ABSTRACT
Background
Sotrovimab is a neutralizing monoclonal antibody (mAb) that seems to remain active against recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. The evidence on its use in kidney transplant (KT) recipients, however, is limited.
Methods
We performed a multicenter, retrospective cohort study of 82 KT patients with SARS-CoV-2 infection {coronavirus disease 2019 [COVID-19]} treated with sotrovimab.
Results
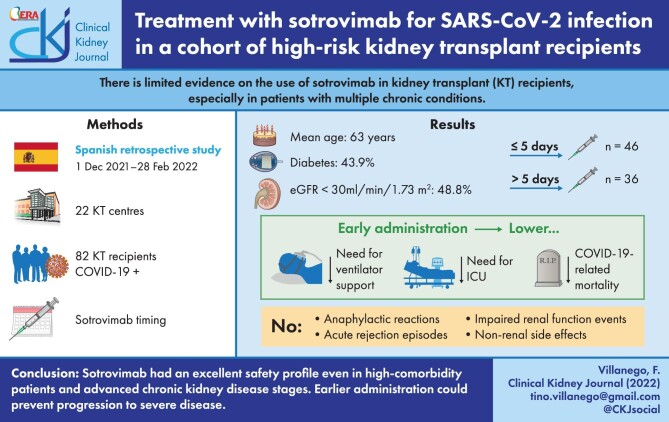
Median age was 63 years. Diabetes was present in 43.9% of patients, and obesity in 32.9% of patients; 48.8% of patients had an estimated glomerular filtration rate under 30 mL/minute/1.73 m2. Additional anti–COVID-19 therapies were administered to 56 patients, especially intravenous steroids (65.9%). Sotrovimab was administered early (<5 days from the onset of the symptoms) in 46 patients (56%). Early-treated patients showed less likely progression to severe COVID-19 than those treated later, represented as a lower need for ventilator support (2.2% vs 36.1%; P < .001) or intensive care admission (2.2% vs 25%; P = .002) and COVID-19–related mortality (2.2% vs 16.7%; P = .020). In the multivariable analysis, controlling for baseline risk factors to severe COVID-19 in KT recipients, early use of sotrovimab remained as a protective factor for a composite outcome, including need for ventilator support, intensive care, and COVID-19–related mortality. No anaphylactic reactions, acute rejection episodes, impaired kidney function events, or non-kidney side effects related to sotrovimab were observed.
Conclusions
Sotrovimab had an excellent safety profile, even in high-comorbidity patients and advanced chronic kidney disease stages. Earlier administration could prevent progression to severe disease, while clinical outcomes were poor in patients treated later. Larger controlled studies enrolling KT recipients are warranted to elucidate the true efficacy of monoclonal antibody therapies.
Keywords: COVID-19, immunosuppression, kidney transplantation, monoclonal antibodies, mortality
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Kidney transplant (KT) recipients have a greater risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection {coronavirus disease 2019 [COVID-19]} [1, 2]. Despite subsequent doses of mRNA vaccine, a significant number of KT patients do not develop an adequate humoral immune response [3, 4]. Thus, COVID-19 breakthrough infections and mortality are still markedly high [1, 2, 5, 6]. Therefore, it is necessary to implement alternative strategies to protect KT recipients from severe COVID-19 as well as new therapies against the virus.
Neutralizing monoclonal antibodies (mAbs) directed at SARS-CoV-2 spike proteins have emerged as a new approach to reduce viral load and mitigate the risk of COVID-19 progression to severe disease [7]. Various formulations have become available in recent months, but most have shown poor efficacy against the new SARS-CoV-2 strains [8]. Sotrovimab has been designed to bind a more conserved region of the receptor-binding domain. Consequently, its potential to remain active against new variants, such as omicron, is greater [9].
Despite solid organ transplant (SOT) patients being considered eligible for mAb therapies, they were not included in the early clinical trials [9]. Some observational studies have reported the experience using bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab in SOT recipients [10–16]. Notwithstanding, there are no large series on the effectiveness and safety of sotrovimab. To date, an isolated clinical case and limited case series have reported favorable results [17–20]. Similar good clinical outcomes were communicated by Chavarot et al. [20] in 25 KT patients compared with controls and by Fernandes et al. in 31 KT recipients [21]. Therefore, we aimed to analyze the outcomes of a national cohort of COVID-19–positive KT patients in Spain treated with this mAb.
MATERIALS AND METHODS
Study design and participants
Sotrovimab was approved in Spain in December, 2021 [22]. We performed a multicenter, retrospective cohort study of KT patients with SARS-CoV-2 infection treated with sotrovimab in Spanish centers according to the Spanish Agency of Medicines and Medical Devices (AEMPS) and the European Medicines Agency criteria [23,24]. In its indication, sotrovimab is preferably administered within 5 days of the onset of symptoms for treating COVID-19 in adults and adolescents (≥12 years of age and weighting at least 40 kg) with negative SARS-CoV-2 serology who do not require supplemental oxygen and who are at increased risk of the disease becoming severe. SOT recipients are included among these patients [23,24]. The absence of hospitalization is not considered a criterion for its use. Special authorization may also be requested for its use in patients with severe COVID-19 who present with negative SARS-CoV-2 serology or in the context of a nosocomial outbreak [23]. In high-risk patients, such as SOT recipients with moderate to severe disease, the need for supplemental oxygen is not a mandatory requirement according to the AEMPS criteria [24].
The analyzed period was from December 1, 2021, to February 28, 2022. During the study period, the predominant variant in Spain was omicron BA.1 in more than 95% of the cases of SARS-CoV-2 infection. In February 2022, the first cases of BA.2 were detected, and this lineage spread rapidly until it became responsible for 20% of infections reported at the end of that month [25]. All centers of the Transplant Working Group of the Spanish Society of Nephrology (SENTRA) who had prescribed sotrovimab to KT recipients were invited to participate.
Variables collected and definitions
Data included information about demographics, comorbidities, transplant characteristics, and immunosuppressive treatment. Hypertension and diabetes were considered when the patient required treatment for these diseases. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) under 60 ml/minute/1.73 m2. The eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation. More detailed epidemiologic and clinical data pertaining to COVID-19 were also required: diagnosis date, symptoms, oxygen saturation, and lymphocyte count at diagnosis; SARS-CoV-2 serology; other anti–COVID-19 therapies; hospitalization; and outcomes.
In all cases, serology was immediately requested before sotrovimab administration. Commercial assays for COVID-19 serology used by the centers appear in supplementary Table S1. All assays were approved by the Spanish Ministry of Health. Serology was considered negative if antibody titers against the spike protein were below the threshold or in the gray zone (undetermined), according to the manufacturer of the test. Hospitalization was indicated according to the Spanish guidelines for COVID-19 clinical management [26]. In addition, in most centers, patients with an indication for sotrovimab treatment were admitted because of the impossibility of administering the drug on an outpatient basis and because they usually presented with high-risk comorbidities. Therefore, hospitalization was not considered an indicator of disease progression. Outcomes were assessed as the need for mechanical ventilation or intensive care and COVID-19–related mortality.
To analyze safety outcomes, we collected adverse effects related to the sotrovimab infusion, acute rejection episodes, other episodes of acute kidney injury, and other non-kidney adverse events likely related to sotrovimab. Acute rejection was defined by histological findings or if the patient required empirical antirejection treatment for clinical suspicion. The criteria from the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline were applied to define acute kidney injury [27]. All eligible patients were approached for discussion about potential benefits and adverse reactions. For patients who agreed, the drug was requested from the AEMPS electronically and administered in less than 24 hours. The dose was 500 mg administered as a single intravenous infusion over 30 minutes [24]. Patients were monitored for at least 1 hour after infusion for related adverse effects.
Immunosuppression management after the COVID-19 diagnosis was similar in all centers. Antimetabolite drugs were withdrawn for 7 to 14 days after the onset of symptoms or reduced in high-immunologic-risk patients. Calcineurin inhibitor doses were decreased only in severely ill patients [28,29].
The study was conducted according to the guidelines dictated by the Declaration of Helsinki. Approval was granted by the Ethical Committee of Puerta del Mar Hospital.
Statistical analyses
Categorical variables were summarized as number and percentage and compared using the Fisher exact test or χ2 test, as appropriated. Continuous variables were presented as median with interquartile range, and the Mann-Whitney U test was used to compare groups. A composite outcome was defined as the need for ventilator support or intensive care or COVID-19–related death. Multivariable logistic regression was performed, including baseline recipient risk factors to severe COVID-19 and time from the onset of symptoms to sotrovimab infusion. Comorbidities already assessed using the Charlson Comorbidity Index were not included in the multivariable model. We used SPSS software, version 25.0 (SPSS Inc.) for statistical analysis. P < .05 was considered statistically significant.
RESULTS
During the period of the study, 82 KT recipients with SARS-CoV-2 infection were treated with sotrovimab in 22 Spanish centers. Genotype assessment was conducted in 41 patients. Omicron variant was detected in 40 cases (97.5%). A patient who acquired the SARS-CoV-2 infection by early December 2021 presented with delta. We did not have data on the lineages of the omicron variant.
Median follow-up was 51 days (minimum, 17 days). Table 1 summarizes baseline characteristics and outcomes. Patients included were older than 18 years, and the median age was 63 years. The median time after KT to COVID-19 diagnosis was 47 months. Three patients became infected during the first month after KT (4, 5, and 17 days after KT) and received sotrovimab in the first 5 days from the onset of symptoms, with recovery in all cases. Without considering CKD, 91% of patients had at least one comorbidity listed in Table 1, and 80% had 2 or more. Furthermore, 86.6% had CKD, and 48.8% had an eGFR under 30 mL/minute/1.73 m2. Tacrolimus-based immunosuppression was the most common drug combination. Respiratory symptoms were the most frequent COVID-19–related symptoms, and 64.6% of patients had pneumonia. Most recipients were hospitalized (81.7%). Eight patients were admitted only for sotrovimab infusion. Ventilatory support was needed for 17% of patients, and 12% required intensive care unit (ICU) admission. Additional anti–COVID-19 therapies were administered to 56 patients, especially intravenous steroids (65.9%). No patient received another mAb therapy. Seven recipients died from COVID-19 (8.5%). These patients were older and had more comorbidities, especially cardiovascular disease. They presented with more severe COVID-19 and with a higher incidence of pneumonia. The patients who died had received sotrovimab later than the patients who recovered.
Table 1.
Characteristics of kidney transplant recipients receiving sotrovimab and outcomes
| All | Recovered | Dead | ||
|---|---|---|---|---|
| Variable | (N = 82) | (n = 75) | (n = 7) | P-value |
| Males, n(%) | 44 (53.7) | 40 (53.3) | 4 (57.1) | >.99 |
| Recipient age, median (IQR), years | 63 (56–70) | 63 (56–69) | 72 (69–80) | .01 |
| Time after KT to COVID-19, median (IQR), monthsa | 47 (23–113) | 44 (22–111) | 52 (26–175) | .56 |
| Vaccination, n(%) | 76 (92.7) | 69 (92) | 7 (100) | >.99 |
| Number of doses of vaccine received | .58 |
|||
| Two doses, n(%) | 10 (13.2) | 10 (14.5) | 0 (0) | |
| Three doses, n(%) | 66 (86.8) | 59 (85.5) | 7 (100) | |
| Type of third dose of vaccine | .66 | |||
| mRNA-1273 Moderna, n(%) | 47 (69.1) | 43 (70.5) | 4 (57.1) | |
| BNT162b2 Pfizer-BioNTech, n(%) | 21 (30.9) | 18 (29.5) | 3 (42.9) | |
| SARS-CoV-2 IgG anti-S | .36 | |||
| Negative, n(%) | 74 (90.2) | 67 (89.3) | 7 (100) | |
| Positive (<100 BAU/mL), n(%) | 8 (9.8) | 8 (10.7) | 0 | |
| Baseline kidney graft function | ||||
| sCr, median (IQR), mg/dl | 1.9 (1.3–2.7) | 1.8 (1.3–2.6) | 2.8 (1.7–3.1) | .14 |
| ACR, median (IQR), mg/g | 37 (3–255) | 39 (2–250) | 35 (16–970) | .54 |
| eGFR, median (IQR), mL/minute/1.73 m2) | 31 (19–50) | 31.4 (19–52) | 20.7 (16–35.2) | .11 |
| eGFR 30–59 mL/minute/1.73 m2, n(%) | 31 (37.8) | 29 (38.7) | 2 (28.6) | .55 |
| eGFR 15–29 mL/minute/1.73 m2, n(%) | 34 (41.5) | 30 (40) | 4 (57.1) | |
| eGFR <15 mL/minute/1.73 m2, n(%) | 6 (7.3) | 5 (6.7) | 1 (14.3) | |
| Other comorbidities | ||||
| Recipient age >65 years, n(%) | 39 (47.6) | 33 (44) | 6 (85.7) | .04 |
| BMI >30 kg/m2, n(%) | 26 (32.9) | 23 (31.9) | 3 (42.9) | .67 |
| Hypertension, n(%) | 72 (87.8) | 65 (86.7) | 7 (100) | .58 |
| Diabetesb, n(%) | 36 (43.9) | 33 (44) | 3 (42.9) | >.99 |
| Cardiovascular disease, n(%) | 22 (26.8) | 17 (22.7) | 5 (71.4) | .01 |
| Cerebrovascular disease, n(%) | 7 (8.5) | 7 (9.3) | 0 (0) | .52 |
| Chronic liver disease, n(%) | 5 (6.1) | 5 (6.7) | 0 (0) | .63 |
| Chronic lung disease, n(%) | 13 (15.9) | 12 (16) | 1 (14.3) | >.99 |
| Active cancer, n(%) | 2 (2.4) | 2 (2.7) | 0 (0) | >.99 |
| CCI, median (IQR) | 5 (3–6) | 5 (3–6) | 8 (6.5–9) | .005 |
| Prior SARS-CoV-2 infection, n(%) | 3 (3.6) | 3 (4) | 0 | .59 |
| Immunosuppressive therapy at COVID-19 diagnosis | ||||
| Prednisone, n(%) | 67 (81.7) | 60 (80.0) | 7 (100) | .34 |
| Tacrolimus, n(%) | 79 (96.3) | 72 (91.1) | 7 (100) | >.99 |
| Tacrolimus through, ng/dL | 7.1 (6–8.7) | 7.1 (5.9–9.1) | 6.8 (6–8) | .75 |
| Mycophenolate, n(%) | 72 (87.8) | 65 (86.7) | 7 (100) | .58 |
| Mycophenolate >1000 mg/day, n(%) | 47 (65.2) | 43 (66.2) | 4 (57.1) | .63 |
| mTOR inhibitor, n(%) | 7 (8.5) | 7 (9.3) | 0 (0) | .52 |
| Cyclosporine, n(%) | 2 (2.4) | 2 (2.7) | 0 (0) | >.99 |
| Immunosuppressive therapy within 2 years before COVID-19 diagnosis | ||||
| Thymoglobulin, n(%) | 10 (12.2) | 10 (13.3) | 0 (0) | .58 |
| Basiliximab, n(%) | 6 (7.3) | 6 (8) | 0 (0) | .57 |
| Rituximab, n(%) | 2 (2.5) | 2 (2.7) | 0 (0) | >.99 |
| Clinical features | ||||
| Fever, n(%) | 49 (59.8) | 44 (58.7) | 5 (71.4) | .69 |
| Upper respiratory tract symptomsa, n(%) | 61 (74.4) | 55 (73.3) | 6 (85.7) | .67 |
| Gastrointestinal symptoms, n(%) | 28 (34.1) | 23 (30.7) | 5 (71.4) | .04 |
| Dyspnea, n(%) | 45 (54.9) | 38 (46.3) | 7 (100) | .01 |
| Oxygen saturation at admission <95%, n(%) | 35 (42.6) | 30 (40) | 5 (71.4) | .43 |
| Pneumonia, n(%) | 53 (64.6) | 46 (61.3) | 7 (100) | .04 |
| Lymphopenia, n(%) | 62 (76.5) | 56 (75.7) | 6 (85.7) | >.99 |
| Lymphocyte countb, median (IQR), (×103/µL) | 400 (200–540) | 400 (270–540) | 210 (112–315) | .05 |
| Time from onset of symptoms to sotrovimab infusion, median (IQR), days | 5 (2–10.2) | 4 (2–10) | 13 (10–22) | .003 |
| Time from onset of symptoms to sotrovimab infusion <5 days, n(%) | 46 (56.1) | 45 (60) | 1 (14.3) | .04 |
| Hospitalized, n(%) | 67 (81.7) | 60 (80) | 7 (100) | .34 |
| Additional COVID-19 therapy | ||||
| Glucocorticoids, n(%) | 54 (65.9) | 47 (62.7) | 7 (100) | .08 |
| Tocilizumab, n(%) | 17 (20.7) | 14 (18.7) | 3 (42.9) | .15 |
| Remdesivir, n(%) | 21 (25.6) | 20 (26.7) | 1 (14.3) | .67 |
| COVID-19 outcomes | ||||
| Ventilator support, n(%) | 14 (17.1) | 7 (9.3) | 7 (100) | <.001 |
| ICU admission, n(%) | 10 (12.2) | 7 (9.3) | 3 (42.9) | .03 |
ACR: albumin-to-creatinine ratio; BAU: binding antibody unit; BMI: body mass index; CCI, Charlson Comorbidity Index; COVID-19: coronavirus disease 2019; eGFR: estimated glomerular filtration rate; ICU: intensive care unit; IgG: immunoglobulin G; IQR: interquartile range; KT: kidney transplant; mTOR: mammalian target of rapamycin; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; sCr: serum creatinine.
aCough, sore throat, rhinorrhea, or expectoration.
bOnly included patients with lymphopenia.
Most patients had been vaccinated with mRNA vaccines, with a median (interquartile range) time of 130 (106–150) days since the last dose of vaccine. More than 85% had received three doses before their COVID-19 diagnosis. Nevertheless, SARS-CoV-2 serology was negative in 74 patients. In the remaining eight cases, the immune response was low (anti–spike antibody titers <100 binding antibody units/mL), so the drug administration was approved by the AEMPS.
The median time from onset of symptoms to sotrovimab infusion was 5 days. Two patients presented with adverse events related to the infusion: one of them with low-grade fever and the other with headache that resolved with a dose of acetaminophen. No anaphylactic reactions were observed. No patients experienced acute rejection episodes, other impaired kidney function events, or non-kidney side effects related to sotrovimab. Nine patients had impaired kidney function events after sotrovimab infusion {median [interquartile range], 13.5 [6.75–26] days}, considered by the investigators to be unrelated to the drug: Eight patients developed acute kidney injury in the setting of severe COVID-19, and one patient presented with an episode of obstructive uropathy.
We compared all the variables listed in Table 1 according to the time from the onset of symptoms to sotrovimab administration. The differences between the two groups are shown in Table 2. Patients treated earlier {<5 days from symptom onset; n = 46 [56%]} tended to be younger, with a lower Charlson Comorbidity Index. Maintenance immunosuppressive therapy was similar, and kidney graft function was significantly better. The clinical picture of COVID-19 was less severe, as reflected by the lower incidence of respiratory and gastrointestinal symptoms, hypoxemia, lymphopenia, and pneumonia as well as the lower need for ventilatory support and admission to the ICU. Only one patient progressed to severe COVID-19, requiring admission to the ICU and ultimately death. Patients treated with sotrovimab later received intravenous steroids and tocilizumab more frequently (P < .001). There was no difference between the two groups in the treatment with remdesivir {n = 14 [30.4%] in the early sotrovimab therapy group vs n = 7 [19.4%] in late therapy group; P = .25}, and the time from the onset of the symptoms to its administration was also similar {6 [5.5–7.0] vs 6.5 [6.00–7.25] days; P = .32}. Regarding vaccination status, no differences were observed. Table S2 summarizes the univariable Cox regression analysis. In the multivariable analysis, adjusting for baseline recipient risk factors, early use of sotrovimab remained as a protective factor for the composite outcome (Table 3).
Table 2.
Comparison between kidney transplant recipients treated with sotrovimab early from the onset of symptoms and those treated late
| ≤5 days | >5 days | ||
|---|---|---|---|
| Variable | (n = 46) | (n = 36) | P-value |
| Recipient age, median (IQR), years | 62.5 (52–69) | 66.5 (58–72) | .07 |
| Baseline kidney graft function | |||
| sCr, median (IQR), mg/dL | 1.7 (1.2–2.4) | 2.4 (1.5–3) | .04 |
| eGFR, median (IQR), mL/minute/1.73 m2 | 35 (21–53) | 24 (19–46) | .09 |
| Other comorbidities | |||
| BMI ≥30 kg/m2, n(%) | 11 (24.4) | 15 (44.1) | .06 |
| Cerebrovascular disease, n(%) | 1 (2.2) | 6 (16.7) | .02 |
| CCI, median (IQR) | 4 (3–6) | 6 (3–7) | .08 |
| Clinical features | |||
| Upper respiratory tract symptomsa, n(%) | 30 (65.2) | 31 (86.1) | .03 |
| Gastrointestinal symptoms, n(%) | 8 (14.4) | 20 (55.6) | <.001 |
| Dyspnea, n(%) | 15 (32.6) | 30 (83.3) | <.001 |
| Oxygen saturation at admission <95%, n(%) | 10 (21.7) | 25 (69.4) | .002 |
| Pneumonia, n(%) | 18 (39.1) | 35 (97.2) | <.001 |
| Lymphopenia, n(%) | 30 (66.7) | 32 (88.9) | .02 |
| Time from onset of symptoms to sotrovimab infusion, median (IQR), days | 2 (1–4) | 11.5 (8–17.7) | <.001 |
| Hospitalized, n(%) | 31 (67.4) | 36 (100) | <.001 |
| Additional COVID-19 therapy | |||
| Glucocorticoids, n(%) | 23 (50) | 31 (86.1) | .001 |
| Tocilizumab, n(%) | 3 (6.5) | 14 (38.9) | <.001 |
| COVID-19 outcomes | |||
| Ventilator support, n(%) | 1 (2.2) | 13 (36.1) | <.001 |
| ICU admission, n(%) | 1 (2.2) | 9 (25) | .002 |
| Dead, n(%) | 1 (2.2) | 6 (16.7) | .02 |
All the variables analyzed in Table 1 were compared. Only those with P < .1 are shown. BMI: body mass index; CCI: Charlson Comorbidity Index; COVID-19: coronavirus disease 2019; eGFR: estimated glomerular filtration rate; ICU: intensive care unit; IQR: interquartile range; sCr: serum creatinine.
aCough, sore throat, rhinorrhea, or expectoration.
Table 3.
Multivariable logistic regression focused on recipient risk factorsa
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Male | 1.291 (0.185–9.016) | .797 |
| Recipient age | 1.138 (1.002–1.291) | .046 |
| CCI | 0.729 (0.416–1.277) | .269 |
| BMI, kg/m2 | 0.887 (0.691–1.139) | .348 |
| KT vintage, days | 1.000 (1.000–1.000) | .886 |
| Mycophenolate ≥1000 mg/day | 0.217 (0.029–1.639) | .139 |
| Polyclonal and/or monoclonal immunosuppressive antibodies in the past 2 yearsb | 0.507 (0.010–26.383) | .737 |
| Sotrovimab administration ≤5 days from onset of symptoms | 0.026 (0.002–0.346) | .006 |
aFor the composite outcome defined as the need for ventilator support, intensive care, or COVID-19–related death. BMI: body mass index; CCI: Charlson Comorbidity Index; CI: confidence interval; COVID-19: coronavirus disease 2019; KT: kidney transplant; OR: odds ratio.
bThymoglobuline, basiliximab, and rituximab.
DISCUSSION
We present the first large series to date reporting on the results sotrovimab use in a national cohort of KT recipients. Unlike other reports, our study includes older patients, with a mean age over 60 years, and with a high incidence of many other risk factors for severe COVID-19, such as CKD, diabetes, obesity, and cardiovascular disease. Despite this, our results illustrate that sotrovimab has an excellent safety profile. In addition, especially when the drug is administered in the first few days after the onset of symptoms, it seems to show effectiveness and may prevent progression to severe disease in these high-risk patients.
We collected 82 KT recipients from a multicenter, collaborative study. Our results identified that deceased patients were older and had a higher comorbidity burden, as previously described [30]. Additionally, recipients who recovered received early administration of sotrovimab more often. Based on the results of the clinical trial COMET-ICE, sotrovimab should be administered as soon as possible, preferably through the fifth day after the onset of symptoms [9]. Thus, the early administration of sotrovimab will achieve a more rapid decline in viral load, modulating the development of a systemic inflammatory response and slowing progression to a more severe form of the disease [7]. In our cohort, KT recipients were more complex than patients included in previous studies with other mAb therapies [10–16, 18–21]. Probably for this reason, more than half of them received other anti–COVID-19 therapies in addition to sotrovimab. Thus, it is not possible to assess the effectiveness of sotrovimab by itself. Despite the multiple chronic conditions predisposing patients to severe COVID-19 and the high presence of hypoxemia and pneumonia, however, only one patient progressed to severe disease and died in the group that received early sotrovimab, which could support its use in the first days. In our experience, controlling for baseline risk factors to severe COVID-19 in KT recipients, early use of sotrovimab remained as a protective factor against the need for ventilator support, ICU admission, and COVID-19–related mortality.
In contrast, the late prescription of sotrovimab has not proven to be effective in the general population [31], but there are no specific data on KT recipients. We have detected a negative evolution in the group treated late: Approximately 30% of patients required ICU admission and ventilator support, and 16.7% died. Despite these patients tending to be older and having a greater comorbidity burden, when we adjusted for these factors, early administration of sotrovimab remained a protective factor. Nevertheless, these patients probably presented with greater severity at admission, as reflected by the higher incidence of hypoxemia. Therefore, we cannot affirm that the late administration of sotrovimab was the only cause of the differences in the outcomes. In any case, results have been poor in this group, which is consistent with the lack of efficacy of the treatment in the most advanced stages of the disease reported in previous clinical trials [31].
We observed an excellent drug tolerance in our KT recipients. The safety of this drug should be highlighted because, unlike other SOTs, impaired kidney function may limit the administration of some treatments for COVID-19 in KT recipients. One of the most widely used drugs against SARS-CoV-2 infection, remdesivir, was contraindicated in patients with an eGFR under 30 ml/minute/1.73 m2. Despite safe experiences in KT recipients, reports are limited in this population, especially among those with advanced CKD, and may not be authorized by the drug agencies [32]. Nirmatrelvir/ritonavir has been shown to reduce the risk of hospitalization or death, but its use is still limited in KT patients for several reasons [33]. Ritonavir is a potent cytochrome P450 (CYP) 3A and P-glycoprotein inhibitor, producing strong pharmacological interactions with the usual immunosuppressive treatment. Nirmatrelvir/ritonavir prescription may also be limited by the difficulty of monitoring trough levels of calcineurin inhibitors in COVID-19–positive outpatients. Furthermore, in patients with moderate kidney impairment (eGFR ≥30 to <60 mL/minute/1.73 m2), the dose of nirmatrelvir/ritonavir must be reduced (with a dose adjustment not clinically tested), and it is not recommended when eGFR is under 30 mL/minute/1.73 m2. In patients with grade 4 or 5 CKD, only sotrovimab and another antiviral, molnupiravir, are approved in Europe, but this drug has shown significantly less effectiveness than sotrovimab in recent data from the omicron era [34].
Information about sotrovimab safety in CKD is based only on pharmacokinetic analyses in patients with mild or moderate kidney impairment [23]. Data on its real-world safety in KT recipients with CKD have not been reported until now. Among our patients, 86% had CKD, and almost half of them had an eGFR under 30 mL/minute/1.73 m2. No differences were noted in safety and clinical course according to eGFR, suggesting that sotrovimab is a valid therapeutic option in KT patients with advanced CKD.
The omicron variant is resistant against most currently developed mAb therapies. In contrast, sotrovimab may have a higher barrier to this resistance as a result of targeting a pan-sarbecovirus epitope [9]. Nonetheless, the efficacy of sotrovimab against omicron subvariant BA.2 is under research. Although in vitro studies suggest that sotrovimab has less neutralizing activity against omicron BA.2, the effects in vivo remain largely unknown [35]. MAbs might act in vivo through a combination of mechanisms that are not fully reflected by in vitro neutralization potency assays. Recently reported data in preprint literature reveal that despite the diminished neutralizing activity, sotrovimab treatment reduced viral RNA and proinflammatory cytokine levels in the lung of BA.2-infected mice substantially, showing resilience against emerging SARS-CoV-2 strains [36]. Furthermore, the researchers observed that sotrovimab protection in vivo was mediated at least in part by the effector Fc function interactions. Consistent with these results, Kawaoka et al. also reported that the drug can restrict viral infection in the respiratory organs of hamsters with BA.2 [37]. Another preprint report based on learning models also concludes that the drug would not be significantly affected by omicron BA.1 and BA.2 variants [38]. Nevertheless, the US Food and Drug Administration communicated a few weeks ago that sotrovimab is no longer authorized because the proportion of COVID-19 cases caused by the BA.2 variant is above 50% in the United States [39]. The new mAb bebtelovimab, not authorized in Europe, is a hopeful alternative because it seems to remain active in vitro against all omicron subvariants, but there are no clinical efficacy data from placebo-controlled trials evaluating its use in patients who are at high risk of progressing to severe COVID-19 [40]. In Europe, the European Medicines Agency has maintained the authorization of sotrovimab because the clinical relevance of the observed decrease in in vitro neutralization against omicron BA.2 is not known and, on May 6, 2022, has updated the product information [24].
Our study presents some limitations. It is a retrospective analysis, with the inherent biases of these studies. Second, we did not have a control group of untreated patients. All the participating centers had applied the Spanish treatment guidelines for COVID-19 in high-risk patients since approval of the drug in Spain, so it was not possible to have this control group [22]. Comparison should not be made with KT patients before sotrovimab approval because the epidemiologic situation and vaccination status were different. This homogenous cohort of seronegative individuals, however, provides a unique picture of progression risk among those lacking humoral immunity. Finally, a significant proportion of patients received other treatments against SARS-CoV-2 infection. For all these reasons, we cannot evaluate the effectiveness of the drug. We have, however, collected the most extensive clinical experience in KT recipients with this mAb, and we have observed that it can be a safe therapy. Finally, sotrovimab could be a replacement for other mAbs with greater efficacy against the new strains. Nevertheless, the knowledge acquired about this drug could guide the use of other mAbs in the setting of these high-risk patients.
CONCLUSION
Our multicenter study supports the safety of sotrovimab in KT recipients with COVID-19, even in high-comorbidity patients and those with advanced-stage CKD. Patients treated early were unlikely to progress to severe COVID-19, while clinical outcomes were poor in those treated later. Therefore, our experience reinforces the importance of an early diagnosis in these patients with a high rate of nonresponse to vaccines. It is necessary, however, to foster larger randomized clinical trials enrolling SOT recipients to elucidate the true effectiveness of mAb therapies in this highly vulnerable population.
Supplementary Material
Contributor Information
Florentino Villanego, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Auxiliadora Mazuecos, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Beatriz Cubillo, Department of Nephrology, Hospital Clínico San Carlos, Madrid, Spain.
M José Merino, Department of Nephrology, Hospital Universitario Juan Ramón Jiménez, Huelva, Spain.
Inmaculada Poveda, Department of Nephrology, Hospital Universitario Torrecárdenas, Almería, Spain.
Isabel M Saura, Department of Nephrology, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain.
Óscar Segurado, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Leónidas Cruzado, Department of Nephrology, Hospital General Universitario de Elche, Elche, Spain.
Myriam Eady, Department of Nephrology, Hospital Universitario de Jerez de la Frontera, Jerez de la Frontera, Spain.
Sofía Zárraga, Department of Nephrology, Hospital Universitario de Cruces, Bilbao, Spain.
M José Aladrén, Department of Nephrology, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Sheila Cabello, Department of Nephrology, Hospital Universitario Son Espases, Palma de Mallorca, Spain.
Verónica López, Department of Nephrology, Hospital Regional Universitario de Málaga, Universidad de Málaga, Instituto de Investigación Biomédica de Málaga, REDinREN (RD16/0009/0006), Málaga, Spain.
Esther González, Department of Nephrology, Hospital Universitario Doce de Octubre, Institute i+12 for Medical Research, Madrid, Spain.
Inmaculada Lorenzo, Department of Nephrology, Complejo Hospitalario Universitario de Albacete, Albacete, Spain.
Jordi Espí-Reig, Department of Nephrology, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
Constantino Fernández, Department of Nephrology, Complexo Hospitalario Universitario de A Coruña, A Coruña, Spain.
July Osma, Department of Nephrology, Hospital Universitario Doctor Peset, Valencia, Spain.
M Carmen Ruiz-Fuentes, Department of Nephrology, Hospital Universitario Virgen de las Nieves, Granada, Spain.
Néstor Toapanta, Department of Nephrology, Hospital Vall d´Hebron, Barcelona, Spain.
Antonio Franco, Department of Nephrology, Hospital General Universitario de Alicante, Alicante, Spain.
Carla C Burballa, Department of Nephrology, Hospital del Mar, Hospital del Mar Medical Research Institute, REDinREN (RD16/0009/0013), Barcelona, Spain.
Miguel A Muñoz, Department of Nephrology, Hospital Universitario de Toledo, Toledo, Spain.
Marta Crespo, Department of Nephrology, Hospital del Mar, Hospital del Mar Medical Research Institute, REDinREN (RD16/0009/0013), Barcelona, Spain.
Julio Pascual, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain; Department of Nephrology, Hospital Clínico San Carlos, Madrid, Spain.
Funding
The Spanish COVID-19 renal registry and the Transplant Working Group of the Spanish Society of Nephrology (SENTRA) are supported by the Spanish Society of Nephrology.
Authors’ Contributions
F.V., A.M., M.C., and J.P. designed the study, analyzed the data, and drafted the article. All authors revised the article, made substantial contributions, and approved the final version of the article. F.V. and A.M. have contributed equally to this work. M.C. and J.P. share senior authorship to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, A.M., upon reasonable request.
Conflict of interest statement
None declared.
REFERENCES
- 1. Qin CX, Moore LW, Anjan Set al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation 2021;105:e265–e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazuecos A, Villanego F, Zarraga Set al. Spanish Society of Nephrology COVID-19 Group . Breakthrough infections following mRNA SARS-CoV-2 vaccination in kidney transplant recipients. Transplantation 2022;106:1430–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stumpf J, Tonnus W, Paliege Aet al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation 2021;105:e267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quiroga B, Soler MJ, Ortiz Aet al. SENCOVAC collaborative network . Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study. Nephrol Dial Transplant 2022;37:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaghan CJ, Mumford L, Curtis RMKet al. NGSBT Organ and Tissue Donation and Transplantation Clinical Team . Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation 2022;106:436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman JR, Wigmore SJ.. Simple vaccination is not enough for the transplant recipient. Transplantation 2022;106:447–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen P, Nirula A, Heller Bet al. BLAZE-1 Investigators . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Eng J Med 2021;384:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreuzberger N, Hirsch C, Chai KLet al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst Rev 2021;9:CD013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta A, Gonzalez-Rojas Y, Juarez Eet al. COMET-ICE Investigators . Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Eng J Med 2021;385:1941–50. [DOI] [PubMed] [Google Scholar]
- 10. Angarone M, Kumar RN, Stosor V.. Organ transplant patients, COVID-19, and neutralizing monoclonal antibodies: the glass is half full. Transpl Infect Dis 2021;23:e13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yetmar ZA, Beam E, O'Horo JCet al. Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients. Open Forum Infect Dis 2021;8:ofab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kutzler HL, Kuzaro HA, Serrano OKet al. Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients. Transpl Infect Dis 2021;23:e13662. [DOI] [PubMed] [Google Scholar]
- 13. Fernandes G, Devresse A, Scohy Aet al. Monoclonal antibody therapy for SARS-CoV-2 infection in kidney transplant recipients: a case series from Belgium. Transplantation 2022;106:e107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarrell BA, Bloch K, El Chediak Aet al. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transplant Infect Dis 2022;24:e13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gueguen J, Colosio C, Del Bello Aet al. Early administration of anti–SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients. Kidney Int Rep 2022;7:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yetmar ZA, Bhaimia E, Bierle DMet al. Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: an analysis of symptomatic cases and monoclonal antibody therapy. Transpl Infect Dis 2022;24:e13779. [DOI] [PubMed] [Google Scholar]
- 17. AlKindi F, Chaaban A, Al Hakim Met al. Sotrovimab use for COVID-19 infection in pregnant kidney transplant recipient. Transplantation 2022;106:e277–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinchera B, Buonomo AR, Scotto ARet al. Federico II COVID team . Sotrovimab in solid organ transplant patients with early, mild/moderate SARS-CoV-2 infection: a single-center experience. Transplantation 2022;106:e343–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhand A, Okumura K, Wolfe Ket al. Sotrovimab for treatment of COVID-19 in solid organ transplant recipients. Transplantation 2022;106:e336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chavarot N, Melenotte C, Amrouche Let al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with omicron infection. Kidney Int 2022;101:1290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandes G, Devresse A, Scohy Aet al. Monoclonal antibody therapy in kidney transplant recipients with delta and omicron variants of SARS-CoV-2: a single-center case series. Kidney Med 2022;4:100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spanish Agency of Medicines and Medical Devices. Meeting of the Committee for Medicinal Products for Human Use in December 2021 [in Spanish]. January 4, 2022. https://www.aemps.gob.es/informa/boletines-aemps/boletin-chmp/2021-boletin-chmp/reunion-del-comite-de-medicamentos-de-uso-humano-chmp-de-diciembre-2021 (May 11, 2022, date last accessed) [Google Scholar]
- 23. Spanish Agency of Medicines and Medical Devices . Criteria to assess the administration of new antiviral therapeutic alternatives against SARS-CoV-2 infection (in order of prioritization) [in Spanish]. April 4, 2022. https://www.aemps.gob.es/medicamentos-de-uso-humano/acceso-a-medicamentos-en-situaciones-especiales/criterios-para-valorar-la-administracion-de-las-nuevas-alternativas-terapeuticas-antivirales-frente-a-la-infeccion-por-sars-cov-2/ (April 15, 2022, date last accessed) [Google Scholar]
- 24. European Medicines Agency . Xevudy. May 6, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/xevudy (May 11, 2022, date last accessed) [Google Scholar]
- 25. Government of Spain Ministry of Health . Update of the epidemiological situation of SARS-CoV-2 variants in Spain [in Spanish]. June 6, 2022. https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20220606.pdf (June 6, 2022, date last accessed) [Google Scholar]
- 26. The Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) . Recommendations of the SEIMC for the clinical management of patients with COVID-19 [in Spanish]. https://covid19.seimc.org/index.php/recomendaciones-seimc-para-el-manejo-clinico-de-pacientes-con-covid-19 (June 8, 2022, date last accessed) [Google Scholar]
- 27. Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- 28. López V, Vázquez T, Alonso-Titos Jet al. Grupo de Estudio GREAT (Grupo Español de Actualizaciones en Trasplante) . Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients. Nefrologia (Engl Ed) 2020;40:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Angelico R, Blasi F, Manzia TMet al. The management of immunosuppression in kidney transplant recipients with COVID-19 disease: an update and systematic review of the literature. Medicina (Kaunas) 2021;57:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villanego F, Mazuecos A, Pérez-Flores IMet al. Spanish Society of Nephrology COVID-19 Group . Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant 2021;21:2573–82. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group . Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021;22:622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buxeda A, Arias-Cabrales C, Pérez-Sáez MJet al. Use and safety of remdesivir in kidney transplant recipients with COVID-19. Kidney Int Rep 2021;6:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salerno DM, Jennings DL, Lange NWet al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant 2022;22:2083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng B, Green ACA, Tazare Jet al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: an observational cohort study using the OpenSAFELY platform. medRxiv 2022.05.22.22275417. 10.1101/2022.05.22.22275417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takashita E, Kinoshita N, Yamayoshi Set al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Eng J Med 2022;386:1475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Case JB, Mackin S, Errico Jet al. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 omicron lineage strains. Nat Commun 2022;13:3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawaoka Y, Uraki R, Kiso Met al. Characterization and antiviral susceptibility of SARS-CoV-2 omicron/BA.2. Res Sq, https://doi.org/10.21203/rs.3.rs-1375091/v1, February 24, 2022, preprint: not peer reviewed. [Google Scholar]
- 38. Chen J, Wei GW.. Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant. Res Sq, 10.21203/rs.3.rs-1362445/v1, February 23, 2022, preprint: not peer reviewed. [DOI] [Google Scholar]
- 39. US Food and Drug Administration . FDA updates sotrovimab emergency use authorization. April 5, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization (April 15, 2022, date last accessed). [Google Scholar]
- 40. National Institutes of Health COVID-19 Treatment Guidelines . Therapeutic management of nonhospitalized adults with COVID-19. April 8, 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults–therapeutic-management/ (April 15, 2022, date last accessed). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.M., upon reasonable request.