Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) presents with inflammation and pathology of multiple organs in the pediatric population in the weeks following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
We characterized the SARS-CoV-2 antigen–specific cytokine and chemokine responses in children with MIS-C, coronavirus disease 2019 (COVID-19), and other infectious diseases.
Results
MIS-C is characterized by elevated levels of type 1 (interferon-γ, interleukin [IL] 2), type 2 (IL-4, IL-13), type 17 (IL-17), and other proinflammatory cytokines (IL-1α, IL-6, IL-12p70, IL-18, and granulocyte-macrophage colony-stimulating factor) in comparison to COVID-19 and other infectious diseases following stimulation with SARS-CoV-2–specific antigens. Similarly, upon SARS-CoV-2 antigen stimulation, CCL2, CCL3, and CXCL10 chemokines were significantly elevated in children with MIS-C in comparison to the other 2 groups. Principal component analysis based on these cytokines and chemokines could clearly distinguish MIS-C from both COVID-19 and other infections. In addition, these responses were significantly diminished and normalized 6–9 months after recovery.
Conclusions
Our data suggest that MIS-C is characterized by an enhanced production of cytokines and chemokines that may be associated with disease pathogenesis.
Keywords: MIS-C, SARS-CoV-2, COVID-19, cytokines, chemokines, pediatric population, in vitro cell culture
Our data suggest that MIS-C is characterized by SARS-CoV-2 antigen–specific enhanced production of cytokines and chemokines that may be associated with disease pathogenesis. Principal component analysis could clearly distinguish cytokines and chemokines of MIS-C children from COVID-19 and other infections.
Coronavirus disease 2019 (COVID-19) is milder in children than adults and accounts for <0.1% of total deaths (Centers for Disease Control and Prevention [CDC] COVID-19 Response Team; American Academy of Pediatrics and Children’s Hospital Association, Children and COVID-19: State Data Report 2020; Pediatric Mortality Investigation Team; https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/). The reasons for differences in pediatric and adult severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are still unclear [1, 2]. Following the early wave of COVID-19, children presented with multisystem inflammatory syndrome in children (MIS-C), a syndrome presenting with vascular involvement and shock [3–7]. MIS-C is proposed to be a delayed infectious event [6, 8] and clinically resembles Kawasaki disease, due to vascular involvement. A major subset of MIS-C children requires intensive care support, indicating that MIS-C is one of the most severe clinical manifestations of COVID-19 in the pediatric population [9, 10].
Studies have shown that MIS-C is associated with elevated inflammatory markers as compared to adult and pediatric COVID-19 [3–5]. In addition, our previous study also reported that there were heightened circulating levels of cytokines, chemokines, acute-phase proteins, and microbial translocation markers in children with MIS-C compared to children with acute COVID-19 and seropositive children [11, 12]. The immunologic responses that drive MIS-C are still being elucidated, but some reports suggest that MIS-C may be correlated with altered adaptive and innate cell frequencies, and also linked with elevated memory T-cell activation, compared with healthy controls [13–15].
We therefore characterized the SARS-CoV-2 antigen–specific immune responses during and after MIS-C and compared them with children with COVID-19 and other infectious diseases. Our results demonstrated that children with MIS-C exhibit heightened levels of cytokines and chemokines in comparison to children with COVID-19 and other infectious diseases. In addition, in the follow-up of children with MIS-C, these cytokine and chemokine responses were normalized 6–9 months after recovery.
METHODS
Ethics Statement
Informed consent was obtained from parent/guardians of all children along with assent where appropriate. The internal ethics committees of the participating institutes approved the study.
Study Population and Procedures
Children of either sex, aged 12 months to 15 years, admitted to the Institute of Child Health, Dr.Mehta’s Children Hospital, Rainbow Children’s Hospital, from 1 December 2020 to 30 May 2021 with MIS-C, acute COVID-19, and other infectious diseases (dengue fever, scrub typhus fever, and Salmonella Typhi infection [enteric fever]) were included in this study (Table 1). For analyses, children were classified into 3 groups: MIS-C (n = 15), acute COVID-19 (n = 10), and other infectious diseases (n = 10). In the MIS-C group, a subset of 8 children was followed up 6–9 months after completion of treatment. Blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes (BD Biosciences) and heparin tubes and processed within 4 hours of collection at the National Institute for Research in Tuberculosis, Chennai. Sampling in all children was done prior to receiving any immunomodulatory treatment. Study staff involved in immunological assays were blinded to any clinical data. Acute COVID-19 disease and severity of COVID-19 were defined according to the Indian Ministry of Health and Family Welfare guidelines [16], and children with MIS-C were diagnosed and treated according to the CDC definition for MIS-C [17, 18]. Dengue fever was confirmed by either serology or NS1 positivity. Scrub typhus was confirmed by serology (immunoglobulin M) or enzyme-linked immunosorbent assay (ELISA). Confirmed culture was used to diagnose enteric fever.
Table 1.
Demographic Characteristics and Hematological Parameters of the Study Population
| Characteristic | MIS-C | Acute COVID-19 (n = 10) |
Other Infectious Diseases (n = 10) |
|
|---|---|---|---|---|
| During MIS-C (n = 15) | After MIS-C (n = 8) |
|||
| Age, y, median (range) | 4.5 (1–13) | 9 (3–12) | 2 (1–13) | 3 (1–10) |
| Male sex, No. (%) | 9 (60) | 7 (63.6) | 5 (50) | 3 (30) |
| RT-PCR positive, No. (%) | 2 (1.3) | NA | 7 (70) | 0 |
| Serology IgG positive, No. (%) | 13 (86.6) | 4 (36.36) | 1 (10) | 3 (30) |
| CRP <3 mg/L, No. (%) | 5 (50) | 0 (0) | 7 (46.6) | 2 (20) |
| WBC count, 103 cells/µL, GM (range) | 8.16 (3.65–13.29) | 7.27 (5.35–9.51) | 5.71 (4.41–8.19) | 11.28 (5.13–29.83) |
| Hemoglobin, g/dL, GM (range) | 10.15 (6–16.1) | 12.17 (10.94–13.14) | 10.68 (8.3–13.09) | 10.9 (7.39–14.33) |
| Lymphocyte, %, median (range) | 39.53 (4.85–63.95) | 44.97 (36.3–62.99) | 71.05 (12.45–80.4) | 57.65 (14.13–63.22) |
| Neutrophils, %, median (range) | 76.50 (32–89) | 39.76 (23.28–53.3) | 26.55 (10.59–82.14) | 31.13 (22.97–83.25) |
| Platelets (200–450) ×109/L, median (range) | 267.3 (84.8–355.5) | 295.54 (151.6–433) | 264.55 (174.8–367.9) | 339.3 (28.7–1044.6) |
| Sodium (135–145 mmol/L), median (range) | 132.5 (124–137) | … | NA | NA |
| Ferritin, ng/mL, median (range) | 762.5 (306.1–5377) | … | NA | NA |
| Duration of stay, d, median (range) | 7.5 (2–13) | NA | 4 (2–6) | 6.5 (3–7) |
| Dengue serology or NS1 positivity | … | … | … | 4 |
| Scrub typhus (IgM) | … | … | … | 4 |
| Enteric fever (culture confirmed) | … | … | … | 2 |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; GM, geometric mean; IgG, immunoglobulin G; IgM, immunoglobulin M; MIS-C, multisystem inflammatory syndrome in children; NA, not applicable; RT-PCR, reverse-transcription polymerase chain reaction.
Antigens
The antigens SARS-CoV-2 S-RBD (spike receptor-binding domain), SARS-CoV-2 ICL (irradiated cell lysate), or Mycobacterium tuberculosis (Mtb) WCL (whole cell lysate) (nonviral antigen control) were used as the antigenic stimuli.
In Vitro Culture
Whole blood cell cultures were performed to determine the levels of cytokines and chemokines. In brief, whole blood was diluted 1:1 with RPMI-1640 medium, supplemented with penicillin/streptomycin (100 U/100 mg/mL), l-glutamine (2 mM), and HEPES (10 mM) (all from Invitrogen, Carlsbad, California) and distributed in 12-well tissue culture plates (Costar). The cultures were then stimulated with SARS-CoV-2 antigens or no stimulation (media alone or baseline) in the presence of the co-stimulatory molecules anti-CD49d and anti-CD28 at 37°C for 48 hours. After 48 hours, culture supernatants were collected for estimating the cytokine and chemokine levels.
Multiplex Assay
The levels of cytokines and chemokines in the culture supernatants were measured using the Magpix multiplex cytokine assay system (Bio-Rad, Hercules, California). Raw cytokine/chemokine values are labeled as cytokine/chemokine values for the unstimulated condition. Net cytokine/chemokine levels are given for the stimulated condition, which indicates cytokine values following subtraction of baseline (unstimulated) values from the total stimulated values. The cytokines analyzed were interferon gamma (IFN-γ), interleukin (IL) 2, tumor necrosis factor alpha (TNF-α), IL-4, IL-5, IL-13, IL-17A, IL-1α, b, IL-6, IL-10, IL-12p70, IL-18, and granulocyte-macrophage colony-stimulating factor (GM-CSF), and chemokines analyzed were CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10, and CXCL11, measured by ELISA using the kit from R&D Systems (Minneapolis, Minnesota).
Statistical Analysis
Geometric means were used for measurements of central tendency. Statistically significant differences between MIS-C, acute COVID-19, and other infectious diseases were analyzed using the Kruskal–Wallis test with Dunn multiple comparisons. P ≤ .05 was considered statistically significant and all tests were 2-sided. Wilcoxon signed-rank test was used to compare levels of culture supernatant cytokine and chemokine concentrations before and after MIS-C treatment. Analyses were performed using GraphPad Prism version 9.0 software (GraphPad, San Diego, California). RStudio was used for plotting principal component analysis (PCA) and heat maps.
RESULTS
Children With MIS-C Have Elevated Type 1, Type 2, and Type 17 Cytokine Responses to SARS-CoV-2 Antigens
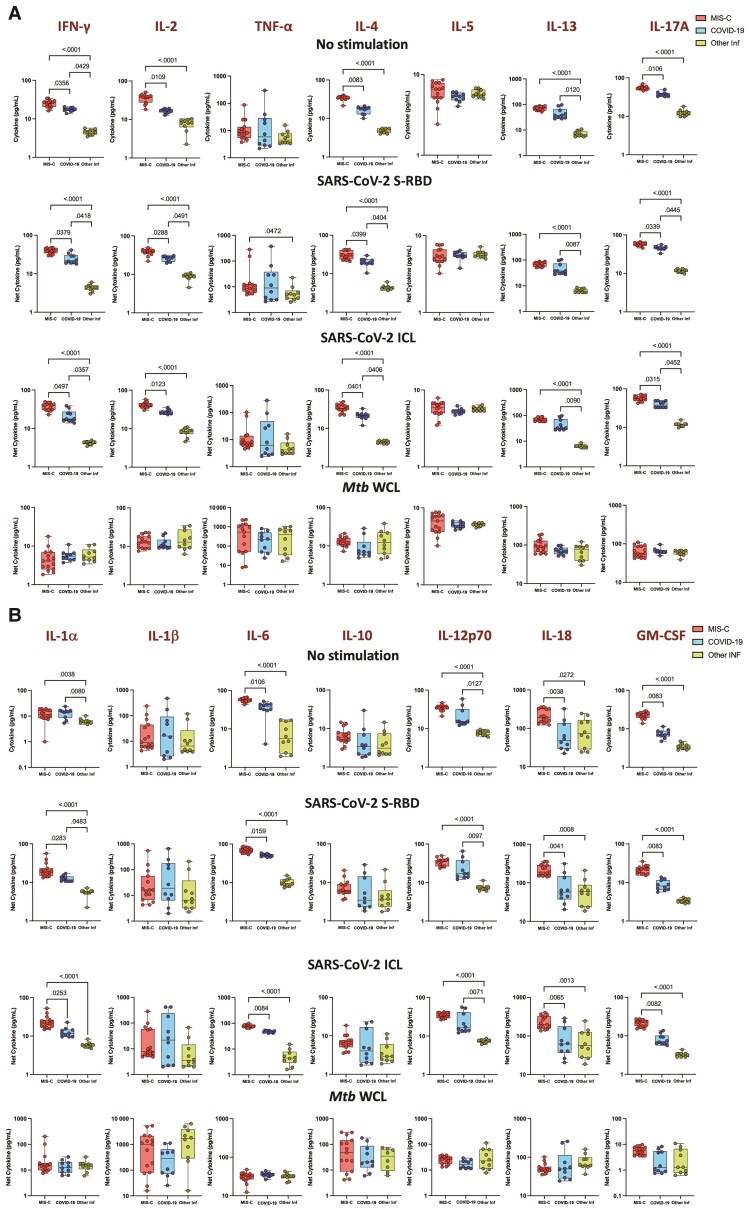
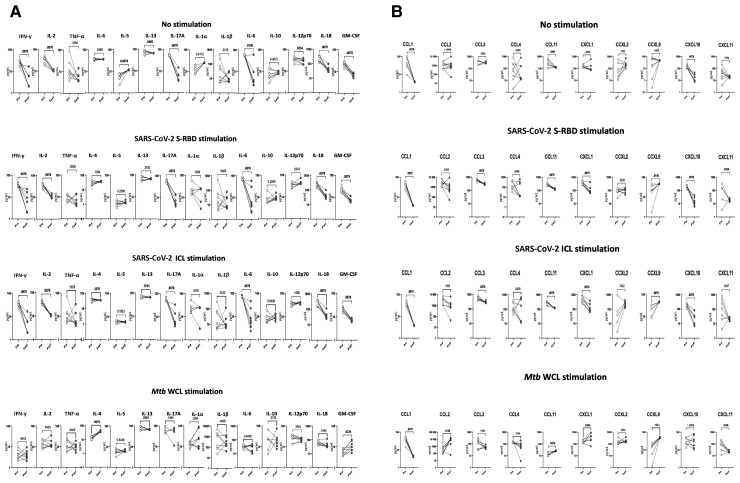
To elucidate the impact of MIS-C on cytokine responses, we assessed their production by whole blood culture supernatants from children with MIS-C, COVID-19, and other infectious diseases with no stimulation or following stimulation with SARS-CoV-2 antigens. As shown in Figure 1A, children with MIS-C exhibited significantly increased production of type 1 (IFN-γ, IL-2), type 2 (IL-4 and IL-13), and type 17 (IL-17A) and other proinflammatory cytokines (IL-1a, IL-6, IL-12p70, IL-18, and GM-CSF) at baseline (no stimulation) (Figure 1B). In response to SARS-CoV-2 S-RBD and SARS-CoV-2 ICL, we observed significantly increased cytokine levels of type 1 (IFN-γ, IL-2), type 2 (IL-4 and IL-13), type 17 (IL-17A) (Figure 1A), and other proinflammatory cytokines (IL-1α, IL-6, IL-12p70, IL-18, and GM-CSF) (Figure 1B) in children with MIS-C compared to children with COVID-19 and those with other infectious diseases. In addition, upon SARS-CoV-2 S-RBD and SARS-CoV-2 ICL stimulation, we also observed elevated levels of IFN-γ, IL-2, IL-4, IL-13, and IL-17A in children with COVID-19 compared to children with other infectious diseases. In contrast, no significant differences were seen in the cytokine levels upon Mtb antigen stimulation (Figure 1).
Figure 1.
Children with multisystem inflammatory syndrome in children (MIS-C) have elevated type 1, type 2, type 17, and other proinflammatory cytokine responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens. Whole blood from children with MIS-C (n = 15), coronavirus disease 2019 (COVID-19) (n = 10), and other infectious diseases (Inf) (n = 10) were stimulated with medium alone, SARS-CoV-2 spike receptor-binding domain (S-RBD), SARS-CoV-2 irradiated cell lysate (ICL), and Mycobacterium tuberculosis whole cell lysate (Mtb WCL) for 12 hours. Levels of type 1, type 2, and type 17 (A) and other proinflammatory (B) cytokines were measured by multiplex assays. Each circle represents a single individual and the bars represent the geometric mean values. P values were calculated using the Kruskal–Wallis test with Dunn multiple comparisons. Cytokine abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; IL, interleukin; TNF-α, tumor necrosis factor.
Children With MIS-C Have Elevated Chemokine Responses to SARS-CoV-2 Antigens
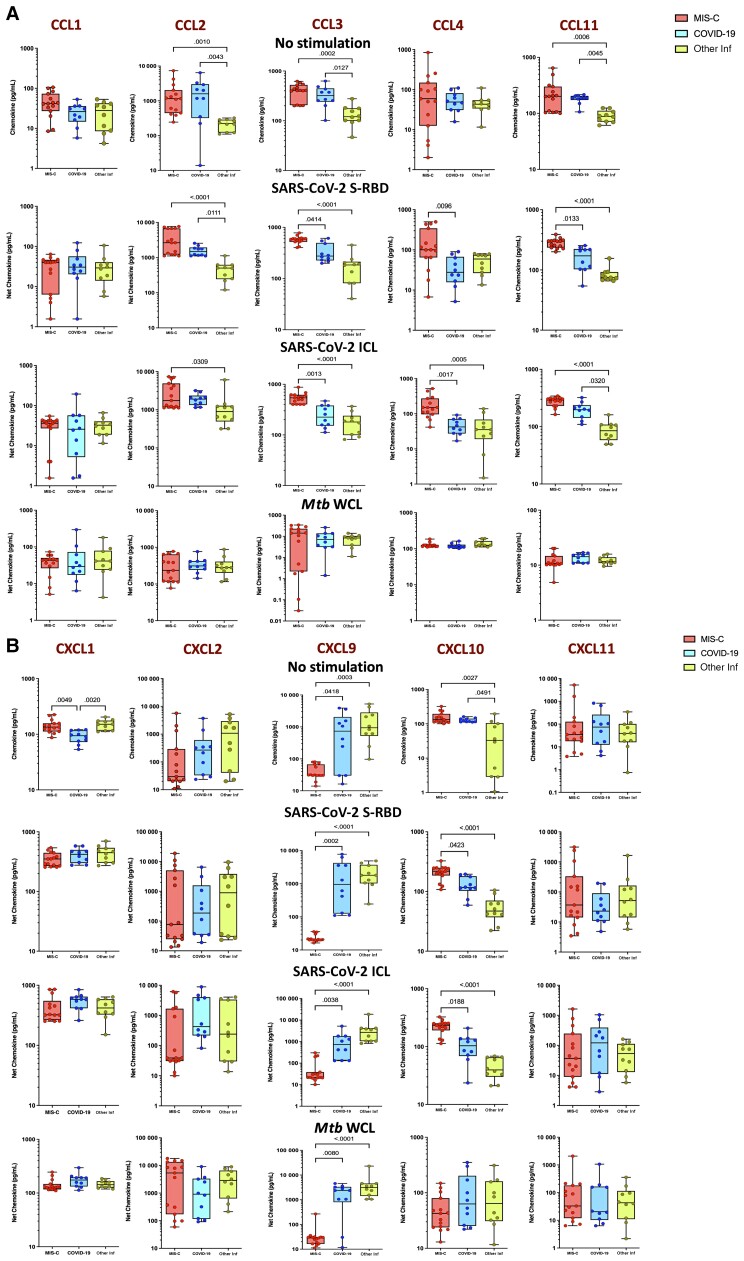
To elucidate the impact of MIS-C on chemokine responses, we assessed their production by whole blood culture supernatants from children with MIS-C, COVID-19, and other infectious diseases with no stimulation or following stimulation with SARS-CoV-2 antigens. Children with MIS-C exhibited significantly increased production of CC chemokines (CCL2, CCL3, and CCL11) and CXC chemokines (CXCL1 and CXCL10) and decreased production of CXCL9 at baseline (no stimulation) (Figure 2A). In response to SARS-CoV-2 S-RBD and SARS-CoV-2 ICL, we observed significantly increased levels of CC chemokines (CCL2, CCL3, CCL4, and CCL11) and CXC chemokines (CXCL10), whereas CXCL9 alone decreased (Figure 2B) in children with MIS-C compared to those with COVID-19 or other infectious diseases. In contrast, no significant differences were seen in the chemokine levels upon Mtb antigen stimulation.
Figure 2.
Children with multisystem inflammatory syndrome in children (MIS-C) have elevated chemokine responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens. Whole blood from children with MIS-C (n = 15), coronavirus disease 2019 (COVID-19) (n = 10), and other infectious diseases (Inf) (n = 10) were stimulated with medium alone, SARS-CoV-2 spike receptor-binding domain (S-RBD), SARS-CoV-2 irradiated cell lysate (ICL), and Mycobacterium tuberculosis whole cell lysate (Mtb WCL) for 12 hours. Levels of CC (A) and CXC (B) chemokines were measured by multiplex assays. Each circle represents a single individual and the bars represent the geometric mean values. P values were calculated using the Kruskal–Wallis test with Dunn multiple comparisons.
Inflammatory Cytokines and Chemokines Can Strongly Distinguish Children With MIS-C From Those With COVID-19 and Other Infectious Diseases
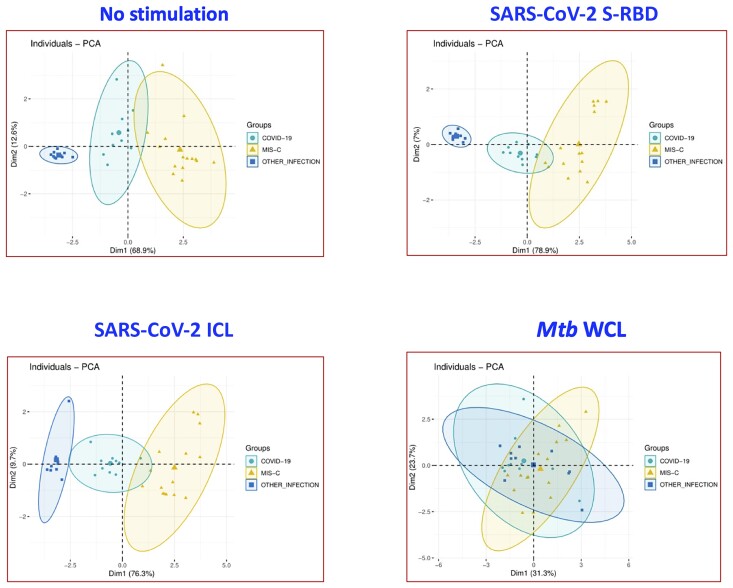
PCA is a statistical procedure that allows us to summarize the information content in big data analysis by means of a smaller set of “summary indices” that can be more easily visualized and analyzed. We performed PCA of IFN-γ, IL-2, IL-4, IL-17, GM-CSF, CCL2, CCL3, and CXCL10 to determine the discriminatory power of cytokines and chemokines in distinguishing children with MIS-C from COVID-19 and other infectious diseases (Figure 3). PCA evidently demonstrates the ability of these markers to differentiate MIS-C from COVID-19 and other infectious diseases, with elevated levels seen in MIS-C and COVID-19 children with no stimulation (captures 81.5% [Dim1: 68.9% + Dim2: 12.6%]) and SARS-CoV-2 S-RBD (captures 85.9% [Dim1: 78.9% + Dim2: 7%]) and SARS-CoV-2 ICL (86% [Dim1: 76.3% + Dim2: 9.7%]) antigen stimulation.
Figure 3.
Principal component analysis (PCA) analysis to estimate the discriminatory power of immune markers in children with multisystem inflammatory syndrome (MIS-C), coronavirus disease 2019 (COVID-19), and other infectious diseases. PCA plot computing normalized multiplex data from levels of selected cytokines and chemokines in combination of 3 different experimental groups. The PCA shows the 2 principal components of variation in medium alone, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike receptor-binding domain (S-RBD), SARS-CoV-2 irradiated cell lysate (ICL), and Mycobacterium tuberculosis whole cell lysate (Mtb WCL) stimulated conditions.
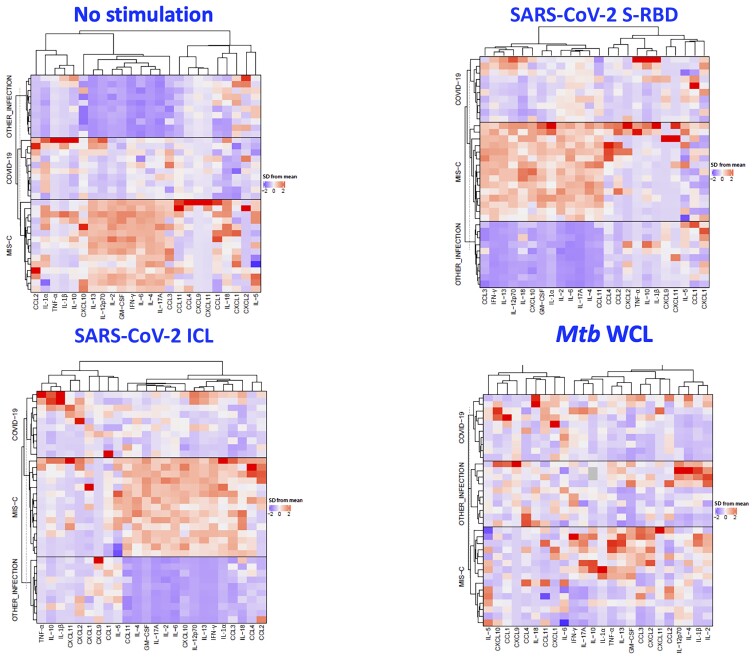
Signature Pattern of Elevated Cytokines and Chemokines in Children With MIS-C
Data were then z-score normalized across the entire cohort and analyzed by hierarchical clustering (Figure 4). For clustering, we used Euclidean distance measure and Ward.d2 method. Two main clusters were identified and showed good separation for MIS-C compared with other infectious diseases, with more overlap between COVID-19 subgroups. The cytokines and chemokines in different disease groups could be clustered separately with only very few misclassifications, and these signature patterns were seen with no stimulation and with SARS-CoV-2 S-RBD and SARS-CoV-2 ICL antigen stimulation.
Figure 4.
Cytokine and chemokine profiling of immune markers in children with multisystem inflammatory syndrome in children (MIS-C), coronavirus disease 2019 (COVID-19), and other infectious diseases. A hierarchical cluster analysis was made to analyze if the combination of biomarkers that were found to be statistically different in MIS-C, COVID-19, or other infectious diseases in univariate analysis could separate the groups according to individual levels of each subject. Data were log10 transformed and z-score normalized. Abbreviations: COVID-19, coronavirus disease 2019; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICL, irradiated cell lysate; IFN-γ, interferon gamma; IL, interleukin; MIS-C, multisystem inflammatory syndrome in children; Mtb, Mycobacterium tuberculosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; S-RBD, spike receptor-binding domain; TNF-α, tumor necrosis factor; WCL, whole cell lysate.
Antigen-Specific Cytokine and Chemokine Levels Are Significantly Diminished Following Recovery
To examine whether the elevated antigen-specific culture supernatant cytokines and chemokines are directly linked with MIS-C, we measured the level of these cytokines and chemokines in MIS-C children during MIS-C and after MIS-C recovery. As shown in Figure 5A, at 6–9 months following recovery, cytokine levels of IFN-γ, IL-2, IL-13, IL-17A, IL-6, IL-1α, IL-1β, IL-18, and GM-CSF were significantly diminished compared to during MIS-C levels at both baseline (no stimulation) and as well as upon SARS-CoV-2 antigen stimulation. Similarly, as shown in Figure 5B, at 6–9 months following recovery, chemokine levels of CCL1, CCL3, CCL11, CXCL1, CXCL10, and CXCL11 were significantly diminished compared to during MIS-C levels at both baseline (no stimulation) as well as upon SARS-CoV-2 antigen stimulation.
Figure 5.
Diminished antigen-specific cytokine and chemokine levels during and after multisystem inflammatory syndrome in children (MIS-C). Culture supernatant cytokine (A) and chemokine (B) levels upon no stimulation and severe acute respiratory syndrome coronavirus 2 antigen stimulation were measured during MIS-C (Pre) and at 6–9 months after recovery from MIS-C (Post). The data are presented as line graphs with each line representing a single individual. P values were calculated using the Wilcoxon signed-rank test. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; ICL, irradiated cell lysate; IFN-γ, interferon gamma; IL, interleukin; Mtb, Mycobacterium tuberculosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; S-RBD, spike receptor-binding domain; TNF-α, tumor necrosis factor; WCL, whole cell lysate.
DISCUSSION
MIS-C is a severe condition associated with COVID-19 infection. The syndrome is rare, and it remains unknown how SARS-CoV-2 infection leads to MIS-C and why it is more specific to the pediatric population. When it comes to treatment and management of MIS-C, a recent systemic review with meta-analysis of data from low- and middle-income countries (LMICs) reported that among the MIS-C–confirmed children, 63% were admitted to the pediatric intensive care unit and almost every one received intravenous immunoglobulin, 58% received corticosteroids, and 19% received alternate agents [9]. However, the exact pathogenesis of MIS-C remains vague, with virus-induced postinfective immune dysregulation appearing to play a leading role. Few other systemic review data reported that overall MIS-C prognosis is good, and reported mortality rates are 0–4% [19].
One assumption is that children with MIS-C might mount an abnormal T-cell response to the coronavirus causing inflammation. Our group previously reported on a comprehensive plasma immune biomarker profile including a large panel of inflammatory markers comprising of cytokines, chemokines, growth factors, acute-phase proteins, and microbial translocation markers in children with MIS-C [11, 12]. A few other studies have demonstrated the immunological responses in children with either mild disease or asymptomatic infection, but antigen-specific T-cell immune responses have been rarely reported [20–22]. Similarly, very few studies till date have described and compared the antigen-specific immunological profile in MIS-C and acute COVID-19 with other infective causes. This comparison and data are crucial in LMICs and tropical countries where diseases such as dengue fever, scrub typhus, and enteric fever are endemic and present an overlapping clinical picture with MIS-C.
In our study, we found higher levels of SARS-CoV-2–specific cytokines and chemokines in culture supernatants in all children with MIS-C compared to those with COVID-19 and other infectious diseases upon antigenic stimulation. Our group has recently reported elevated baseline plasma levels of cytokine and chemokines in children with MIS-C [11]. The elevated levels of cytokines might have systemic effects and may in turn cause organ dysfunction [23]. This may possibly be the reason for children with MIS-C exhibiting multiorgan dysfunction. These findings suggest that enhancement or elevation of T-cell–specific immune responses is greater in the MIS-C and COVID-19 group than the other infectious diseases group. In addition, we have used a nonviral antigen control Mtb WCL, where we observed no statistical significant differences between the 3 study groups, indicating that reported changes seen in cytokine and chemokine levels are only SARS-CoV-2 antigen–mediated responses.
Current knowledge of MIS-C is largely inadequate, and most of the immunological findings are reported during MIS-C [10, 19], but the postrecovery (after MIS-C) manifestation and immune responses have not been thoroughly examined. Our current finding demonstrates that antigen-specific cytokines and chemokine levels significantly diminished after 6–9 months after recovery. This suggests that the elevated proinflammatory responses during MIS-C are normalized after recovery, indicating that immune homeostasis is achieved in these children.
Our outcomes offer some fundamental insights into the potential drivers of immune pathogenesis in MIS-C. Our study suggests that MIS-C is characterized by an enhanced immune response with increased expression of cytokines and chemokines; moreover, our longitudinal data suggest there was a diminished immune activation in the later stage of the syndrome or following recovery. Altogether, studies of antigen specificity will be important in understanding lymphocyte activation and immune dysregulation in MIS-C. Our study has limitations, as we studied a small number of children in all groups including longitudinal follow-up.
In conclusion, the findings from the current study improve our knowledge on the immunology of MIS-C and COVID-19 in children, and may benefit clinicians in the differentiating MIS-C from other common tropical conditions.
Notes
Acknowledgments. We thank the Director of the National Institute for Research in Tuberculosis (NIRT) for the constant support throughout the study; we also thank all of the pediatric consultants and infection control nurses at Kanchi Kamakoti CHILDS Trust Hospital, Institute of Child Health and Hospital for Children, Chennai, India; Dr.Mehta’s Children’s Hospital, Chennai, India; and Rainbow Children’s Hospital, Chennai, India, for their support and contributions. We also sincerely thank the NIRT International Center for Excellence in Research staff for technical support.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, and the National Institute for Research in Tuberculosis–International Center for Excellence in Research.
Potential conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Nathella Pavan Kumar, Department of Immunology, National Institute for Research in Tuberculosis, Indian Council of Medical Research, Chennai, India.
Aishwarya Venkataraman, Department of Immunology, National Institute for Research in Tuberculosis, Indian Council of Medical Research, Chennai, India.
Arul Nancy, ICER, National Institute for Research in Tuberculosis, National Institutes of Health–International Center for Excellence in Research, Chennai, India.
Kadar Moideen, ICER, National Institute for Research in Tuberculosis, National Institutes of Health–International Center for Excellence in Research, Chennai, India.
Poovazhagi Varadarjan, Pediatric Intensive Care Unit, Institute of Child Health and Hospital for Children, Chennai, India.
Elilarasi Selladurai, Pediatric Intensive Care Unit, Institute of Child Health and Hospital for Children, Chennai, India.
Thankgavelu Sangaralingam, General Pediatrics, Dr.Mehta’s Children’s Hospital, Chennai, India.
Ramya Selvam, General Pediatrics, Dr.Mehta’s Children’s Hospital, Chennai, India.
Akshith Thimmaiah, General Pediatrics, Dr.Mehta’s Children’s Hospital, Chennai, India.
Suresh Natarajan, Pediatric Pulmonology, Rainbow Children’s Hospital, Chennai, India.
Ganesh Ramasamy, Pediatric Pulmonology, Rainbow Children’s Hospital, Chennai, India.
Syed Hissar, Department of Immunology, National Institute for Research in Tuberculosis, Indian Council of Medical Research, Chennai, India.
Umadevi Radayam Ranganathan, Department of Immunology, National Institute for Research in Tuberculosis, Indian Council of Medical Research, Chennai, India.
Subash Babu, ICER, National Institute for Research in Tuberculosis, National Institutes of Health–International Center for Excellence in Research, Chennai, India.
References
- 1. Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus–associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 2. Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics 2018; 141:e20172918. [DOI] [PubMed] [Google Scholar]
- 3. Dufort EM, Koumans EH, Chow EJ, et al. . Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feldstein LR, Rose EB, Horwitz SM, et al. . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiotos K, Bassiri H, Behrens EM, et al. . Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc 2020; 9:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20:453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies P, Evans C, Kanthimathinathan HK, et al. . Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health 2020; 4:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandes DM, Oliveira CR, Guerguis S, et al. . Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr 2021; 230:23–31.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sood M, Sharma S, Sood I, Sharma K, Kaushik A. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection: a systematic review with meta-analysis. SN Compr Clin Med 2021; 3:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMurray JC, May JW, Cunningham MW, Jones OY. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis-a critical review of its pathogenesis and treatment. Front Pediatr 2020; 8:626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkataraman A, Kumar NP, Hanna LE, et al. . Plasma biomarker profiling of PIMS-TS, COVID-19 and SARS-CoV2 seropositive children—a cross-sectional observational study from southern India. EBioMedicine 2021; 66:103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar NP, Venkataraman A, Hanna LE, et al. . Systemic inflammation and microbial translocation are characteristic features of SARS-CoV-2-related multisystem inflammatory syndrome in children. Open Forum Infect Dis 2021; 8:ofab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consiglio CR, Cotugno N, Sardh F, et al. . The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gruber CN, Patel RS, Trachtman R, et al. . Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020; 183:982–95.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carter MJ, Fish M, Jennings A, et al. . Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–7. [DOI] [PubMed] [Google Scholar]
- 16. Ministry of Health and Family Welfare (MOHFW). COVID-19 guidelines. 2021. https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf.
- 17.Centers for Disease Control and Prevention. Definition for multisystem inflammatory syndrome in children. 2020. https://www.cdc.gov/mis/index.html.
- 18.World Health Organization. Definition for multisystem inflammatory syndrome in children. 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- 19. Malviya A, Mishra A. Childhood multisystem inflammatory syndrome: an emerging disease with prominent cardiovascular involvement-a scoping review. SN Compr Clin Med 2021; 3:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia R, Wang X, Liu P, et al. . Mild cytokine elevation, moderate CD4(+) T cell response and abundant antibody production in children with COVID-19. Virol Sin 2020; 35:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moratto D, Giacomelli M, Chiarini M, et al. . Immune response in children with COVID-19 is characterized by lower levels of T-cell activation than infected adults. Eur J Immunol 2020; 50:1412–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tosif S, Neeland MR, Sutton P, et al. . Immune responses to SARS-CoV-2 in three children of parents with symptomatic COVID-19. Nat Commun 2020; 11:5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020; 383:2255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]