Abstract
Background
Remdesivir and sotrovimab both have clinical trial data in the outpatient setting demonstrating reduction in the risk of hospitalizations and emergency department (ED) visits related to COVID-19.
Objectives
To evaluate the effectiveness of remdesivir in comparison with sotrovimab and matched high-risk control patients in preventing COVID-19-related hospitalizations and ED visits during the Omicron B.1.1.529 surge.
Patients and methods
This retrospective cohort study included outpatients positive for SARS-CoV-2, with non-severe symptoms for ≤7 days and deemed high-risk for severe COVID-19 by an internal scoring matrix. Patients who received remdesivir or sotrovimab from 27/12/2021 to 04/02/2022 were included (n = 82 and n = 88, respectively). These were compared with a control cohort of high-risk COVID-19 outpatients who did not receive therapy (n = 90). The primary outcome was a composite of 29 day COVID-19-related hospitalizations and/or ED visits. Pre-specified secondary outcomes included components of the primary endpoint, 29 day all-cause mortality and serious adverse drug events.
Results
Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset (11% versus 23.3%; OR = 0.41, 95% CI = 0.17–0.95). Patients receiving sotrovimab were also less likely to be hospitalized or visit the ED (8% versus 23.3%; OR = 0.28, 95% CI = 0.11–0.71). There was no difference in the incidence of hospitalizations/ED visits between sotrovimab and remdesivir.
Conclusions
Our highest-risk outpatients with Omicron-related COVID-19 who received early sotrovimab or remdesivir had significantly lower likelihoods of a hospitalization and/or ED visit.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), continues to impact global healthcare with >539 million cases reported worldwide as of 21 June 2022.1 COVID-19 produces a wide spectrum of illness ranging from asymptomatic disease to death, making severity risk stratification a vital component of care. There is an abundance of data illustrating the positive correlation between age, quantity of underlying medical conditions and severe COVID-19 disease.2–4
The evolution of SARS-CoV-2 and emergence of multiple variants has compromised the efficacy of COVID-19 therapeutics and vaccines. The B.1.1.529 (Omicron) variant was first identified in November 2021 and quickly replaced its predecessor B.1.617.2 (Delta) as the predominant cause of COVID-19 globally. Omicron’s mutations increased its transmissibility and immune system evasion while compromising the efficacy of anti-SARS-CoV-2 mAbs aside from sotrovimab and bebtelovimab.5–7 Sotrovimab obtained emergency use authorization (EUA) in May 2021 for the treatment of mild-to-moderate COVID-19 disease in non-hospitalized patients at high risk for progression to severe disease.7 A Phase three randomized, placebo-controlled study found a significant decrease in the composite endpoint of hospitalization or death (1% versus 7%; relative risk reduction = 85%, 95% CI = 44% to 96%) when sotrovimab was given within 5 days of symptom onset.8 Sotrovimab became the only viable mAb treatment option for the Omicron surge, but demand significantly outweighed supply in late December 2021 and January 2022.
Remdesivir, a direct-acting nucleotide inhibitor of SARS-CoV-2 RNA-dependent RNA polymerase, was the first FDA-approved medication for COVID-19 and received FDA approval in October 2020 for use in hospitalized adults and paediatric patients for the treatment of COVID-19.9 In late December 2021, a randomized, double-blinded, placebo-controlled trial involving unvaccinated, high-risk outpatients with COVID-19 who received remdesivir within 7 days from symptom onset was published.10 Study patients randomly assigned to receive remdesivir for 3 days (200 mg on day 1, followed by 100 mg on days 2 and 3) had an 87% lower composite risk of hospitalization or death. Based on these results, the NIH, IDSA and WHO COVID-19 guidelines added remdesivir as a treatment option for high-risk outpatients.11–13 On 21 January 2022, FDA expanded the approved indication for remdesivir to include adults and paediatric outpatients with mild-to-moderate COVID-19 at high risk for progression to severe COVID-19, including hospitalization or death.9,14
Our hospital proactively monitored the emergence of the Omicron B.1.1.529 variant in our region and modified outpatient treatment recommendations in late December 2021 to include agents expected to have activity against B.1.1.529. The purpose of this study was to evaluate the effectiveness of outpatient remdesivir in comparison with sotrovimab and a matched high-risk control group in preventing COVID-19-related hospitalizations and emergency department (ED) visits during the Omicron surge.
Patients and methods
Study design
This was a single-centre, retrospective cohort study of outpatients with confirmed COVID-19 infection, who had mild-to-moderate symptoms without an increasing need for oxygen compared with their baseline and had multiple risk factors for progression to severe COVID-19 based on the CDC’s data and sotrovimab’s EUA provider factsheet.3,4,7 The study protocol was reviewed and approved by the University of South Florida (USF) institutional review board.
Patients
All patients had confirmed COVID-19 infection (either by antigen or PCR testing), were ≥12 years of age and weighed ≥40 kg. In addition, all patients were classified as having mild-to-moderate symptoms for ≤7 days at the time of inclusion and at high-risk for progression to severe COVID-19. Patients were excluded if they received a COVID-19-directed oral antiviral (e.g. nirmatrelvir/ritonavir or molnupiravir), if they received community-administered mAbs or if there were limited records for follow-up.
Since 18 November 2020, our institution’s COVID-19 ambulatory infusion clinic has accepted internal and external referrals for SARS-CoV-2 mAb treatments. This infusion clinic has administered >5000 mAb doses. Patients are referred via an internal electronic medical record (EMR) order or online community referral form. Due to limited sotrovimab supply and infusion chair space, all referrals during the Omicron surge were screened for positive test results, symptom onset timing and risk factors for severe COVID-19 disease by three infectious diseases-trained, antimicrobial stewardship pharmacists. To ensure equitable and timely administration of treatment, we devised an internal scoring system (Table S1, available as Supplementary data at JAC Online) to prioritize patients at highest risk. Our weighted scoring system, which mirrored Mayo Clinic’s Monoclonal Antibody Screening Score, incorporated CDC and EUA risk factors for severe COVID-19 and NIH patient prioritization for outpatient anti-SARS-CoV-2 therapies.3,4,7,11,15 This scoring algorithm was weighted to offer treatment to severely immunocompromised patients and unvaccinated patients with multiple comorbidities. Our institution’s threshold score to offer either therapeutic agent was ≥10.
Patients who received a dose of sotrovimab 500 mg (n = 88) or were initiated on a 3 day course of remdesivir (200 mg on day 1, followed by 100 mg daily on days 2 and 3) (n = 82) from 27 December 2021 to 4 February 2022 were included. The decision to administer remdesivir or sotrovimab was primarily dependent on the weekly allocation of sotrovimab at our institution. If available, sotrovimab was our preferred therapeutic agent. We only offered remdesivir to outpatients when sotrovimab was unavailable, which occurred during a 3 week span in the middle of the study time period. Based on mounting evidence that SARS-CoV-2 mAbs should be administered early, patients were eligible to receive sotrovimab within 7 days from symptom onset.7,16 Due to limited infusion chair space and poor external validity of PINETREE for both the Omicron variant and in vaccinated high-risk outpatients, remdesivir was only offered to patients if they had symptoms ≤5 days. This decision to use a stricter time from symptom onset was based on the median duration of symptoms before first infusion of 5 days (IQR = 3–6 days) in PINETREE.10
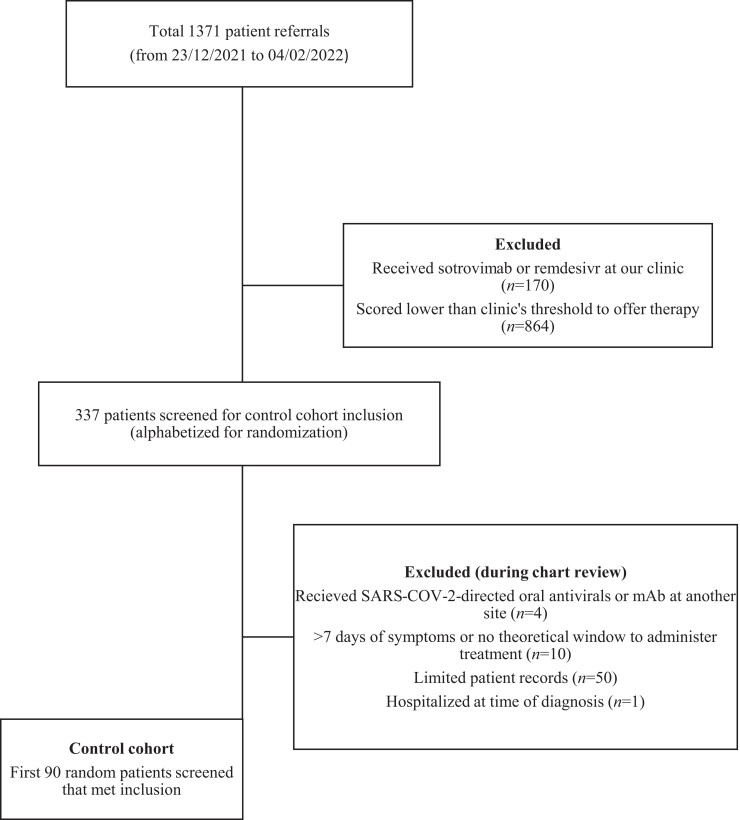
Our control cohort consisted of randomly selected high-risk outpatients who did not receive remdesivir or sotrovimab (n = 90). These patients were referred to our clinic, scored high enough to be allocated treatment and were offered either remdesivir or sotrovimab; however, they declined treatment, were unable to be contacted for scheduling before timing out from symptom onset, had transportation issues or had a major drug interaction (e.g. chronic hydroxychloroquine) with remdesivir. An initial list of 1371 referrals during the study period (Figure 1) was generated from our EMR (Epic Systems Corp., Verona, WI, USA). This list incorporated our internal risk factor scoring comments, enabling exclusion of patients with scores lower than our threshold to offer therapy. After cross-referencing patients who received treatment with remdesivir or sotrovimab at our clinic, the list was uploaded into a spreadsheet and alphabetized based on last name for randomization. Patients were screened for inclusion starting from the beginning of the alphabet. During chart review, patients were excluded if they received a SARS-CoV-2-directed oral antiviral or mAb (inactive or active against Omicron) at another site, had more than 7 days of symptoms from referral date or were immediately hospitalized at the time of initial COVID-19 diagnosis. To make a fair comparison with treatment cohorts, we excluded patients who did not have a theoretical window of ≥48 h from referral date for treatment administration in the outpatient setting. A pre-specified targeted number of control patients was determined to be 90 to closely match the volume of patients in each of the treatment arms. Thus, the first 90 randomly listed patients meeting inclusion were included as controls.
Figure 1.
Control cohort patient selection flow diagram.
Beginning in January 2021, our institution’s Esoteric/R&D Laboratory began to monitor the molecular epidemiology of SARS-CoV-2 by randomly sequencing samples from inpatients and outpatients with a confirmed COVID-positive PCR test and cycle threshold value ≤28. PCR positivity and virus lineage identification were tracked on an internal COVID-19 dashboard and sequences were submitted to the Global Initiative on Sharing Avian Influenza Data (GISAID) database. With this data, we were able to precisely determine when Omicron surpassed Delta and became the most predominant variant in our region, which occurred before Christmas Day 2021 (Table 1). Additionally, it heavily informed our decision to stop offering casirivimab/imdevimab before its EUA revocation and switch to sotrovimab or remdesivir on 27 December 2021.
Table 1.
Onset of Omicron (B.1.1.529) predominance – institutional sequencing data
| Date | Number of positive cases per week, n | Number of samples sequenced, n (%) | Number of samples identified as B.1.1.529, n (%) |
|---|---|---|---|
| 11/12/2021 | 42 | 30 (71.4) | 3 (10) |
| 18/12/2021 | 82 | 37 (45.1) | 20 (54) |
| 25/12/2021 | 316 | 58 (18.4) | 53 (91.4) |
| 01/01/2022 | 1150 | 48 (4.2) | 45 (94) |
| 08/01/2022 | 1616 | 108 (6.7) | 108 (100) |
Primary and secondary outcomes
The primary outcome was a composite of COVID-19-related hospitalizations and ED visits within 29 days from symptom onset (day zero) for all cohorts. Hospitalization was denoted as an acute care stay of ≥24 h. A patient’s initial COVID-19 diagnosis made in the ED did not count as an ED visit. Pre-specified secondary outcomes included the incidence of each component of the primary endpoint, 29 day all-cause mortality and adverse drug events in the treatment cohorts.
Statistical analyses
Descriptive statistics were used to summarize patient and disease characteristics, with continuous variables summarized as mean ± SD and categorical variables summarized as rates. The difference in continuous variables amongst COVID-19 subjects receiving versus not receiving treatment was assessed using one-way analysis of variance (ANOVA) and 3 × 2 χ2 test for categorical variables. The unadjusted associations between categorical variables and compared groups were assessed and summarized as OR along with 95% CI. Alpha level was set at 0.05 for all analyses. The percentage of patients who were hospitalized or visited the ED by day 29 was determined with Kaplan–Meier analysis. All data analyses were performed using Stata v17.0 statistical analysis software (StataCorp, College Station, TX, USA).
Results
Patient demographics and characteristics
As shown in Table 2, treatment and control groups were well matched. A higher proportion of Hispanic individuals was observed in the control arm; a majority (93%) of these patients either refused our offer for treatment or were unavailable for scheduling. A significantly higher proportion of patients in the sotrovimab arm were immunocompromised (92%; P = 0.00008), which included patients with solid organ transplants, active cancer on chemotherapy and humoral immunity deficits. The most predominant immunocompromised population in all cohorts were solid organ transplant patients, specifically kidney transplants (Table 3). Other significant differences between cohorts included higher rates of unvaccinated and cardiovascular disease in the control arm, with chronic kidney disease being more predominant in the sotrovimab cohort. In fully vaccinated patients, type of vaccine and receipt of a booster were similar between groups.
Table 2.
Patient demographics and baseline clinical characteristics
| Control (n = 90) | Remdesivir (n = 82) | Sotrovimab (n = 88) | P | |
|---|---|---|---|---|
| Age (years), mean (SD) | 55.2 (16.8) | 58 (14.2) | 55.8 (12.5) | 0.42 |
| Female, n (%) | 44 (49) | 45 (54.9) | 44 (50) | 0.71 |
| Race, n (%) | ||||
| Caucasian | 55 (61) | 51 (62.2) | 59 (67) | 0.68 |
| African American | 20 (22.3) | 15 (18.3) | 11 (12.5) | 0.23 |
| Hispanic | 14 (15.6) | 6 (7.3) | 4 (4.5) | 0.03 |
| other | 1 (1.1) | 10 (12.2) | 14 (15.9) | 0.006 |
| BMIa, mean (SD) | 30.8 (7.9) | 31.6 (7.3) | 29.8 (6.5) | 0.29 |
| Risk factors for severe COVID-19, n (%) | ||||
| ≥65 years | 31 (34.4) | 33 (40.2) | 22 (25) | 0.1 |
| BMIa ≥35 | 25 (27.8) | 27 (32.9) | 16 (18.2) | 0.08 |
| diabetes | 37 (41.2) | 38 (46.3) | 28 (32) | 0.14 |
| chronic kidney disease | 23 (25.6) | 25 (30.5) | 40 (45.5) | 0.014 |
| hypertension | 66 (73.4) | 55 (67.1) | 58 (66) | 0.52 |
| cardiovascular disease | 36 (40) | 20 (24.4) | 12 (13.6) | 0.0003 |
| chronic lung disease | 17 (18.9) | 22 (26.8) | 21 (23.9) | 0.46 |
| immunocompromised | 66 (73.3) | 53 (64.6) | 81 (92) | 0.00008 |
| pregnant | 2 (2.3) | 0 (0) | 0 (0) | 0.87 |
| unvaccinated | 31 (34.4) | 14 (17) | 21 (23.9) | 0.03 |
| total risk factors, mean (SD) | 4.1 (1.3) | 3.9 (1.4) | 3.7 (1.6) | 0.17 |
| Initial vaccine series completed, n (%) | 59 (65.6) | 68 (83) | 67 (76.1) | 0.03 |
| BNT162b2 (Pfizer-BioNTech) | 33 (56) | 39 (57.3) | 50 (74.6) | 0.05 |
| mRNA-1273 (Moderna) | 19 (32) | 23 (33.8) | 14 (20.9) | 0.2 |
| Ad26.COV2.S (Johnson & Johnson) | 2 (3) | 1 (1.5) | 0 (0) | 0.92 |
| unknown | 5 (9) | 5 (7.4) | 3 (4.5) | 0.65 |
| time from completed initial vaccine series to positive test (days), mean (SD) | 274.5 (77.1) | 271.8 (79.9) | 291.7 (57.4) | 0.26 |
| Booster dose received, n (%) | 32 (35.6) | 36 (43.9) | 43 (48.9) | 0.18 |
| time from booster dose to positive test (days), mean (SD) | 113.4 (44.9) | 93.8 (50.2) | 116.1 (48.4) | 0.14 |
| Referral timeliness | ||||
| time from positive test to referral (days), mean (SD) | 1.2 (1.3) | 1.1 (1.3) | 0.9 (1.3) | 0.66 |
| time from symptom onset to referral (days), mean (SD) | 2.9 (1.4) | 2.3 (1.4) | 2.6 (1.6) | 0.026 |
| time from symptom onset to 1st dose (days), mean (SD) | - | 4 (1.4) | 4.4 (1.7) | 0.09 |
Calculated as weight in kg divided by height in m2.
Table 3.
Distribution of immunocompromised patients
| Control (n = 66) | Remdesivir (n = 53) | Sotrovimab (n = 81) | |
|---|---|---|---|
| Solid organ transplant, n (%) | 49 (74.2) | 36 (67.9) | 57 (70) |
| kidney | 28 (42.4) | 21 (39.6) | 34 (42) |
| liver | 4 (6.1) | 2 (3.8) | 3 (3.7) |
| pancreas | 0 (0) | 1 (1.9) | 0 (0) |
| heart | 11 (16.7) | 9 (17) | 10 (12.3) |
| lung | 2 (5.6) | 1 (1.9) | 8 (9.9) |
| dual organ | 4 (6.1) | 2 (3.8) | 2 (2.5) |
| Haematology/oncology, n (%) | 5 (7.6) | 4 (7.5) | 6 (7.4) |
| Other, n (%) | 13 (19.7) | 14 (26.4) | 18 (22.2) |
Our control group consisted of patients at highest risk for severe COVID-19, but were not able to receive either therapy. These patients refused therapy (n = 47, 52.2%), were unavailable to be scheduled (n = 38, 42.2%), had a remdesivir drug interaction with chronic hydroxychloroquine (n = 3, 3.3%) or were unable to get to clinic (n = 2, 2.3%). Of note, 18 control patients who declined therapy were unvaccinated at the time of refusal. Additionally, four control patients were prescribed alternative COVID-19 therapeutics.
Mean ± SD duration from symptom onset to administration of sotrovimab and remdesivir was similar (4.4 ± 1.7 and 4 ± 1.4 days, respectively). There was a statistically significant difference in time from symptom onset to referral placement amongst the cohorts; however, this difference was not numerically dissimilar. Of the 82 remdesivir patients, the majority (95%) received a full 3 day treatment.
Primary outcome
Patients treated with remdesivir were significantly less likely to be hospitalized or visit the ED within 29 days from symptom onset compared with control patients (11% versus 23.3%), resulting in an OR = 0.41 (95% CI = 0.17–0.95) and an absolute risk reduction (ARR) = 12.4% (Table 4). This significant reduction represents a number needed to treat (NNT) = 9 (95% CI = 4.3–76.6).
Table 4.
Primary outcome
| Control (n = 90) | Remdesivir (n = 82) | Sotrovimab (n = 88) | P | |
|---|---|---|---|---|
| Composite of COVID-19-related 29 day hospitalizations and/or ED visits, n (%) | 21 (23.3) | 9 (11) | 7a (8) | 0.008 |
| Remdesivir versus control | unadjusted OR = 0.41 (95% CI = 0.17–0.95) | 0.04 | ||
| Sotrovimab versus control | unadjusted OR = 0.28 (95% CI = 0.11–0.71) | 0.007 | ||
| Sotrovimab versus remdesivir | unadjusted OR = 0.7 (95% CI = 0.25–1.98) | 0.5 | ||
One patient in the sotrovimab arm had a hospitalization and ED visit within 29 days and was only counted once.
Patients in the sotrovimab cohort were also less likely to be hospitalized or visit the ED within 29 days when compared with control patients (8% versus 23.3%), resulting in an OR = 0.28 (95% CI = 0.11–0.71) and an ARR = 15.4%. This significant reduction represents an NNT = 7 (95% CI = 3.9–20.1).
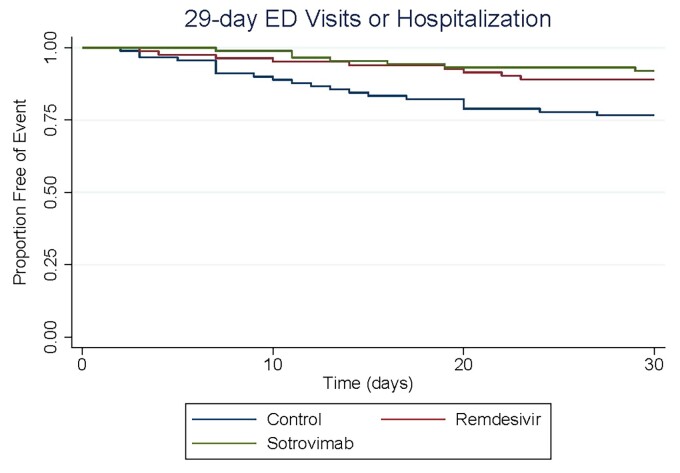
When comparing treatment groups, there was no difference in the primary outcome between sotrovimab and remdesivir (P = 0.5). Figure 2 shows the Kaplan–Meier analysis of time to 29 day hospitalization and/or ED visit related to COVID-19 (primary endpoint) for all three cohorts.
Figure 2.
Kaplan–Meier curve for primary outcome.
Secondary outcomes
Patients treated with remdesivir or sotrovimab were significantly less likely to visit the ED within 29 days from symptom onset (2.4% versus 1.2%, respectively) in comparison with control patients (11.1%; P = 0.004). Remdesivir-treated patients were significantly less likely to visit the ED within 29 days compared with control patients (OR = 0.2, 95% CI = 0.04–0.94) with an ARR = 8.7% (Table 5). This significant reduction represents an NNT = 12 (95% CI = 6.3–72.9). The sotrovimab cohort was associated with an OR = 0.09 (95% CI = 0.01–0.73) and an ARR = 10% for this same endpoint. This significant reduction represents an NNT = 11 (95% CI = 5.9–32.1).
Table 5.
Secondary outcomes
| Control (n = 90) | Remdesivir (n = 82) | Sotrovimab (n = 88) | P | |
|---|---|---|---|---|
| COVID-19-related 29 day hospitalization, n (%) | 11 (12.2) | 7 (8.5) | 7 (8) | 0.58 |
| COVID-19-related 29 day ED visit, n (%) | 10 (11.1) | 2 (2.4) | 1 (1.2) | 0.004 |
| remdesivir versus control | unadjusted OR = 0.2 (95% CI = 0.04–0.94) | 0.04 | ||
| sotrovimab versus control | unadjusted OR = 0.09 (95% CI = 0.01–0.73) | 0.02 | ||
| COVID-19-related 14 day hospitalization, n (%) | 8 (8.9) | 4 (5) | 3 (3.4) | 0.27 |
| COVID-19-related 14 day ED visit, n (%) | 6 (6.7) | 1 (1.2) | 1 (1.2) | 0.05 |
| 29 day all-cause mortality, n (%) | 1 (1.1) | 0 (0) | 0 (0) | 0.39 |
| Adverse drug event, n (%) | - | 1 (1.2) | 1 (1.2) | 0.96 |
Incidence of 29 day all-cause mortality was low in all arms, with only one death occurring in the control group. One patient in each treatment cohort experienced a serious adverse drug event requiring intervention. The sotrovimab patient developed right eye, lip and nostril blisters ∼1.5 days after infusion, requiring treatment with valaciclovir for probable herpes simplex reactivation. Of note, this occurred in a heart transplant recipient with no change in immunosuppression. The remdesivir adverse drug event occurred in a myasthenia gravis patient who experienced transient subjective confusion, left lower extremity numbness and right upper extremity numbness after their second infusion. This event resulted in an ED visit and was classified as a possible myasthenia gravis exacerbation secondary to remdesivir by the ED provider. This patient was instructed not to receive their third remdesivir dose.
Discussion
Our observational study shows that remdesivir and sotrovimab are effective interventions that significantly reduce the risk of hospitalization and/or ED visits in our highest-risk COVID-19 outpatients. Sotrovimab-treated patients had 72% reduced odds of the 29 day composite outcome with an absolute difference of 15.3%. Our sotrovimab data are similar to those of the COMET-ICE trial, which showed a 66% relative risk reduction in the composite endpoint of hospitalizations, ED visits and death.8 Remdesivir was associated with 59% reduced odds of the 29 day composite outcome with an absolute difference of 12.3%. In comparison, PINETREE showed remdesivir reduced the risk of 28 day hospitalization and death by 87%, which was predominantly driven by reduction in hospitalizations.10 Our study showed a lower NNT of 8 (versus 22 from the original trial). Of interest, patient compliance with three consecutive days of remdesivir at our infusion clinic was better than anticipated, with 95% of patients completing all three infusions. To the best of our knowledge, this is the first publication to demonstrate the real-world effectiveness of early, outpatient remdesivir during the era of SARS-CoV-2 vaccines and variants in addition to its direct comparison against an in vitro-active mAb.
The primary endpoint for both therapies was driven predominately by reduction in ED visits. Patients who received remdesivir or sotrovimab had 80% and 91% reduced odds of ED visits within 29 days from symptom onset, respectively. These results are consistent with the reduced severity observed with Omicron and effectiveness of vaccine prevention of severe COVID-19 disease and hospitalizations.17–19 Omicron is less likely to cause lower respiratory tract infections due to its reduced replication competence in lung parenchyma cells, which may help prevent severe disease in immunocompromised patients who have reduced vaccine response.20,21 Blunted vaccine response is especially evident in solid organ transplant patients, who accounted for 55% of our total study group. A concern with outpatient remdesivir is its reduced generalizability to fully vaccinated, high-risk patients (with/without booster doses) since PINETREE was conducted before widespread availability of SARS-CoV-2 vaccines. Our study demonstrates that the use of remdesivir is clinically impactful in the correct patient population (e.g. immunocompromised patients with possibly poor vaccine response).
Overall, patients receiving remdesivir and sotrovimab tolerated therapy well. We reported only one serious adverse event for each agent. In remdesivir’s outpatient trial, five (2%) patients were noted to have developed a serious adverse event, which lead to discontinuation in two individuals.10 Our remdesivir serious adverse event was a possible myasthenia gravis exacerbation. A case series reported three COVID-19 myasthenia gravis patients who tolerated remdesivir well and the package insert does not mention any contraindications.9,22 Sotrovimab was similarly well tolerated in its clinical trial, with 2% of participants experiencing a treatment-related adverse event.8 Our experience with both agents is comparable to previously published studies and reinforces their safety profiles.
Our study has several limitations. Due to its retrospective nature, this study may have been affected by confounding variables that a placebo-controlled, randomized clinical trial design would have eliminated. Since sotrovimab and remdesivir have already been shown to reduce healthcare exposure and received approval by FDA, it was unethical to withhold treatment to conduct a placebo-controlled study in our real-world setting. Due to small sample sizes, we could not determine whether specific comorbidities were independently associated with the primary outcome. However, there are a bevy of data demonstrating unvaccinated individuals and immunocompromised patients are at highest risk for severe COVID-19, hospitalization and death.3,11 Even though our groups were matched, a higher proportion of patients were unvaccinated in the control arm. Of these unvaccinated control patients, 58% (18 out of 31) refused our offer for treatment and 35.5% (11 out of 31) were unavailable for scheduling. These findings are not unexpected since patients with vaccine hesitancy are more likely to mistrust other forms of medical management.23
Patients who were hospitalized or visited the ED at another institution could have been missed in the primary endpoint; however, both treatment and control groups were at the same risk for this limitation. Also, we inherently introduced selection bias due to allocating therapies and matching them with a control group that was at highest risk for severe COVID-19. The higher rates of hospitalizations and ED visits amongst our groups may be an overestimation of their true incidence in the entire at-risk patient population, especially in patients with only one or two risk factors. Thus, our findings are most generalizable to immunocompromised patients and patients who are unvaccinated with multiple comorbidities.
As this manuscript was in preparation, sotrovimab distribution in the USA was stopped due to in vitro activity concerns against the BA.2 subvariant. Our internal sequencing results showed <2% of circulating SARS-CoV-2 were BA.2 in the Tampa Bay region from December 2021 to February 2022. It is likely our approach and results can be extrapolated to bebtelovimab, which has retained in vitro activity against BA.2.6 Additionally, the widespread availability of the oral COVID-19 antiviral agents (nirmatrelvir/ritonavir and molnupiravir) was sparse during our study period. Both antiviral medications may have similar clinical impact for these highest-risk outpatients in the era of vaccination; however, clinical trial data to support this are unavailable at this time.
In conclusion, our study demonstrates that outpatients with multiple risk factors for severe COVID-19 have reduced odds of hospitalization and ED visits when remdesivir or sotrovimab is administered early in disease. Our results support the clinical utility of mAbs as outpatient treatment for susceptible SARS-CoV-2 variants and provide evidence for the effectiveness of a 3 day course of remdesivir in our highest-risk outpatients. Although there are logistical and operational concerns for outpatient administration, remdesivir is a viable treatment clinicians can offer for COVID-19. Our active local molecular surveillance of circulating variants enhanced our ability to promptly adjust clinical treatment algorithms while our high-risk scoring matrix allowed us to optimize resource allocation while prioritizing patient outcomes.
Supplementary Material
Acknowledgements
Tampa General Hospital’s esoteric laboratory SARS-CoV-2 sequencing was made possible by the generous support of the TGH Foundation. We would like to thank John Couris, Dr Peggy Duggan and the TGH leadership team for their unstinting support of evidence-based clinical care.
Contributor Information
Nicholas Piccicacco, Department of Pharmacy, Tampa General Hospital, Tampa, FL, USA.
Kristen Zeitler, Department of Pharmacy, Tampa General Hospital, Tampa, FL, USA.
Austin Ing, Department of Pharmacy, Vanderbilt University Medical Center, Nashville, TN, USA.
Jose Montero, Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine, Tampa, FL, USA.
Jonathan Faughn, Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital, Tampa, FL, USA.
Suzane Silbert, Esoteric Testing/R&D and Microbiology Laboratories, Tampa General Hospital, Tampa, FL, USA.
Kami Kim, Division of Infectious Diseases, Department of Internal Medicine, University of South Florida Morsani College of Medicine, Tampa, FL, USA; Global Emerging Diseases Institute, Tampa General Hospital, Tampa, FL, USA.
Funding
This study was carried out as part of our routine work.
Transparency declarations
K.K. is a site investigator for several clinical trials in COVID-19 (Regeneron Pharmaceuticals, Inc.; Pfizer) and currently sits on the editorial board for the Sanford Guide. All other authors: none to declare.
Author contributions
Concept and design: N.P., K.Z., A.I. and J.M. Acquisition, analysis or interpretation of data: N.P., K.Z., A.I. and J.M. Drafting of manuscript: N.P., A.I., K.Z., J.F. and S.S. Critical revision of the manuscript for important intellectual content: K.K. and J.M. Statistical analysis: N.P. and K.Z.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Johns Hopkins University . COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html.
- 2. Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC . Underlying Medical Conditions Associated With Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Updated 15 June 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html.
- 4. Yek C, Warner S, Wiltz J et al. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 19–25. 10.15585/mmwr.mm7101a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Planas D, Saunders N, Maes P et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602: 671–7. 10.1038/s41586-021-04389-z [DOI] [PubMed] [Google Scholar]
- 6. FDA . Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Bebtelovimab. Updated June 2022. https://www.fda.gov/media/156152/download.
- 7. FDA . Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Sotrovimab. Updated March 2022. https://www.fda.gov/media/149534/download.
- 8. Gupta A, Gonzalez-Rojas Y, Juarez E et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327: 1236–46. 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilead Sciences, Inc . Veklury, Package Insert. Revised March 2022.
- 10. Gottlieb RL, Vaca CE, Paredes R et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386: 305–15. 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NIH . COVID-19 Treatment Guidelines. Updated 31 May 2022. https://www.covid19treatmentguidelines.nih.gov/.
- 12. Bhimraj A, Morgan RL, Shumaker AH et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. IDSA, 2022; Version 9.0.0. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
- 13. Agarwal A, Rochwerg B, Lamontagne F et al. A living WHO guideline on drugs for covid-19. BMJ 2020; 370: m3379. 10.1136/bmj.m3379. Erratum in: BMJ 2022; 377: o1045. [DOI] [PubMed] [Google Scholar]
- 14. FDA . FDA Takes Actions to Expand Use of Treatment for Outpatients With Mild-to-Moderate COVID-19. Updated 21 January 2022. https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19.
- 15. Razonable R, Ganesh R, Bierle DM. Clinical prioritization of antispike monoclonal antibody treatment of mild to moderate COVID-19. Mayo Clin Proc 2022; 97: 26–30. 10.1016/j.mayocp.2021.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piccicacco N, Zeitler K, Montero J et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients. Open Forum Infect Dis 2021; 8: ofab292. 10.1093/ofid/ofab292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iuliano A, Brunkard J, Boehmer T et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 146–52. 10.15585/mmwr.mm7104e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tseng HF, Ackerson BK, Luo Y et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med 2022; 28: 1063–71. 10.1038/s41591-022-01753-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrews N, Stowe J, Kirsebom F et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022; 386: 1532–46. 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hui K, Ho J, Cheung M et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022; 603: 715–20. 10.1038/s41586-022-04479-6 [DOI] [PubMed] [Google Scholar]
- 21. Lee A, Wong S, Chai L et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376: e068632. 10.1136/bmj-2021-068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters BJ, Rabinstein A, DuBrock HM. Use of remdesivir in myasthenia gravis and COVID-19. Pharmacotherapy 2021; 41: 546–50. 10.1002/phar.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stolle L, Nalamasu R, Pergolizzi J et al. Fact vs fallacy: the anti-vaccine discussion reloaded. Adv Ther 2020; 37: 4481–90. 10.1007/s12325-020-01502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.