review of the existing literature. J Clinical Med 2020; 9: 1420. 10.3390/jcm9051420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zahid U, Ramachandran P, Spitalewitz S et al. Acute kidney injury in COVID-19 patients: an inner city hospital experience and policy implications. Am J Nephrol 2020; 51: 786–96. 10.1159/000511160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gerges Harb J, Noureldine HA, Chedid G et al. SARS, MERS and COVID-19: clinical manifestations and organ-system complications: a mini review. Pathog Dis 2020; 78: ftaa033. 10.1093/femspd/ftaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data is available in supplementary Table S1.