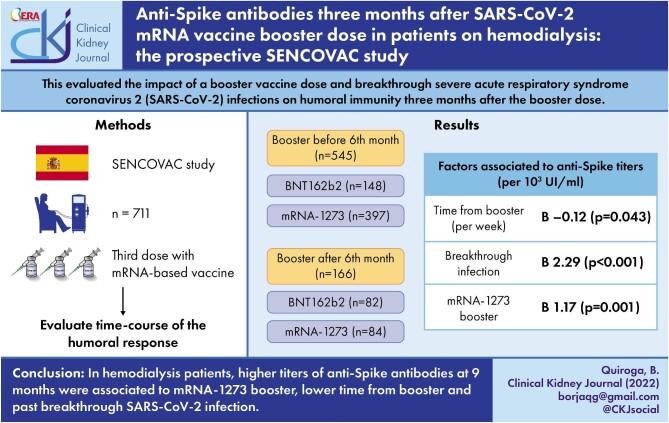
ABSTRACT
Background
Patients on hemodialysis are at high-risk for complications derived from coronavirus disease 2019 (COVID-19). The present analysis evaluated the impact of a booster vaccine dose and breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections on humoral immunity 3 months after the booster dose.
Methods
This is a multicentric and prospective study assessing immunoglobulin G anti-Spike antibodies 6 and 9 months after initial SARS-CoV-2 vaccination in patients on hemodialysis that had also received a booster dose before the 6-month assessment (early booster) or between the 6- and 9-month assessments (late booster). The impact of breakthrough infections, type of vaccine, time from the booster and clinical variables were assessed.
Results
A total of 711 patients [67% male, median age (range) 67 (20–89) years] were included. Of these, 545 (77%) received an early booster and the rest a late booster. At 6 months, 64 (9%) patients had negative anti-Spike antibody titers (3% of early booster and 29% of late booster patients, P = .001). At 9 months, 91% of patients with 6-month negative response had seroconverted and there were no differences in residual prevalence of negative humoral response between early and late booster patients (0.9% vs 0.6%, P = .693). During follow-up, 35 patients (5%) developed breakthrough SARS-CoV-2 infection. Antibody titers at 9 months were independently associated with mRNA-1273 booster (P = .001), lower time from booster (P = .043) and past breakthrough SARS-CoV-2 infection (P < .001).
Conclusions
In hemodialysis patients, higher titers of anti-Spike antibodies at 9 months were associated with mRNA-1273 booster, lower time from booster and past breakthrough SARS-CoV-2 infection.
Keywords: booster, COVID-19, hemodialysis, SARS-CoV-2, vaccination
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients undergoing hemodialysis present higher mortality secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. SARS-CoV-2 vaccines attenuate the risk of complications such as severe coronavirus disease 2019 (COVID-19), hospitalizations or the need for intensive care admission [2]. However, the initial vaccination schedule against SARS-CoV-2 could be suboptimal in hemodialysis patients as anti-Spike antibodies decline over time [3]. Indeed, some reports have established the duration of anti-Spike antibodies in patients on hemodialysis between 4 and 6 months after vaccination, raising some concerns on the real effectivity of the initial vaccine schedule in this vulnerable population [4].
To avoid the clinical consequences of losing humoral immunity against SARS-CoV-2, many countries are offering a SARS-CoV-2 vaccine booster dose. The most widely prescribed booster is an mRNA vaccine (BNT162b2 or mRNA-1273). These mRNA vaccines enter many cell types but only some of them (i.e. immune cells) express the mRNA-encoded protein (Spike), which triggers the development of antibodies [5]. Although BNT162b2 and mRNA-1273 have similar mechanisms of action, the amount of mRNA differs (30 vs 100 µg, respectively). The development of anti-Spike antibodies seems to be lower in hemodialysis patients than in other cohorts, so additional strategies are required for maintaining their protection against COVID-19 [6].
A booster dose may protect from both spontaneous loss of anti-Spike antibodies and from new virus variants, such as Omicron, especially in immunosuppressed patients [7]. Recent small studies have shown that a booster dose in patients on hemodialysis helps in seroconverting patients with previous negative humoral responses [8, 9]. However, evidence is not strong enough to establish the ideal timing for prescribing a booster dose of SARS-CoV-2 vaccines in hemodialysis. In addition, the impact of breakthrough infections on humoral immunity has not been determined.
In the current study, we evaluated the time-course of the humoral response after a booster dose of SARS-CoV-2 RNA-based vaccines among patients on hemodialysis. In addition, we also determined the impact of breakthrough SARS-CoV-2 infections on anti-Spike antibody development.
MATERIALS AND METHODS
Study design
SENCOVAC is a multicentric study promoted by the Spanish Society of Nephrology. All patients in the present analysis had chronic kidney disease (CKD) on hemodialysis and had received both an initial SARS-CoV-2 vaccination schedule and a booster dose, following the local health authority's guidelines. Prescribed initial vaccines were BNT162b2 (Pfizer-BioNTech®), mRNA-1273 (Moderna®), ChAdOx1-S (AstraZeneca®) or Ad26.COV.2 (Janssen®). In contrast, the booster was an mRNA-based vaccine [BNT162b2 (Pfizer-BioNTech®) or mRNA-1273 (Moderna®)] for all patients. The original protocol included prespecified assessment of vaccine response at 3, 6 and 12 months and a 9-month assessment was later added as an amendment. In Spain, local authorities determined the type and timing of vaccines, stratifying by age and comorbidities. Hemodialysis patients were the second group that received the initial schedule (only after the elderly) and most of them were prescribed mRNA-based vaccines as they were the only available at that moment. For booster dosing in Spain, all patients received mRNA-based vaccine between 3 and 6 months after completing the initial schedule. The type of vaccine was determined by the local healthcare center, depending on availability of the different vaccines. Patients did not have the opportunity to choose the type of vaccine. Since this was an observational study and booster doses were recommended by health authorities after the study had been initiated, independently from the study investigators, the analysis of the impact of booster doses may be considered a post hoc analysis of prospectively drawn samples at prespecified 6- and 9-month timepoints.
Population
This is a pre-specified analysis that included all hemodialysis patients with anti-Spike antibody assessment 6 and 9 months after completing the initial vaccination schedule. Exclusion criteria were the lack of anti-Spike antibody assessment, solid organ transplantation, active oncological or hematological disease, primary immunodeficiency, human immunodeficiency virus and immunosuppressive treatment 6 months prior to vaccination [3, 10, 11]. Due to the different timelines of different regions and hospitals throughout Spain, some patients received the booster dose before the 6-month assessment (early booster) of anti-Spike antibodies and others between the 6- and 9-month assessments (late booster). This situation allowed dividision of the sample in two groups based on the timing of the booster, in a real-world pragmatic approach for evaluating the humoral response to the booster. Investigators did not intervene when determining the timing of the third dose, which fully depended on local health authorities.
Outcomes
The primary outcome was to evaluate the impact of a booster dose on humoral immunity, defined by anti-Spike immunoglobulin G (IgG) antibodies. Secondary outcomes included assessing anti-Spike IgG antibody development after breakthrough infections and exploring differences between RNA-based vaccines.
Variables and outcomes
At baseline, epidemiological variables and comorbidities were registered. CKD etiology, vascular access, dialysis modality and hemodialysis adequacy parameters were collected. Previous COVID-19 was defined as an infection before initial vaccination confirmed by any of the available techniques [real-time polymerase chain reaction (rt-PCR), antigen test or antibodies against SARS-CoV-2]. Humoral immunity was assessed 6 and 9 months after initial vaccination with blood samples that were sent to a central laboratory for testing by a CE-marked quantitative chemiluminescence immunoassay (CLIA, COVID-19 Spike Quantitative Virclia® IgG Monotest, Vircell SL, Spain), with a sensitivity and specificity of 96% and 100%, respectively, which detects IgG antibodies against the SARS-CoV-2 Spike protein. This assay was calibrated against the First WHO International Standard for anti-SARS-CoV-2 human immunoglobulin (NIBSC code: 20/136) and results were expressed as IU/mL. According to performance studies by the manufacturer, titers ≤32 IU/mL were considered negative, between 32 and 36 equivocal and >36 IU/mL positive, reflecting the presence of anti-Spike IgG antibodies resulting from previous infection or vaccination.
Breakthrough infections during follow-up
SARS-CoV-2 infections were registered during follow-up from 6 to 9 months. A positive rt-PCR or antigen test were required for confirming the SARS-CoV-2 infection.
Ethical concerns
The study was approved in February 2021 by the Ethical Committee (code SENCOVAC, ER_EO020-21_FJD-HRJC-HGV-HIE).
Statistical methods
Data are expressed as median (interquartile range). Categorical variables were compared using Fisher test and continuous variables with Mann–Whitney U test. Continuous variables from more than two groups were compared with the Kruskal–Wallis test. Logistic regression analysis was used to evaluate factors associated with humoral response after the booster dose. Multivariate logistic regression models adjusted for variables with P < .1 in univariate analysis and confounders were constructed. SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistics and GraphPad Prism version 9.02 (GraphPad Holdings, LLC) for plotting.
RESULTS
Baseline characteristics
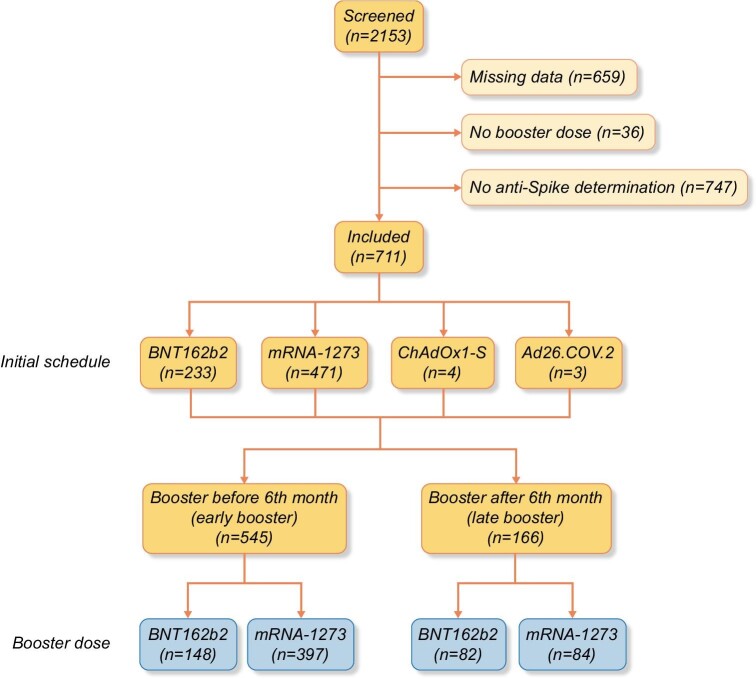
Among the initial 2153 patients enrolled in SENCOVAC, 711 (33%) were included in the present analysis as they had anti-Spike antibody data for both the pre-specified timepoints of 6 and 9 months: 67% male, median age (range) 67 (20–89) years (Table 1, Fig. 1). The initial vaccination schedule was mainly based on RNA-based vaccines [233 (33%) BNT162b2, 471 (66%) mRNA-1273, 4 (<1%) ChAdOx1-S and 3 (<1%) Ad26.COV.2]. Before the 6-month assessment, 545 (77%) patients had received a booster dose in contrast to 166 (23%) that received the booster between Months 6 and 9. In 230 (32%) patients, the booster dose was BNT162b2 and in 481 (68%) mRNA-1273 (Fig. 1).
Table 1.
Baseline characteristics of the included hemodialysis patients by time of booster dosing: after the 6th month assessment (late booster) and before the 6th month assessment (early booster)
| Total N = 711 | Late booster N = 166 | Early booster N = 545 | P | ||
|---|---|---|---|---|---|
| Sex (male), n (%) | 475 (66.8) | 113 (68.1) | 362 (66.4) | .623 | |
| Age (years), median (range) | 67 (20–89) | 68 (27–86) | 67 (20–89) | .316 | |
| Diabetic kidney disease, n (%) | 212 (29.8) | 53 (31.9) | 159 (29.2) | .497 | |
| Hemodialysis technique, n (%) | |||||
| HFHD | 321 (45.2) | 84 (50.6) | 237 (43.6) | .226 | |
| HDx | 36 (5.1) | 6 (3.6) | 30 (5.5) | ||
| OL-HDF | 353 (49.7) | 76 (45.8) | 277 (50.9) | ||
| Vascular access, n (%): | |||||
| AVF | 469 (67.4) | 103 (62.4) | 366 (68.9) | .119 | |
| Catheter | 227 (32.6) | 62 (37.6) | 165 (31.1) | ||
| Anticoagulants, n (%) | 113 (15.9) | 33 (19.9) | 80 (14.7) | .109 | |
| Antiplatelet agents, n (%) | 294 (41.4) | 63 (38.0) | 231 (42.4) | .310 | |
| RAASi, n (%) | 237 (33.3) | 60 (36.1) | 177 (32.5) | .380 | |
| ESA, n (%) | 536 (75.4) | 116 (69.9) | 420 (77.1) | .060 | |
| Vaccine (initial), n (%) | |||||
| BNT162b2 | 233 (32.8) | 78 (47.0) | 155 (28.4) | .001 | |
| mRNA-1273 | 471 (66.2) | 86 (51.8) | 385 (70.6) | ||
| ChAdOx1-S | 4 (0.6) | 2 (1.2) | 2 (0.4) | ||
| Ad26.COV.2 | 3 (0.4) | 0 (0.0) | 3 (0.6) | ||
| Vaccine (booster dose), n (%): | |||||
| BNT162b2 | 230 (32.3) | 82 (49.4) | 148 (27.2) | .001 | |
| mRNA-1273 | 481 (67.7) | 84 (50.6) | 397 (72.8) | ||
| COVID-19 before vaccination, n (%) | 83 (11.7) | 19 (11.4) | 64 (11.7) | .917 | |
Data are presented as n (%), unless otherwise noted. ESA, erythropoiesis stimulating agent; HD, hemodialysis; HFHD, high flux hemodialysis; HDx, expanded hemodialysis therapy; OL-HDF, online hemodiafiltration; AVF, arteriovenous fistulae; RAASi, renin–angiotensin–aldosterone inhibitors; COVID-19: coronavirus disease 2019.
FIGURE 1:
Flow chart.
Kinetics of anti-Spike antibodies after the booster dose
Amongst patients that received the booster before the 6-month assessment, anti-Spike titers at 6 months were significantly higher than in patients without booster by 6 months (P < .001); however, they significantly decreased from 6 to 9 months [3788 (989–10 000) UI/mL vs 2807 (985–10 000) UI/mL] (P = .001). In contrast, in patients who received the booster dose between the 6- and 9-month assessments, anti-Spike antibodies significantly increased from 6 to 9 months [142 (29–1666) UI/mL vs 6021 (1405–10 000) UI/mL] (P < .001) (Fig. 2). As shown in Supplementary data, Fig. S1, patients that received an mRNA-1273 booster before the 6-month assessment developed higher titers of anti-Spike antibodies at 9 months than those receiving BNT162b2 before the 6-month assessment [3776 (1156–10 000) UI/mL vs 1712 (676–8423) UI/mL] (P < .001). In contrast, in patients who received the booster from 6 to 9 months there were no significant differences in antibody titers at 9 months between types of RNA-based vaccines (P = .142), although the same trend was observed.
FIGURE 2:
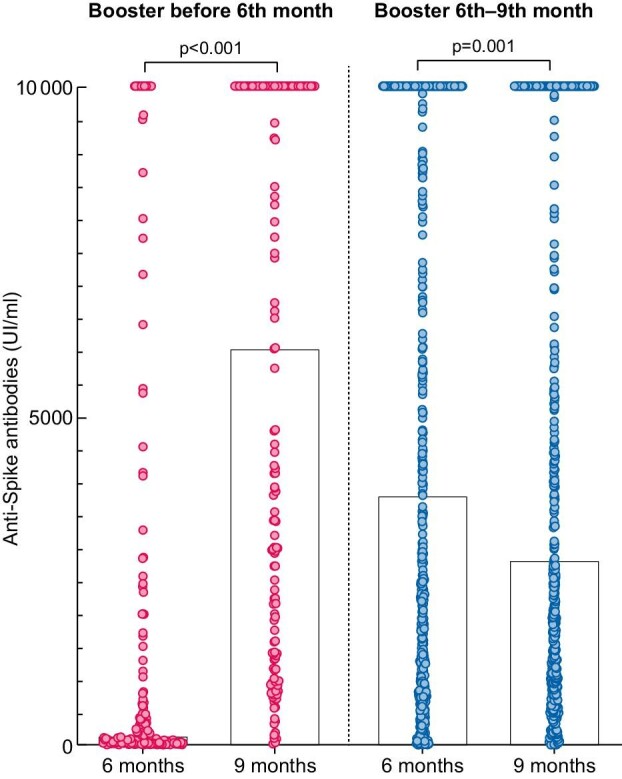
Anti-Spike antibodies 6 and 9 months after the initial SARS-CoV-2 vaccination among hemodialysis patients that had received a booster dose: without a booster dose before the 6th month assessment (late booster, left) and with a booster dose before the 6th month assessment (early booster, right).
Impact of breakthrough SARS-CoV-2 infection on anti-Spike antibodies
During follow-up from Month 6 to Month 9, 35 patients (5%) developed a SARS-CoV-2 infection: 22/545 (4.0%) in the early booster and 13/166 (7.8%) in the later booster cohort (P = .063). Baseline characteristics of infected and non-infected patients are shown in Supplementary data, Table S1. At the 9-month assessment, patients with an infection during follow-up had higher titers of anti-Spike antibodies than those in the early booster [10000 (4178–10 000) UI/mL vs 2665 (959–10 000) UI/mL] (P = .003) and in the late booster cohorts [10000 (8014–10 000) UI/mL vs 4443 (1365–10 000) UI/mL] (P = .021) (Supplementary data, Fig. S2).
Anti-Spike antibody seroconversion
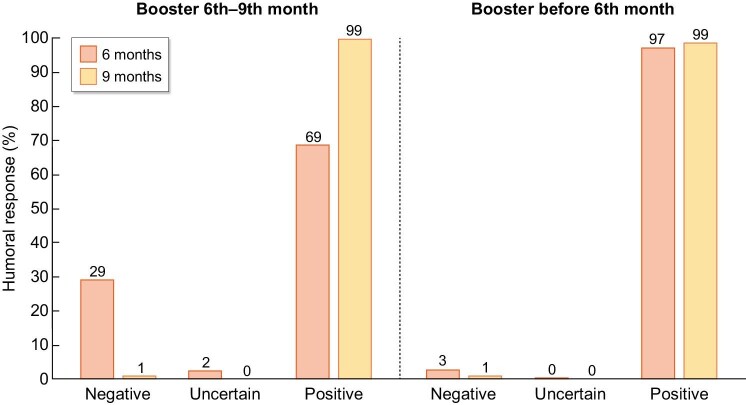
Six months after the initial vaccine, 64 (9%) patients presented a negative humoral response, i.e. anti-Spike antibodies were not detected. Most had not yet received the booster dose (48/166, 29% in the later booster cohort vs 16/545, 3% in the early booster group) (P < .001) (Fig. 3). Beyond the booster, factors associated to negative humoral response at 6 months included no pre-vaccine COVID-19 (P < .001) and BNT162b2 as initial vaccine (vs mRNA-1273) (P = .018). Of patients with negative humoral response at 6 months, 58 (91%) seroconverted at 9 months. Six patients had persistent negative humoral response at 9 months: 5/545 (0.9%) in the early booster cohort and 1/166 (0.6%) in the late booster cohort (P = .693) (Fig. 3). None of the seroconversions was attributed to a SARS-CoV-2 breakthrough infection.
FIGURE 3:
Humoral response among SARS-CoV-2-vaccinated hemodialysis patients that had received a booster dose: without a booster dose before the 6th month assessment (late booster, left) and with a booster dose before the 6th month assessment (early booster, right).
Factors associated to higher anti-Spike titers
Factors associated with higher anti-Spike titers at 9 months in univariate analysis were younger age (P = .025), late booster group, i.e. booster closer to antibody assessment (P = .030), less time from booster to antibody assessment (P = .004), breakthrough SARS-CoV-2 infection (P < .001) and mRNA-1273 booster (P = .001) (Table 2). An adjusted linear regression showed that less time from booster to assessment (B –0.12, P = .043), breakthrough SARS-CoV-2 infection (B 2.29, P < .001) and mRNA-1273 booster (B 1.17, P = .001) were independently associated to higher anti-Spike titers at 9 months (Table 2).
Table 2.
Linear regression for factors associated to anti-Spike titers (per 103 UI/mL)
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | P | |
| Age (per year) | –0.03 (–0.05, –0.01) | 0.025 | ||
| Gender (male) | 0,33 (–0.03, –0.97) | 0.302 | ||
| Late booster (yes) | 1.08 (0.37, 1.78) | 0.030 | ||
| Time from booster (per week) | –0.21 (–0.19, –0.04) | 0.004 | –0.12 (–0.23, –0.01) | 0.043 |
| SARS-CoV-2 infection during follow-up (yes) | 3.01 (1.68, 4.28) | <0.001 | 2.29 (1.46, 4.39) | <0.001 |
| Type of booster (mRNA-1273) | 1.05 (0.41, 1.68) | 0.001 | 1.17 (0.45, 1.89) | 0.001 |
Linear regression adjusted for age, gender and date of booster.
DISCUSSION
The key finding of the present study is that persistent stimulation of the immune system, such as vaccine boosters and breakthrough infections, contributed to stronger antibody responses when compared with isolated stimulation, namely single or double vaccine dose, especially if the additional stimuli were recent. In addition, the controversial issue of humoral response duration and the impact of an early or late booster was addressed. Our data show that 6 months after initial vaccination, 29% of hemodialysis patients lacked IgG anti-Spike antibodies. However, most patients seroconverted after the booster dose. Indeed, only one patient lacked anti-Spike antibodies after the booster. As anti-Spike antibodies have been related to the severity of COVID-19 and monoclonal anti-Spike antibodies are used to treat COVID-19, it seems realistic to use the humoral response as a biomarker to stratify the risk of vulnerable patients [12].
Breakthrough infections enhance antibody development. In accordance with previous reports, our results showed that even in patients having received only the initial vaccination, infections increased anti-Spike antibody titers to at least the same level as the booster dose at 6 months [13]. Indeed, previous reports from the SENCOVAC study showed that previous COVID-19 (even asymptomatic) was associated with higher humoral responses [3, 8]. As many SARS-CoV-2 infections remain asymptomatic, especially in vaccinated individuals, the only method available to assess anti-SARS-CoV-2 immunity would be to monitor anti-Spike antibodies to avoid hyperstimulation of immune cells by repeated boosters [14].
The type of vaccine is also a key driver for anti-Spike antibody development. As previously demonstrated for the initial vaccination schedule, patients that received the mRNA-1273 booster achieved stronger humoral responses [8]. This finding agrees with a prior study that compared two different types of a booster and supports that dose is directly related to antibody titer development, at least in hemodialysis patients, as mRNA-1273 contains approximately 3-fold more mRNA than BNT162b2 [15]. The choice of mRNA vaccine should be of especial interest in vulnerable patients with a rapid decline in pre-existing antibodies to optimize their antibody response [3, 8]. To our knowledge, non-mRNA vaccines, such as AZD1222 or Ad26.COV2.S, have not been used to booster hemodialysis patients. Their use in initial vaccination schedules resulted in lower antibody titers than mRNA-based vaccines [16, 17]. Importantly, a recent study has shown that heterologous vaccination (initial schedule and booster) with AZD1222 and BNT162b2 was insufficient to generate antibodies against Omicron [7]. Anecdotally, one small report has shown that a fourth vaccine dose is safe and increases anti-Spike antibodies in dialysis patients [18].
Our study presents some limitations. First, breakthrough infections may have been underestimated, as asymptomatic SARS-CoV-2 infections may be unnoticed (i.e. local protocol for detecting SARS-CoV-2 infections differed and ranged from periodic screening of the hemodialysis population to testing only symptomatic individuals or contacts) and false-negative results may occur in PCR and antigen tests. However, severe SARS-CoV-2 infections increase anti-Spike titers to higher levels than asymptomatic infections, and the registered cases of our study were mostly symptomatic [19]. Second, for logistic reasons we do not have data on cellular immunity. This is a multicentric national study with more than 50 participating centers that presents serious challenges to the centralization of samples for this purpose. Beyond this, humoral immunity is probably the best choice for assessing protection against COVID-19 as it is easily measurable and affordable in routine clinical practice. Finally, observational studies have inherent biases. However, SENCOVAC was designed to be a prospective real-world study using a pragmatic approach.
In conclusion, breakthrough SARS-CoV-2 infections, time elapsed from booster dosing and type of booster are associated with higher anti-Spike titers in hemodialysis patients. Assessing the humoral response could be useful to stratify the need for close monitoring of SARS-CoV-2 infection, maintaining isolation measures and social distance, or the use of further booster doses.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the involved centers and healthcare workers, especially the nurse team, for their involvement in this project. In addition, we want to thank the support of the Sociedad Española de Enfermería Nefrológica (SEDEN), Organización Nacional de Trasplantes (ONT) and Sociedad Española de Trasplante (SET). We also thank Ethical Committee of Fundación Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz which approved the study.
APPENDIX
The SENCOVAC collaborative network:
Marta Puerta Carretero, Daniel Gaitán Tocora, Mª Teresa Jaldo Rodríguez, Tamar Talaván Zanón, Esther Rodriguez Suárez, Alfredo José Sáenz Santolaya, Raquel Cerrajero Calero, Patricia Arribas Cobo, Patricia Muñoz Ramos, Carolina Gracia-Iguacel, Catalina Martin-Cleary, Jinny Sánchez-Rodríguez, Ana Ramos-Verde, Yohana Gil Giraldo, Pablo Ruano Suárez, Antonio Fernández Perpén, Andrés Fernández Ramos, Laura Salanova Villanueva, Alejandra Cortiñas, Pablo A. Díez Arias, Alicia Cabrera Cárdenas, Antonio de Santos, Almudena Núñez, Guillermina Barril Cuadrado, Raquel Repollet, Francesc Moreso, María Antonieta Azancot, Natalia Ramos, Oriol Bestard, Ignacio Cidraque, Sheila Bermejo, Irene Agraz, Oreto Prat, Carlota Medina, Emma Pardo, Alejandro Saiz, Nicolás Menéndez Granados, María Jesús Corton Cabo, Walter López Alarcón, Simona Alexandru, Laura García Puente Suarez, Saul Pampa Saico, Marisol Poma Tapia, Laura Rodríguez Osorio, Rocío Zamora, Paloma Leticia Martin Moreno, Noelia Ania González, Ana Sabalza Ortiz, María Nieves Bastida Iñarrea, Teresa García, Carlos Narváez, Cristhian Orellana, José Luis Pizarro León, Manuel Antonio Martínez García, Benaldina García Jiménez, Juan de Dios Ramiro Moya, Diana López Espinosa, Alejandro Jiménez Herrador, Manuel Navarro Zurita, Leonardo Díaz Álvarez, Álvaro González Martínez, Sandra Báez Arroyo, Raquel Reina Fernández, Marlyn Janella Suárez Vargas, Rocío Calurano Casero, Gustavo Useche, Carmen Santamaría de Miguel, Ángel Palacios, Brenda Henningsmeyer, Esther Orero Calve, José Lacueva Moya, Sandra Castellano Gash, Lara Ruíz Martínez, Virginia Lopez De La Manzanara Perez, Marta Calvo Arevalo, Jose Antonio Herrero Calvo, Mercedes Salgueira, Nuria Aresté, María de los Ángeles Rodríguez, Rocío Collantes, Ana Isabel Martínez, María Jesús Moyano, Elena Jiménez Víbora, Aurelio Pastor Rodríguez Hernández, María Sagrario García Rebollo, Juana Margarita Rufino Hernández, Esther Torres Aguilera, Rolando Tello Alea, Margie Soledad Del Rosario Saldaña, Ana María Urraca de la Pisa, Lidia Sendino Monzon, Karina Ampuero Anachuri, Esther Hernández Garcia, Victoria Oviedo Gomez, Ignacio Manzur Cavalotti, Itziar Navarro Zorita, Sol Otero López, Sara Outon González, Carlos Soto Montañez, Manuel Ramírez de Arellano Serna, Luis Guirado Perich, Eva Cotilla de la Rosa.
Contributor Information
Borja Quiroga, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
María José Soler, Nephrology Department, Vall d'Hebrón University Hospital, Barcelona, Spain; RICORS2040 (Kidney Disease).
Alberto Ortiz, RICORS2040 (Kidney Disease); IIS-Fundación Jiménez Diaz, School of Medicine, Universidad Autónoma de Madrid, Fundación Renal Iñigo Álvarez de Toledo-IRSIN, REDinREN, Instituto de Investigación Carlos III, Madrid, Spain.
Carlos Jesús Jaravaca Mantecón, Diaverum Andalucía, Spain.
Nathasha Nava Pérez, Diaverum Andalucía, Spain.
Marta Serra Martín, Diaverum Valencia, Spain.
Yurika Sato, Diaverum Valencia, Spain.
Antonio José Marin Franco, Diaverum Castilla León-Galicia, Spain.
Diana Flor Pazmiño Zambrano, Diaverum Castilla León-Galicia, Spain.
Rafael Lucena Valverde, Nephrology Department, Hospital Universitario Infanta Leonor – Universidad Complutense de Madrid, Madrid, Spain.
Mayra Ortega Diaz, Nephrology Department, Hospital Universitario Infanta Leonor – Universidad Complutense de Madrid, Madrid, Spain.
Carmen Calderón González, Nephrology Department, Complejo Asistencial de Palencia, Palencia, Spain.
Juan Manuel Cazorla López, Nephrology Department, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Mónica Pereira, IIS-Fundación Jiménez Diaz, School of Medicine, Universidad Autónoma de Madrid, Fundación Renal Iñigo Álvarez de Toledo-IRSIN, REDinREN, Instituto de Investigación Carlos III, Madrid, Spain.
Emilio González Parra, IIS-Fundación Jiménez Diaz, School of Medicine, Universidad Autónoma de Madrid, Fundación Renal Iñigo Álvarez de Toledo-IRSIN, REDinREN, Instituto de Investigación Carlos III, Madrid, Spain.
Ana Sánchez Horrillo, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
Carmen Sánchez González, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
Néstor Toapanta, Nephrology Department, Vall d'Hebrón University Hospital, Barcelona, Spain.
Secundino Cigarrán Guldris, Nephrology Department, Hospital Da Mariña, Lugo, Spain.
Rosa Sánchez Hernández, Nephrology Department, Hospital Universitario General de Villalba, Madrid, Spain.
Soledad Pizarro Sánchez, Nephrology Department, Hospital Rey Juan Carlos, Madrid, Spain.
María Muñiz Rincón, Nephrology Department, Hospital Clínico San Carlos, Madrid, Spain.
Nuria Garcia-Fernández, Nephrology Department, Clínica Universidad de Navarra, Navarra, Spain.
Natalia Blanco Castro, Nephrology Department, QuirónSalud A Coruña, A Coruña, Spain.
Rocío Collantes Mateo, Nephrology Department, Hospital Virgen de la Macarena, Sevilla, Spain.
Manuel Augusto Quiroz Morales, Nephrology Department, Consorci Sanitari Alt Penedès-Garraf, Barcelona, Spain.
Beatriz Escamilla-Cabrera, Nephrology Department, Hospital Universitario de Canarias, Canarias, Spain.
Isabel Berdud Godoy, FMC San Rafael, Córdoba, Spain.
Beatriz Gil-Casares Casanova, Nephrology Department, Hospital Universitario del Sureste, Madrid, Spain.
Alba Leyva, R&D Department, VIRCELL SL, Granada, Spain.
José Rojas, R&D Department, VIRCELL SL, Granada, Spain.
Ron T Gansevoort, Dept Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Patricia de Sequera, RICORS2040 (Kidney Disease); Nephrology Department, Hospital Universitario Infanta Leonor – Universidad Complutense de Madrid, Madrid, Spain.
SENCOVAC collaborative network:
Marta Puerta Carretero, Daniel Gaitán Tocora, Mª Teresa Jaldo Rodríguez, Tamar Talaván Zanón, Esther Rodriguez Suárez, Alfredo José Sáenz Santolaya, Raquel Cerrajero Calero, Patricia Arribas Cobo, Patricia Muñoz Ramos, Carolina Gracia-Iguacel, Catalina Martin-Cleary, Jinny Sánchez-Rodríguez, Ana Ramos-Verde, Yohana Gil Giraldo, Pablo Ruano Suárez, Antonio Fernández Perpén, Andrés Fernández Ramos, Laura Salanova Villanueva, Alejandra Cortiñas, Pablo A Díez Arias, Alicia Cabrera Cárdenas, Antonio de Santos, Almudena Núñez, Guillermina Barril Cuadrado, Raquel Repollet, Francesc Moreso, María Antonieta Azancot, Natalia Ramos, Oriol Bestard, Ignacio Cidraque, Sheila Bermejo, Irene Agraz, Oreto Prat, Carlota Medina, Emma Pardo, Alejandro Saiz, Nicolás Menéndez Granados, María Jesús Corton Cabo, Walter López Alarcón, Simona Alexandru, Laura García Puente Suarez, Saul Pampa Saico, Marisol Poma Tapia, Laura Rodríguez Osorio, Rocío Zamora, Paloma Leticia Martin Moreno, Noelia Ania González, Ana Sabalza Ortiz, María Nieves Bastida Iñarrea, Teresa García, Carlos Narváez, Cristhian Orellana, José Luis Pizarro León, Manuel Antonio Martínez García, Benaldina García Jiménez, Juan de Dios Ramiro Moya, Diana López Espinosa, Alejandro Jiménez Herrador, Manuel Navarro Zurita, Leonardo Díaz Álvarez, Álvaro González Martínez, Sandra Báez Arroyo, Raquel Reina Fernández, Marlyn Janella Suárez Vargas, Rocío Calurano Casero, Gustavo Useche, Carmen Santamaría de Miguel, Ángel Palacios, Brenda Henningsmeyer, Esther Orero Calve, José Lacueva Moya, Sandra Castellano Gash, Lara Ruíz Martínez, Virginia Lopez De La Manzanara Perez, Marta Calvo Arevalo, Jose Antonio Herrero Calvo, Mercedes Salgueira, Nuria Aresté, María de los Ángeles Rodríguez, Rocío Collantes, Ana Isabel Martínez, María Jesús Moyano, Elena Jiménez Víbora, Aurelio Pastor Rodríguez Hernández, María Sagrario García Rebollo, Juana Margarita Rufino Hernández, Esther Torres Aguilera, Rolando Tello Alea, Margie Soledad Del Rosario Saldaña, Ana María Urraca de la Pisa, Lidia Sendino Monzon, Karina Ampuero Anachuri, Esther Hernández Garcia, Victoria Oviedo Gomez, Ignacio Manzur Cavalotti, Itziar Navarro Zorita, Sol Otero López, Sara Outon González, Carlos Soto Montañez, Manuel Ramírez de Arellano Serna, Luis Guirado Perich, and Eva Cotilla de la Rosa
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
B.Q. has received honoraria for conferences, consulting fees and advisory boards from Vifor-Pharma, Astellas, Amgen, Bial, Ferrer, Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme and Otsuka. M.J.S. is the CKJ Editor-in-Chief and reports honorarium for conferences, consulting fees and advisory boards from AstraZeneca, NovoNordsik, Esteve, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, ICU Medical and Boehringer. A.O. is the former CKJ Editor-in-Chief and has received consultancy or speaker fees or travel support from Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma, and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes. C.J.J.M. has received honoraria for one conference from Vifor-Pharma. S.C.G. has received honoraria for conferences, consulting fees and Advisory Boards from Vifor-Pharma, Astellas, Amgen, Novartis, Novo Nordisk, Chiesi, AstraZeneca, Sanofi-Genzyme, Otsuka, Chemo-Centrix, Boheringher and Rovi. N.G.-F. has received horonaria from Mundipharma and Astellas, and from Baxter, Vifor, Fresenius and Fresenius-Kabi to attend meetings. B.E.-C. has received honoraria for conferences, consulting fees and advisory boards from Astellas and AstraZeneca. P.d.S. reports honorarium for conferences, consulting fees and advisory boards from Amgen, Astellas, AstraZeneca, Baxter, Braun, Fresenius, Nipro and Vifor-Pharma. She is the present president of the Spanish Society of Nephrology. N.N.P., M.S.M., Y.S., A.J.M.F., D.F.P.Z., R.L.V., M.O.D., C.C.G., J.M.C.L., M.P., E.G.P., A.S.H., C.S.G., N.T., R.S.H., S.P.S., M.M.R., N.B.C., R.C.M., M.A.Q.M., I.B.G., B.G.-C.C., A.L., J.R. and R.T.G. do not present conflict of interests.
FUNDING
The present project has been supported by Fresenius Medical Care, Diaverum, Vifor Pharma, Vircell, Fundación Renal Iñigo Álvarez de Toledo and ISCIII FEDER funds RICORS2040 (RD21/0005).
REFERENCES
- 1. Sánchez-Alvarez E, Macía M, de Sequera Ortiz P.. Kidney360 2020;1:1254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews N, Tessier E, Stowe Jet al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med 2022;386:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quiroga B, Soler MJ, Ortiz Aet al. Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study. Nephrol Dialysis Transplant 2022;37:994–9(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dulovic A, Strengert M, Ramos GMet al. Diminishing immune responses against variants of concern in dialysis patients 4 months after SARS-CoV-2 mRNA vaccination. Emerg Infect Dis 2022;28:743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Federico M. Biological and immune responses to current Anti-SARS-cov-2 mRNA vaccines beyond anti-spike antibody production. J Immunol Res 2022; 2022(in press). Published online 14 May 2022. doi: 10.1155/2022/4028577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yau K, Abe KT, Naimark Det al. Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Network Open 2021;4:e2123622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carr EJ, Wu M, Harvey Ret al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet North Am Ed 2022;399:800–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bensouna I, Caudwell V, Kubab Set al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis 2022;79:185–92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Housset P, Kubab S, Pardon Aet al. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J Nephrol 2022;35:783–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quiroga B, Soler MJ, Ortiz Aet al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dialysis Transplant 2021. doi: 10.1093/ndt/gfab313. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quiroga B, Soler MJ, Ortiz Aet al. Humoral response to third dose of SARS-CoV-2 vaccines in the CKD spectrum. Clin J Am Soc Nephrol 2022;17:872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boudhabhay I, Serris A, Servais Aet al. COVID-19 outbreak in vaccinated patients from a hemodialysis unit: antibody titers as a marker of protection from infection. Nephrol Dialysis Transplant 2022;37:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Karoui K, De Vriese AS.. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int 2022;101:883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalifehzadeh-Esfahani Z, Fattahi S, Heidari Haratemeh Zet al. The role of immune regulatory molecules in COVID-19. Viral Immunol 2022;35:359–64. [DOI] [PubMed] [Google Scholar]
- 15. Broseta JJ, Rodríguez-Espinosa D, Cuadrado Eet al. Humoral response after three doses of mRNA-1273 or BNT162b2 SARS-CoV-2 vaccines in hemodialysis patients. Vaccines 2022;10:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu CM, Weiner DE, Manley HJet al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over 6 months. Clin J Am Soc Nephrol 2022;17: 403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carr EJ, Wu M, Harvey Ret al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet North Am Ed 2021;398:1038–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Housset P, Kubab S, Hanafi Let al. Humoral response after a fourth “booster” dose of a COVID-19 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int 2022;101:1289–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang L, Xu Q, Yang Bet al. IgG antibody titers against SARS-CoV-2 nucleocapsid protein correlate with the severity of COVID-19 patients. BMC Microbiol 2021;21:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.