Abstract
Our study in 21 pediatric cancer patients demonstrates that 3 doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA vaccine (BioNTech/Pfizer) elicited both humoral and cellular immunity in most patients during chemotherapy. Immunity was stronger in children with solid tumors and during maintenance therapy compared to those with hematological malignancies or during intensive chemotherapy.
Clinical Trials Registration. German Registry for Clinical Trials (DRKS00025254).
Keywords: child, cancer, vaccination, SARS-CoV-2
Compared with healthy individuals, children and adolescents treated for cancer have a higher risk of critical illness during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. When vaccination against SARS-CoV-2 became available by the end of 2020, the Standing Vaccination Commission (STIKO) in Germany recommended that all cancer patients receiving chemotherapy should be vaccinated against SARS-CoV-2 [2]. As uncertainties remained whether and to what extent the vaccine was effective in pediatric patients treated for cancer, we performed a prospective exploratory, longitudinal, observational study to examine the cellular and humoral immune response to 3 doses of messenger RNA (mRNA) vaccine in this patient population.
PATIENTS AND METHODS
The study enrolled pediatric cancer patients <18 years irrespective of the underlying malignancy who were on active treatment, had received at least 1 cycle of chemotherapy, and had a life expectancy of at least 3 months. Previous or ongoing infection with SARS-CoV-2, pregnancy. and primary immunodeficiency were exclusion criteria. Patients received 2 doses of the mRNA vaccine BNT162b2 (Comirnaty, BioNTech/Pfizer) within 3–6 weeks, and a booster vaccination between 4 weeks and 6 months after the second vaccination, which were administered preferentially at lymphocyte counts ≥1000 cells/µL. Vaccination could be delayed due to cancer treatment or complications of therapy. Primary study endpoints were humoral and cellular immune responses to vaccination against SARS-CoV-2, and the secondary endpoint was infection with SARS-CoV-2. The study was approved by the local Ethical Committees Frankfurt (2021-128), Münster (2021-467-b-S), and Freiburg (2021-1382), and was registered with the German Registry for Clinical Trials (DRKS00025254). All patients and caregivers provided written informed consent.
A prior infection was ruled out by assessing antibodies against the nucleocapsid antigen and the receptor-binding domain (RBD) of SARS-CoV-2 (Abbott Alinity i platform, Abbott Laboratories, Abbott Park, Illinois). During the study, all patients underwent regular respiratory polymerase chain reaction (PCR) testing irrespective of clinical symptoms (eg, prior to each hospitalization).
Two to 3 weeks after the first and second vaccination, respectively, blood was drawn for a complete blood count, lymphocyte subsets, immunoglobulin G (IgG) level, anti-RBD-IgG, neutralizing antibodies against the Delta variant (after the third vaccination also against the Omicron variant) in an authentic neutralizing assay, SARS-CoV-2–specific T cells, and antigen-specific memory B cells. The end of the study was defined by the time of the assessment of complete blood count, anti-RBD-IgG of SARS-CoV-2, and neutralizing antibodies 4–6 weeks after the third vaccination (Supplementary Figure 1). Tests could be postponed as long as the patient was pancytopenic after chemotherapy.
Neutralizing antibodies were assessed as described previously [3]. The enzyme-linked immunosorbent spot assay was used to detect SARS-CoV-2–specific T cells (T‐SPOT.COVID, Oxford Immunotec) and antigen-specific memory B cells [4, 5].
Differences between groups were analyzed using the Wilcoxon rank test for paired samples, and the Mann–Whitney U test for unpaired groups. The Spearman rank coefficient of correlation was calculated to evaluate the correlation between laboratory parameters such as leukocyte counts and SARS-CoV-2. A P value (2-tailed) < .05 was considered statistically significant. Analyses were performed using GraphPad Prism software version 5.0.2 (Graph Pad Software, San Diego, California).
RESULTS
Between March and October 2021, 21 pediatric patients (11 female) with a median age of 16.3 years (range, 13.2–17.9 years) were included in the study (Supplementary Table). Patients suffered from hematological (n = 14) and solid malignancies (n = 7), and 10 and 11 received intensive or lower-intensity maintenance chemotherapy, respectively. All but 1 patient, who died of tumor progression after the second vaccination, received 3 vaccinations. None of the 21 study participants had evidence of an infection with SARS-CoV-2 until the end of the study.
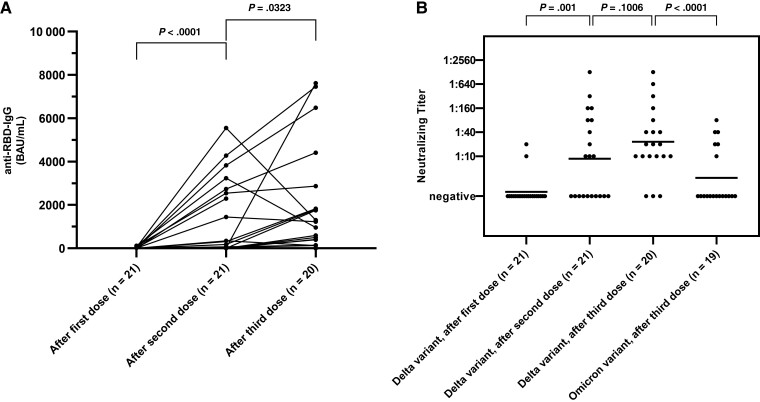
After 1 vaccine dose, 38.1% (8/21) had detectable antibodies against the RBD of SARS-CoV-2, and 76.2% (16/21) and 90% (18/20) after 2 and 3 doses, respectively, with statistically significant increases of geometric mean antibody titers of 3.3 (geometric standard deviation [GSD], 14.86), 103.7 (GSD, 27.97), and 612.5 (GSD, 8.905) binding antibody units (BAU)/mL (P < .0001 and P = .032) (Figure 1A). No significant correlation was observed between the lymphocyte count prior to vaccination and the anti-RBD-IgG concentration after the respective vaccine dose, and the antibody response of patients with lymphocyte counts ≥1000 cells/µL prior vaccination did not significantly differ from those with lymphocyte counts <1000 cells/µL, Supplementary Figure 2). Neutralizing antibodies against the Delta variant of SARS-CoV-2 with a titer >1:10 were detected in 4.7% (1/21) after the first dose, in 38.1% (8/21) after the second dose and in 55% (11/20) after the third dose of vaccine. After the third dose, 20.0% (5/20) had neutralizing antibodies against the Omicron variant (Figure 1B).
Figure 1.
A, Longitudinal results of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti–receptor-binding domain immunoglobulin G (IgG) test. Data points from individual study participants are connected. B, Neutralizing titers against the Delta variant of SARS-CoV-2 after the first, second, and third vaccine dose, and neutralizing titers against the Omicron variant after the third dose. The horizontal line indicates the median titer. Differences between groups were assessed using the Wilcoxon matched-pairs signed-rank test. Abbreviations: BAU, binding antibody units; IgG, immunoglobulin G; RBD, receptor-binding domain.
When comparing patients with solid and hematological malignancies, all 6 surviving patients with solid tumors (100%) and 12 of the 14 patients (85.7%) with hematological malignancies had detectable SARS-CoV-2 anti-RBD-IgG after the third dose (the deceased patient already had detectable SARS-CoV-2 anti-RBD-IgG after the second vaccination) (Supplementary Figure 3). Five of 6 patients with solid tumors (83.3%) and 7 of 14 patients (50%) with hematological malignancies had detectable neutralizing antibodies against the Delta variant (titer ≥1:20) after the third dose. After all 3 vaccine doses, patients with solid tumors had higher geometric mean anti-RBD-IgG concentrations and neutralizing titer against the Delta variant of SARS-CoV-2, which was statistically significant for anti-RBD-IgG after the third dose (P = .041).
Compared to the 10 pediatric patients who started vaccination during intensive chemotherapy, the 11 patients (10 at the third time point) starting vaccination during maintenance therapy developed higher levels of anti-RBD-IgG antibodies at all 3 time points (significant after the second vaccine dose; P = .0019) (Supplementary Figure 4). After the third dose, the mean geometric concentration of anti-RBD-IgG was 355.6 BAU/mL for patients on intensive anticancer treatment, and 1063 BAU/mL for those on maintenance therapy. Patients on maintenance therapy also demonstrated higher neutralizing antibody titers against the Delta variants after all 3 vaccine doses (significant after second and third vaccine dose; P = .003 and P = .026, respectively).
SARS-CoV-2–specific T cells could be evaluated in 15 and 18 patients after the first and second vaccination, respectively. Five patients (33%) had a positive result after the first vaccination (median number of spots, 3 [range, 0–25]) and 12 patients (67%) after the second vaccination (median, 10.5 [range, 0–181]) (P = .056). After the second vaccination, SARS-CoV-2–specific T cells did not significantly differ between patients with hematological and solid malignancies (7/12 vs 5/6; P = .29), but were detected in significantly more patients receiving maintenance than in those with intensive therapy (8/9 vs 4/9; P = .046). Discordant anti-RBD-IgG and T-cell responses after the first and second vaccination were observed in 7 of 15 (47%) and 4 of 18 patients (22%), respectively (first vaccination: 4/15 patients with detectable SARS-CoV-2 anti-RBD-IgG, but undetectable SARS-CoV-2–specific T cells, and 3/15 patients with negative results of SARS-CoV-2 anti-RBD-IgG but detectable SARS-CoV-2–specific T cells; second vaccination: 2/18 each). Similar results were observed when comparing Delta variant neutralizing antibodies and SARS-CoV-2–specific T cells (data not shown). The number of SARS-CoV-2–specific T cells assessed prior to the third vaccination correlated with the antibody response after booster (P = .0479, ρ = 0.4895) (Supplementary Figure 5).
SARS-CoV-2–specific memory B cells could be assessed after the first and second vaccine dose in 18 and 19 patients, respectively. Specific memory B cells were detected in 1 patient (6%) after the first vaccination and in 8 patients (42%) after the second vaccination. Positive results for memory B cells against SARS-CoV-2 did not significantly differ between hematological and solid malignancies and intensive and maintenance therapy, respectively. The frequency of SARS-CoV-2–specific memory B cells after the second vaccine dose did not correlate with the number of leukocytes, lymphocytes, SARS-CoV-2–specific T cells, and the production of antibodies against the SARS-CoV-2 RBD (ρ values between −0.22 and 0.36; Supplementary Figure 6).
DISCUSSION
In this exploratory study of 21 pediatric patients receiving treatment for cancer, a seroconversion of anti-spike RBD-IgG antibodies was observed in 90% of the patients after 3 mRNA vaccine doses, and each vaccine dose significantly increased the geometric mean antibody concentration. After 2 vaccine doses, the seroconversion rate was 76.2%, which, however, is lower compared to healthy adolescents [6]. Each of the 3 vaccine doses increased the median neutralizing antibody titer against the Delta variant, but notably, neutralization against the Omicron variant was lower, which has also been reported in a small group of adult cancer patients [7].
The humoral response was lower for patients with hematological malignancies compared to patients with solid tumors, for whom high immunogenicity of the mRNA vaccine has been reported [8]. Humoral response markers were also lower in patients who received intensive therapy compared to those receiving maintenance therapy, which has a lower immunosuppressive intensity.
The use of serology as the sole measure of protection might lead to an underestimation of the proportion of protected individuals, specifically when looking into protection against severe disease [9]. Our data showed that the specific T-cell response after 2 doses of SARS-CoV-2 vaccine was lower in children with cancer compared to healthy adults, but was higher than that of adult patients receiving immunosuppressive therapy, in whom only less than half of the patients elicited a specific T-cell response [10, 11]. Notably, in contrast to healthy individuals, adult patients with cancer often have discordant results in antibody and T-cell responses [12]. In line with our observation in pediatric cancer patients, it was reported that SARS-CoV-2–specific T cells were detectable in patients older than 18 years receiving chemotherapy for various malignancies even if an antibody response was absent [13]. In our study, SARS-CoV-2–specific T cells after the second, but not the first, vaccine dose correlated with the anti-RBD antibody response.
Memory B cells play an important role in viral control by generating antibody responses upon reexposure to the pathogen. Our data show that SARS-CoV-2–specific memory B cells can be detected after the second vaccination in about 40% of patients. In immunocompetent adults, SARS-CoV-2 spike and RBD-specific memory B cells increase after infection [14], and about two-thirds of adults exhibit SARS-CoV-2–specific memory B-cell responses after 1 year [5].
We recognize that our analysis has important limitations, as the number of patients included in our study is small, patients suffered from a variety of different malignancies, and strict adherence to the planned schedule of vaccination was not possible in some patients, as they suffered from treatment-related adverse events. In addition, due to different assays assessing the immune response, caution is needed when comparing study results.
Our data are unique and demonstrate that both humoral and cellular immune responses can be elicited in a significant proportion of pediatric patients receiving intensive or maintenance therapy for cancer. Our ongoing studies will reveal the benefit of the second booster dose and evaluate the long-term immunity against SARS-CoV-2 in this vulnerable patient population. However, it is unclear whether additional boosters will be needed, in particular in view of the emerging variants of SARS-CoV-2.
Supplementary Material
Contributor Information
Thomas Lehrnbecher, Division of Pediatric Hematology and Oncology, Hospital for Children and Adolescents, University Hospital, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
Ulrich Sack, Institute of Clinical Immunology, Medical Faculty, University of Leipzig, Leipzig, Germany.
Carsten Speckmann, Division of Pediatric Hematology and Oncology, Department of Pediatric and Adolescent Medicine, University Medical Center Freiburg, University of Freiburg, Freiburg, Germany.
Andreas H Groll, Infectious Disease Research Program, Center for Bone Marrow Transplantation, Department of Pediatric Hematology/Oncology, University Children’s Hospital, Muenster, Muenster, Germany.
Andreas Boldt, Institute of Clinical Immunology, Medical Faculty, University of Leipzig, Leipzig, Germany.
Benjamin Siebald, Division of Pediatric Hematology and Oncology, Hospital for Children and Adolescents, University Hospital, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
Simone Hettmer, Division of Pediatric Hematology and Oncology, Department of Pediatric and Adolescent Medicine, University Medical Center Freiburg, University of Freiburg, Freiburg, Germany.
Eva Maria Demmerath, Division of Pediatric Hematology and Oncology, Department of Pediatric and Adolescent Medicine, University Medical Center Freiburg, University of Freiburg, Freiburg, Germany.
Barbara Schenk, Institute of Medical Virology, University Hospital Frankfurt, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
Sandra Ciesek, Institute of Medical Virology, University Hospital Frankfurt, Johann Wolfgang Goethe University, Frankfurt am Main, Germany; Fraunhofer Institute for Translational Medicine and Pharmacology, Frankfurt am Main, Germany; German Center for Infection Research, External Partner Site Frankfurt, Braunschweig, Germany.
Jan Henning Klusmann, Division of Pediatric Hematology and Oncology, Hospital for Children and Adolescents, University Hospital, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
Christian Jassoy, Institute for Medical Microbiology and Virology, University Hospital and Medical Faculty, University of Leipzig, Leipzig, Germany.
Sebastian Hoehl, Institute of Medical Virology, University Hospital Frankfurt, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Katrin Lehner and Daniel Janisch (Institute of Medical Virology, Goethe University Frankfurt), Tina König, Katrin Bräutigam, and Katja Hintz (Institute for Medical Microbiology and Virology, University of Leipzig), and Judith Reemtsma (Institute of Clinical Immunology, University of Leipzig) for their technical assistance.
Potential conflicts of interest. T. L. received consulting fees and honoraria from Pfizer. S. C. received consulting fees and honorarium from BioNTech for serving on an advisory board. C. J. reports payment for expert testimony from Landschaftsverband Rheinland, Department for Social Decompensation and Institut für Medizinische und Pharmazeutische Prüfungsfragen. J. H. K. reports grants or contracts unrelated to this work from Deutsche Forschungsgemeinschaft (KL2374/3-1, KL2374/5-1), European Research Council for the European Union’s Horizon 2020 research and innovation program (grant agreement number 714226), St Baldrick’s Robert Arceci Innovations Award (551589), and the German Federal Ministry of Education and Research (01GM1911D myPred); consulting fees from Bluebird Bio, Novartis, Roche, and Jazz Pharmaceuticals; and participation on a data safety and monitoring board or advisory board for Bluebird Bio, Novartis, Roche, and Jazz Pharmaceuticals. S. Ho. reports speaker’s fees from Streamedup GmbH and Sanofi Genzyme and receipt of materials and reagents for other study from Roche Diagnostics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 2021; 22:1416–26. doi: 10.1016/S1470-2045(21)00454-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vygen-Bonnet S, Koch J, Berner R, et al. STIKO decision on the 4th update of the COVID-19 vaccination recommendation and the associated scientific justification [in German]. Epidemiol Bull 2021; 23:33–9. doi: 10.25646/8596 [DOI] [Google Scholar]
- 3. Delbrück M, Hoehl S, Toptan T, et al. Characterization of the humoral immune response to BNT162b2 in elderly residents of long-term care facilities five to seven months after vaccination. medRxiv [Preprint]. Posted online 10 November 2021. doi: 10.1101/2021.11.09.21266110 [DOI] [Google Scholar]
- 4. Kruse M, Dark C, Aspden M, et al. Performance of the T-SPOT.COVID test for detecting SARS-CoV-2-responsive T cells. Int J Infect Dis 2021; 113:155–61. doi: 10.1016/j.ijid.2021.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kannenberg J, Trawinski H, Henschler R, Buhmann R, Honemann M, Jassoy C. Antibody course and memory B-cell response in the first year after SARS-CoV-2 infection [manuscript published online ahead of print 1 February 2022]. J Infect Dis 2022. doi: 10.1093/infdis/jiac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021; 385:239–50. doi: 10.1056/NEJMoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fendler A, Shepherd STC, Au L, et al. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet 2022; 399:905–7. doi: 10.1016/S0140-6736(22)00147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Revon-Riviere G, Ninove L, Min V, et al. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer 2021; 154:30–4. doi: 10.1016/j.ejca.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fendler A, de Vries EGE, GeurtsvanKessel CH, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol 2022; 19:385–401. doi: 10.1038/s41571-022-00610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell imm responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021; 39:1034–6. doi: 10.1016/j.ccell.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586:594–9. doi: 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 12. McKenzie DR, Munoz-Ruiz M, Monin L, et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell 2021; 39:1445–7. doi: 10.1016/j.ccell.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol 2022; 196:548–58. doi: 10.1111/bjh.17877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med 2021; 2:100354. doi: 10.1016/j.xcrm.2021.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.