Abstract
Background
Households are common places for spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We investigated factors associated with household transmission and acquisition of SARS-CoV-2.
Methods
Households with children age <18 years were enrolled into prospective, longitudinal cohorts and followed from August 2020 to August 2021 in Utah, September 2020 to August 2021 in New York City, and November 2020 to October 2021 in Maryland. Participants self-collected nasal swabs weekly and with onset of acute illness. Swabs were tested for SARS-CoV-2 using reverse transcription polymerase chain reaction. We assessed factors associated with SARS-CoV-2 acquisition using a multilevel logistic regression adjusted for household size and clustering and SARS-CoV-2 transmission using a logistic regression adjusted for household size.
Results
Among 2053 people (513 households) enrolled, 180 people (8.8%; in 76 households) tested positive for SARS-CoV-2. Compared with children age <12 years, the odds of acquiring infection were lower for adults age ≥18 years (adjusted odds ratio [aOR], 0.34; 95% CI, 0.14–0.87); however, this may reflect vaccination status, which protected against SARS-CoV-2 acquisition (aOR, 0.17; 95% CI, 0.03–0.91). The odds of onward transmission were similar between symptomatic and asymptomatic primary cases (aOR, 1.00; 95% CI, 0.35–2.93) and did not differ by age (12–17 years vs <12 years: aOR, 1.08; 95% CI, 0.20–5.62; ≥18 years vs <12 years: aOR, 1.70; 95% CI, 0.52–5.83).
Conclusions
Adults had lower odds of acquiring SARS-CoV-2 compared with children, but this association might be influenced by coronavirus disease 2019 (COVID-19) vaccination, which was primarily available for adults and protective against infection. In contrast, all ages, regardless of symptoms and COVID-19 vaccination, had similar odds of transmitting SARS-CoV-2. Our findings underscore the importance of SARS-CoV-2 mitigation measures for persons of all ages.
Keywords: household transmission, COVID-19, SARS-CoV-2
As of June 2022, >1 million people have died from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United States [1]. Households are common places for SARS-CoV-2 to spread [2], and studies of households can be used to learn more about factors associated with virus transmission and susceptibility. Previous work has assessed the influence of personal characteristics, such as a person’s age, symptom development, or coronavirus disease 2019 (COVID-19) vaccination status, on SARS-CoV-2 transmission and acquisition in the household [3–19]; however, most of these studies were limited by retrospective design [4, 7, 8, 11, 13, 17, 18] or use of data collected before the Delta wave [3–17].
We built upon previous work to investigate risk factors associated with transmission and acquisition of SARS-CoV-2 among children and adults in household-based, longitudinal cohorts in New York City, Utah, and Maryland [20, 21]. Following participants through the Alpha and Delta waves, we hypothesized that a person’s age would not be associated with SARS-CoV-2 acquisition or transmission, COVID-19 vaccination status would protect against viral acquisition or transmission, and presence of symptoms would increase odds of transmission.
METHODS
Study Design
In response to the COVID-19 pandemic, the US Centers for Disease Control and Prevention (CDC) initiated 2 household-based, prospective cohort studies to estimate the incidence and within-household transmission of SARS-CoV-2: (i) the Coronavirus Household Evaluation and Respiratory Testing (C-HEaRT) study in New York City and Utah [20] and (ii) the SARS-CoV-2 Epidemiology And Response in Children (SEARCh) study in Maryland [21]. C-HEaRT recruited households with at least 1 child age <18 years from previously established cohorts and the broader community [22, 23]. SEARCh recruited households with at least 1 child age 0–4 years from pediatric primary care practices. Households were followed from enrollment until study end in C-HEaRT and for 8 months from enrollment in SEARCh. Study enrollment and follow-up occurred from August 2020 to August 2021 in Utah, September 2020 to August 2021 in New York City, and November 2020 to October 2021 in Maryland.
At enrollment, serum specimens were collected, and participants completed online surveys describing demographic characteristics, history of COVID-19 testing and infection, and household features. Throughout follow-up, participants completed a weekly survey, online or via text, about symptoms experienced in the preceding week and, if symptoms were experienced, mitigation measures they took like isolating and wearing a mask. SARS-CoV-2 vaccination information was collected through medical record abstraction of immunization records and self-reported on follow-up surveys. Specific questionnaires used are available in the Supplementary Data. Participants self-collected nasal swabs weekly and additional swabs with onset of acute illness with COVID-19-like symptoms; parents collected nasal swabs for young children at these same time points instead of the children self-swabbing.
Swabs were tested for SARS-CoV-2 using reverse transcription polymerase chain reaction (RT-PCR; Quidel Lyra SARS-CoV-2 Assay [24] or ThermoFisher TaqPath COVID-19 Combo Kit [25]) at Marshfield Clinic Research Institute in Wisconsin [20]. The CDC conducted viral whole-genome sequencing [26, 27] on an individual’s first nasal swab specimen with a cycle threshold value ≤30 for the SARS-CoV-2 nucleocapsid target using the IDT xGen SARS-CoV-2 library prep kit [28]. Libraries were sequenced using 2 × 150 base pair Illumina Chemistry on a MiSeq or NovaSeq instrument. Demultiplexed data were down-sampled to 1 million reads per sample, primers were trimmed with BBDuk (BBMap, version 38.87) [29], and a single consensus genome for each sample was generated with Iterative Refinement Meta-Assembler (IRMA), version 1.0.2, using the default CoV configuration [30]. We performed clade assignments using Nextclade, version 1.13.2 [31], and assigned lineages using Pangolin, version 3.1.20 (pangoLEARN 1.2.123, Scorpio 0.3.16) [32].
Acquisition of SARS-CoV-2
To assess household acquisition of SARS-CoV-2, the analysis was restricted to households with ≥1 RT-PCR-confirmed SARS-CoV-2 infection among enrolled household members. Participants with a positive test were classified as having a primary, secondary, or tertiary household infection. Primary cases were defined as participants with the first symptom onset or positive SARS-CoV-2 test, whichever occurred earlier, in the households. Co-primary cases were participants who had symptom onset or a positive test <2 days after the household’s initial SARS-CoV-2 infection [33]. Participants who were secondary cases had a positive SARS-CoV-2 test ≥2 days after the household’s first detected infection, and tertiary cases tested positive >2 days after a household’s initial secondary infection date [33]. Tertiary cases were excluded from estimation of the crude secondary infection risk (SIR). Primary or co-primary cases were excluded from the acquisition analysis.
We then evaluated the association between household member characteristics and the odds of SARS-CoV-2 acquisition among people who were a secondary case or later using multilevel logistic regressions controlling for household size (households with <5 or ≥5 people) and clustering (household-level random intercept). The household size categorization threshold was chosen based on the median household size (5) of the participants in the analysis set. Models did not adjust for COVID-19 vaccination status because not all members were eligible for vaccination. Household member characteristics assessed included (i) study site (New York, Utah, or Maryland); (ii) age group (<12, 12–17, and ≥18 years); (iii) gender; (iv) presence of ≥1 high-risk medical condition; (v) vaccination status, defined as primary COVID-19 vaccination series receipt (1 dose of a vector-based vaccine or 2 doses of an mRNA vaccine) ≥14 days before the household was affected by SARS-CoV-2; and (vi) having a prior SARS-CoV-2 infection. A participant had a prior SARS-CoV-2 infection if at enrollment they self-reported a previous SARS-CoV-2 infection diagnosis or had SARS-CoV-2 antibodies present in enrollment serology based on results from the Roche Elecsys Anti-SARS-CoV-2 Assay (Maryland) [34] and Luminex xMAP-SARS-CoV-2 Multiantigen Assay (New York and Utah) [35].
Further analyses investigated how COVID-19 vaccination status impacted the relationship between a participant’s age and the likelihood of SARS-CoV-2 acquisition. A multilevel logistic regression model adjusted for household size, and clustering was repeated using a composite variable of each participant’s age group and COVID-19 vaccination status before household infection: (i) unvaccinated children (<12 years); (ii) unvaccinated adolescents (12–17 years); (iii) vaccinated adolescents; (iv) unvaccinated adults (≥18 years); and (v) vaccinated adults.
Transmission of SARS-CoV-2
Next, we evaluated the association between households’ primary SARS-CoV-2 case characteristics and the odds of transmitting SARS-CoV-2 to ≥1 household member. Often, several household members acquired the infection at the same time, resulting in co-primary cases and challenging identification of which primary case was the source of infection for others in the household. To remove the influence of this uncertainty, we excluded households with co-primary cases from the transmission analysis. We supplemented the analysis by repeating it using a previously published probabilistic transmission trees approach that accounted for co-primary cases [36], with methods presented in the Supplementary Data.
Across the remaining primary cases, we assessed primary case characteristics related to SARS-CoV-2 transmission to ≥1 other nonprimary case household member. Characteristics assessed included (i) study site; (ii) age group; (iii) gender; (iv) presence of ≥1 high-risk medical condition; (v) receipt of primary COVID-19 vaccination series; (vi) prior SARS-CoV-2 infection; (vii) and symptomatic status, (viii) lineage (Delta [defined as B.1.617.2 and AY lineages] or other variant), and (ix) mitigation measures taken during the current SARS-CoV-2 infection. These associations were evaluated using a logistic regression model adjusted for household size; no household-level clustering was considered as models included only 1 individual per household. A sensitivity analysis assessed the association of (i) symptomatic status and (ii) SARS-CoV-2 lineage and odds of transmitting SARS-CoV-2 to a household member, additionally adjusting for the primary case’s age. Additional sensitivity analyses were conducted to assess the association of primary case characteristics with onward transmission by (i) using the proportion of susceptible household contacts who were infected by the primary case as the main outcome in an adjusted Poisson regression; (ii) estimating an adjusted risk ratio of transmission using a log-risk regression model; and (iii) re-calculating the adjusted odds ratio of transmission across mitigation measures on a subset of symptomatic primary cases.
All analyses were conducted using the packages “tidyverse” and “lme4” in R, version 4.0.4 [37–39].
RESULTS
Study Population
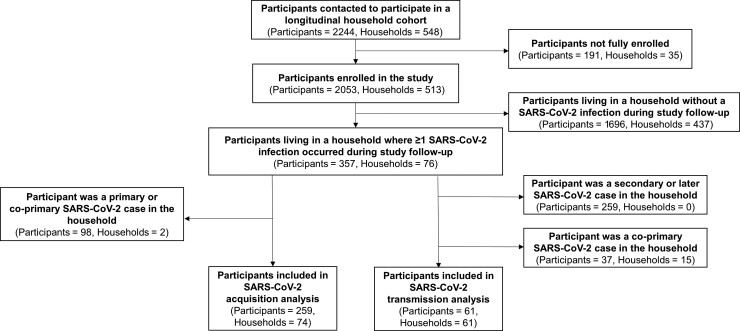
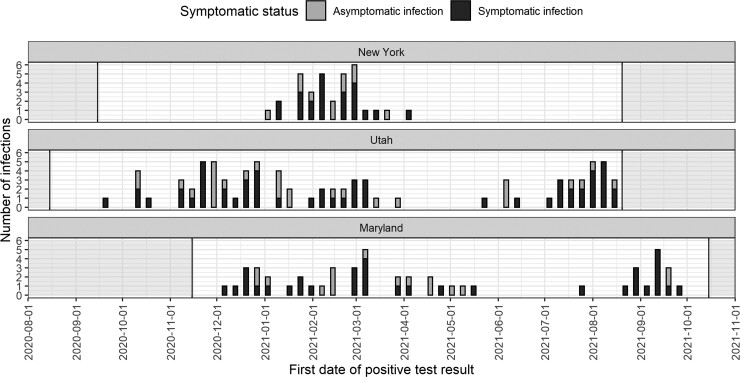
Of 2244 people (548 households) contacted to participate in the cohorts, 2053 participants (513 households) enrolled in the longitudinal study across 3 states: 508 (24.7%) in New York, 852 (41.5%) in Utah, and 693 (33.8%) in Maryland (Figure 1; Supplementary Table 1). Participants were enrolled if they consented to the study and completed an individual enrollment questionnaire. From August 2020 to October 2021, 180 people (8.8%; 76 households) tested positive for SARS-CoV-2. Infections were observed throughout the period of observation, but some clustered in time corresponding to increased community circulation (Figure 2) [1]. Most households with infections were in Utah (53.9%). Our analysis focused on each household’s first cluster of infections, so we excluded 3 SARS-CoV-2 infections that occurred in participants ≥3 months after their households were initially affected. After excluding these infections, the analysis included 357 people, 177 with SARS-CoV-2, in 76 households with ≥1 member who tested positive for SARS-CoV-2 (Table 1).
Figure 1.
Participants included in analysis data set. This chart depicts how many participants were contacted, fully enrolled in the study, and included in the final analysis data set. Only participants living in a household with at least 1 SARS-CoV-2 infection during study follow-up were included in the analysis data set. Additional criteria were enforced for the acquisition and transmission analyses. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
Distribution of SARS-CoV-2 cases over time across longitudinal household cohorts. The distribution of SARS-CoV-2 cases over time is shown stratified by the 3 cohort sites: New York, Utah, and Maryland. Asymptomatic infections are indicated by the gray bars and symptomatic infections by the black bars. The period of follow-up for each study site is indicated by the white area. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Characteristics of Participants Living in a Household With a SARS-CoV-2-Positive Participant
| C-HEaRT | SEARCh | Total | ||
|---|---|---|---|---|
| New York | Utah | Maryland | ||
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Total households | 13 | 41 | 22 | 76 |
| Total participants | 52 | 211 | 94 | 357 |
| Age groups | ||||
| <12 y | 17 (32.7) | 95 (45.0) | 45 (47.9) | 157 (44.0) |
| 12–17 y | 7 (13.5) | 31 (14.7) | 5 (5.3) | 43 (12.0) |
| ≥18 y | 28 (53.8) | 85 (40.3) | 44 (46.8) | 157 (44.0) |
| Gender | ||||
| Male | 28 (53.8) | 99 (46.9) | 46 (48.9) | 173 (48.5) |
| Female | 23 (44.2) | 112 (53.1) | 48 (51.1) | 183 (51.3) |
| Nonbinary/third gender | 1 (1.9) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Race/ethnicity | ||||
| White, non-Hispanic | 6 (11.5) | 191 (90.5) | 73 (77.7) | 270 (75.6) |
| Black, non-Hispanic | 2 (3.8) | 1 (0.5) | 2 (2.1) | 5 (1.4) |
| Asian, non-Hispanic | 0 (0.0) | 0 (0.0) | 3 (3.2) | 3 (0.8) |
| Other, non-Hispanic | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Multiracial, non-Hispanic | 3 (5.8) | 4 (1.9) | 14 (14.9) | 21 (5.9) |
| Hispanic | 41 (78.8) | 13 (6.2) | 2 (2.1) | 56 (15.7) |
| Unknown | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| High-risk medical conditiona | ||||
| No | 35 (67.3) | 149 (70.6) | 61 (64.9) | 245 (68.6) |
| Yes | 17 (32.7) | 62 (29.4) | 33 (35.1) | 112 (31.4) |
| Primary vaccination series before household infection | ||||
| No | 52 (100.0) | 180 (85.3) | 82 (87.2) | 314 (88.0) |
| Yes | 0 (0.0) | 31 (14.7) | 12 (12.8) | 43 (12.0) |
| Previous SARS-CoV-2 infectionb | ||||
| No | 36 (69.2) | 187 (88.6) | 66 (70.2) | 289 (81.0) |
| Yes | 16 (30.8) | 24 (11.4) | 27 (28.7) | 67 (18.8) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (0.3) |
| SARS-CoV-2 infection during study follow-upc | ||||
| No | 19 (36.5) | 118 (55.9) | 43 (45.7) | 180 (50.4) |
| Yes | 33 (63.5) | 93 (44.1) | 51 (54.3) | 177 (49.6) |
Abbreviations: C-HEaRT, Coronavirus Household Evaluation and Respiratory Testing study; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEARCh, SARS-CoV-2 Epidemiology And Response in Children study.
High-risk medical conditions included were asthma, chronic lung disease, active tuberculosis, chronic bronchitis, cystic fibrosis, chronic obstructive pulmonary disease, obstructive sleep apnea, chronic metabolic disease, diabetes, thyroid problems, blood disorders, hypertension, cardiovascular disease, bladder disease, liver disease, immunocompromised condition, leukemia, cancer, long-term steroid treatment, organ transplant, neurologic condition, or autoimmune condition.
Defined using self-reported prior SARS-CoV-2 infection or antibodies detected in serology at enrollment.
Three people in the Utah cohort had SARS-CoV-2 infection during follow-up 3 months or more after their households’ initial SARS-CoV-2 introduction. Households were censored from the analysis after their initial SARS-CoV-2 introduction, so these 3 infections were excluded from the analysis and not presented here.
Among 177 people with SARS-CoV-2, 81 (45.8%) were children age <12 years, 18 (10.2%) were adolescents age 12–17 years, and 78 (44.1%) were adults age ≥18 years (Supplementary Table 2). During their infection, from the first week of SARS-CoV-2 detection to the last week of detection preceding 2 negative or missed weekly swabs, 120 (67.8%) people reported symptoms. Thirty-two (18.1%) people infected during follow-up had evidence of prior SARS-CoV-2 infection, from self-report or serologic testing, and 16 (9.0%) people infected had received the primary vaccination series. Supplementary Table 3 further describes vaccination information in the cohort, including brand and age eligibility. Across the 177 SARS-CoV-2 infections, 61 (34.5%) were classified as single primary cases, 37 (20.9%) as co-primary cases, 61 (34.5%) as secondary cases, and 18 (10.2%) as tertiary cases. The crude SIR among household members excluding tertiary cases was 23.6% (95% CI, 0.19–0.29; SIR range by site, 22.4%–29.4%).
Risk Factors for Acquisition of SARS-CoV-2
Excluding participants who were primary or co-primary SARS-CoV-2 cases in their households, the association between household member characteristics and SARS-CoV-2 acquisition was assessed among 259 participants in 74 households (Table 2). There was no association with odds of infection in Utah (adjusted odds ratio [aOR], 0.30; 95% CI, 0.03–2.72) and Maryland (aOR, 0.35; 95% CI, 0.03–3.74) compared with New York. Compared with children age <12 years, adults (aOR, 0.34; 95% CI, 0.14–0.87) were less likely to acquire SARS-CoV-2 from a household member; however, no association was found between children age <12 years and adolescents age 12–17 years and acquisition of SARS-CoV-2 (aOR, 0.33; 95% CI, 0.07–1.45), possibly due to limited sample size. Participants who received the primary COVID-19 vaccination series before household SARS-CoV-2 introduction had significantly lower odds of SARS-CoV-2 infection (aOR, 0.17; 95% CI, 0.03–0.91). When unvaccinated adults were compared with unvaccinated children age <12 years, there was no longer an association with odds of acquiring SARS-CoV-2 (aOR, 0.51; 95% CI, 0.18–1.45); vaccinated adults compared with unvaccinated children age <12 years had significantly lower odds of infection (aOR, 0.10; 95% CI, 0.01–0.65). Prior SARS-CoV-2 infection was not associated with odds of SARS-CoV-2 re-acquisition (aOR, 0.82; 95% CI, 0.20–3.36); this pattern was also observed when prior infection was defined only based on presence of SARS-CoV-2 antibodies at enrollment (aOR, 2.27; 95% CI, 0.45–11.46).
Table 2.
Odds of SARS-CoV-2 Acquisition After Household Introduction Across Household Member Characteristics
| No. of Susceptible Participants After Household Introductiona | No. of SARS-CoV-2 Infections | Odds of Infection After Household Introduction Adjusted for Household Size and Clustering | |
|---|---|---|---|
| No. (%) | No. (% of Row Total) | aOR (95% CI)b | |
| Total participants | 259 | 79 (30.5) | … |
| Total households | 74 | 37 (50.0) | … |
| Study site | |||
| C-HEaRT: New York | 34 (13.1) | 15 (44.1) | Ref |
| C-HEaRT: Utah | 161 (62.2) | 43 (26.7) | 0.30 (0.03–2.72) |
| SEARCh: Maryland | 64 (24.7) | 21 (32.8) | 0.35 (0.03–3.74) |
| Age group | |||
| <12 y | 123 (47.5) | 47 (38.2) | Ref |
| 12–17 y | 32 (12.4) | 7 (21.9) | 0.33 (0.07–1.45) |
| ≥18 y | 104 (40.2) | 25 (24.0) | 0.34 (0.14–0.87) |
| Gender, No. (%)c | |||
| Male | 122 (47.3) | 40 (32.8) | Ref |
| Female | 136 (52.7) | 39 (28.7) | 1.00 (0.43–2.34) |
| High-risk medical conditiond | |||
| No | 182 (70.3) | 56 (30.8) | Ref |
| Yes | 77 (29.7) | 23 (29.9) | 1.46 (0.54–3.97) |
| Primary vaccination series before household infection | |||
| No | 228 (88.0) | 75 (32.9) | Ref |
| Yes | 31 (12.0) | 4 (12.9) | 0.17 (0.03–0.91) |
| Previous SARS-CoV-2 infectione,f | |||
| No | 212 (82.2) | 68 (32.1) | Ref |
| Yes | 46 (17.8) | 11 (23.9) | 0.82 (0.20–3.36) |
Abbreviations: aOR, adjusted odds ratio; C-HEaRT, Coronavirus Household Evaluation and Respiratory Testing study; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEARCh, SARS-CoV-2 Epidemiology And Response in Children study.
Primary and co-primary cases are excluded.
Results from a multilevel logistic regression model adjusted for household size and clustering.
Nonbinary/third gender was excluded from this association due to data sparsity (n = 1 observation).
High-risk medical conditions included were asthma, chronic lung disease, active tuberculosis, chronic bronchitis, cystic fibrosis, chronic obstructive pulmonary disease, obstructive sleep apnea, chronic metabolic disease, diabetes, thyroid problems, blood disorders, hypertension, cardiovascular disease, bladder disease, liver disease, immunocompromised condition, leukemia, cancer, long-term steroid treatment, organ transplant, neurologic condition, or autoimmune condition.
Defined using self-reported prior SARS-CoV-2 infection or antibodies detected in serology at enrollment.
One observation was missing and excluded from this association.
Risk Factors for Transmission of SARS-CoV-2
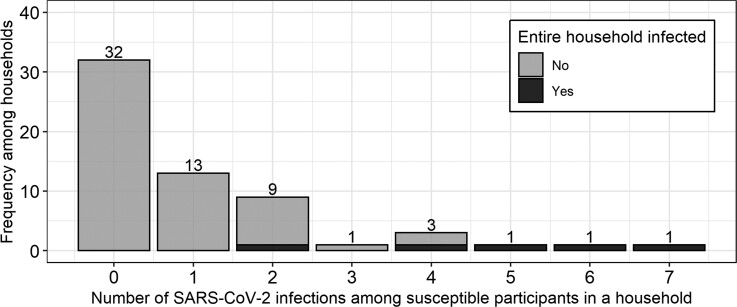
After excluding households with co-primary cases (Supplementary Table 4), risk factors for SARS-CoV-2 transmission from infected primary cases to their household members were assessed. Across 61 households with single primary cases, 32 (52.5%) did not transmit SARS-CoV-2 to another enrolled household member, whereas 29 (47.5%) did. Of the 29 households with onward transmission, 13 (44.8%) experienced transmission to only 1 other person in the household, and 5 (17.2%) had all enrolled members infected (range of household size, 2–7 people) (Figure 3).
Figure 3.
Distribution of number of susceptible people who acquired SARS-CoV-2 in their households from the primary case among households with only 1 primary case. The distribution of SARS-CoV-2 infections susceptible people acquired in their households is displayed. Households with co-primary cases were excluded. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
No demographic characteristics of people infected with SARS-CoV-2 were found to significantly modify their odds of transmitting the virus to at least 1 other household member (Table 3). The odds that the primary case transmitted SARS-CoV-2 to a household member were not significantly different across study sites (Utah vs New York: aOR, 0.44; 95% CI, 0.07–2.27; Maryland vs New York: aOR, 0.41; 95% CI, 0.07–2.11). Compared with children age <12 years, adolescents (aOR, 1.08; 95% CI, 0.20–5.62) and adults (aOR, 1.70; 95% CI, 0.52–5.83) had overall similar odds of transmitting SARS-CoV-2 to other household members. There was no association between the odds of SARS-CoV-2 transmission from participants who completed the primary COVID-19 vaccination series vs those who were unvaccinated (aOR, 0.44; 95% CI, 0.06–2.57). Compared with those without prior infection, people with prior SARS-CoV-2 infection (aOR, 0.96; 95% CI, 0.24–3.65) had similar odds of SARS-CoV-2 transmission to another household member; results were similar when prior infection was defined only based on presence of SARS-CoV-2 antibodies at enrollment (aOR, 1.61; 95% CI, 0.37–7.58). When we cast the outcome as the proportion of susceptible household members who became infected, we found that adult primary cases transmitted within the household at a significantly greater rate than children age <12 years (adjusted rate ratio [aRR], 1.97; 95% CI, 1.07–3.91) (Supplementary Table 5), as did primary cases with a high-risk medical condition (aRR, 2.69; 95% CI, 1.51–4.68).
Table 3.
Odds of SARS-CoV-2 Transmission to a Household Member Across Characteristics of Primary Cases
| No. of Primary Casesa | No. of Primary Cases who Transmitted SARS-CoV-2 | Odds of Transmission to a Household Member Adjusted for Household Size | |
|---|---|---|---|
| No. (%) | No. (% of Row Total) | aOR (95% CI)b | |
| Characteristics of participants | |||
| Total households | 61 | 29 (47.5) | … |
| Study site | |||
| C-HEaRT: New York | 9 (14.8) | 6 (66.7) | Ref |
| C-HEaRT: Utah | 34 (55.7) | 15 (44.1) | 0.44 (0.07–2.27) |
| SEARCh: Maryland | 18 (29.5) | 8 (44.4) | 0.41 (0.07–2.11) |
| Age group | |||
| <12 y | 17 (27.9) | 7 (41.2) | Ref |
| 12–17 y | 10 (16.4) | 4 (40.0) | 1.08 (0.20–5.62) |
| ≥18 y | 34 (55.7) | 18 (52.9) | 1.70 (0.52–5.83) |
| Genderc | |||
| Male | 30 (49.2) | 17 (56.7) | Ref |
| Female | 31 (50.8) | 12 (38.7) | 0.45 (0.15–1.25) |
| High-risk medical conditiond | |||
| No | 39 (63.9) | 17 (43.6) | Ref |
| Yes | 22 (36.1) | 12 (54.5) | 1.61 (0.56–4.75) |
| Primary vaccination series before household infection | |||
| No | 55 (90.2) | 27 (49.1) | Ref |
| Yes | 6 (9.8) | 2 (33.3) | 0.44 (0.06–2.57) |
| Previous SARS-CoV-2 infectione | |||
| No | 50 (82.0) | 24 (48.0) | Ref |
| Yes | 11 (18.0) | 5 (45.5) | 0.96 (0.24–3.65) |
| SARS-CoV-2 infection characteristics | |||
| Symptomatic status | |||
| Asymptomatic infection | 21 (34.4) | 10 (47.6) | Ref |
| Symptomatic infection | 40 (65.6) | 19 (47.5) | 1.00 (0.35–2.93) |
| Presence of specific symptomsf | |||
| Shortness of breath | 6 (9.8) | 3 (50.0) | 1.05 (0.18–6.19) |
| Chills | 5 (8.2) | 3 (60.0) | 2.25 (0.32–19.53) |
| Cough | 13 (21.3) | 7 (53.8) | 1.36 (0.39–4.84) |
| Nausea | 3 (4.9) | 1 (33.3) | … |
| Vomiting | 0 (0.0) | 0 (0.0) | … |
| Diarrhea | 4 (6.6) | 2 (50.0) | 1.22 (0.14–11.06) |
| Fatigue | 10 (16.4) | 5 (50.0) | 1.18 (0.29–4.79) |
| Fever | 12 (19.7) | 5 (41.7) | 0.77 (0.20–2.77) |
| Headache | 14 (23.0) | 7 (50.0) | 1.22 (0.36–4.22) |
| Body aches | 12 (19.7) | 5 (41.7) | 0.77 (0.20–2.77) |
| Joint pain | 5 (8.2) | 4 (80.0) | … |
| Abdominal pain | 3 (4.9) | 1 (33.3) | … |
| Chest pain | 2 (3.3) | 1 (50.0) | … |
| Nasal congestion | 20 (32.8) | 10 (50.0) | 1.16 (0.39–3.44) |
| Sore throat | 14 (23.0) | 7 (50.0) | 1.14 (0.34–3.85) |
| Loss of taste or smell | 6 (9.8) | 4 (66.7) | 2.42 (0.43–18.63) |
| Conjunctivitis | 1 (1.6) | 0 (0.0) | … |
| SARS-CoV-2 lineage | |||
| Other lineage | 30 (49.2) | 15 (50.0) | Ref |
| Delta lineage | 9 (14.8) | 5 (55.6) | 1.23 (0.27–5.88) |
| Unknown | 22 (36.1) | 9 (40.9) | … |
| Mitigation measuresg | |||
| Used own bathroom | |||
| No | 17 (27.9) | 10 (58.8) | Ref |
| Yes | 9 (14.8) | 3 (33.3) | 0.35 (0.06–1.81) |
| Unknown | 35 (57.4) | 16 (45.7) | … |
| Did not receive care from another member | |||
| No | 14 (23.0) | 5 (35.7) | Ref |
| Yes | 13 (21.3) | 8 (61.5) | 3.00 (0.64–15.95) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
| Avoided close contact | |||
| No | 20 (32.8) | 11 (55.0) | Ref |
| Yes | 7 (11.5) | 2 (28.6) | 0.33 (0.04–1.94) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
| Wore mask | |||
| No | 18 (29.5) | 9 (50.0) | Ref |
| Yes | 9 (14.8) | 4 (44.4) | 0.81 (0.15–4.08) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
| Avoided sharing drinks and utensils | |||
| No | 5 (8.2) | 3 (60.0) | Ref |
| Yes | 22 (36.1) | 10 (45.5) | 0.52 (0.06–3.87) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
| Slept in alone in room | |||
| No | 14 (23.0) | 7 (50.0) | Ref |
| Yes | 13 (21.3) | 6 (46.2) | 0.85 (0.18–3.90) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
| Stayed in separate room | |||
| No | 18 (29.5) | 11 (61.1) | Ref |
| Yes | 9 (14.8) | 2 (22.2) | 0.17 (0.02–0.97) |
| Unknown | 34 (55.7) | 16 (47.1) | … |
Abbreviations: aOR, adjusted odds ratio; C-HEaRT, Coronavirus Household Evaluation and Respiratory Testing study; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEARCh, SARS-CoV-2 Epidemiology And Response in Children study.
Households with co-primary cases were excluded.
Results from a logistic regression model adjusted for household size.
No primary cases identified as nonbinary/third gender.
High-risk medical conditions included were asthma, chronic lung disease, active tuberculosis, chronic bronchitis, cystic fibrosis, chronic obstructive pulmonary disease, obstructive sleep apnea, chronic metabolic disease, diabetes, thyroid problems, blood disorders, hypertension, cardiovascular disease, bladder disease, liver disease, immunocompromised condition, leukemia, cancer, long-term steroid treatment, organ transplant, neurologic condition, or autoimmune condition.
Defined using self-reported prior SARS-CoV-2 infection or antibodies detected in serology at enrollment.
For specific symptoms listed, referent is absence of that symptom during illness with SARS-CoV-2. Adjusted odds ratios were not calculated for some specific symptoms due to sparse data.
Those who did not report use of mitigation measures and those who responded as “unknown” about use of mitigation measures were categorized as “unknown” in the table and excluded from analyses.
People with symptomatic compared with asymptomatic infections (aOR, 1.00; 95% CI, 0.35–2.93) had comparable odds of SARS-CoV-2 transmission, which was also reflected in the crude frequencies of asymptomatic (47.6%) and symptomatic (47.5%) primary cases who transmitted SARS-CoV-2 in their households (Table 3). No association with odds of transmission was seen among people experiencing chills (aOR, 2.25; 95% CI, 0.32–19.53), cough (aOR, 1.36; 95% CI, 0.39–4.84), diarrhea (aOR, 1.22; 95% CI, 0.14–11.06), headache (aOR, 1.22; 95% CI, 0.36–4.22), or loss of taste or smell (aOR, 2.42; 95% CI, 0.43–18.63) during the course of their illness; however, data were underpowered to detect associations across many specific symptoms. A sensitivity analysis of odds of transmission by a person’s symptomatic status adjusted for age in addition to household size produced a similar point estimate (symptomatic vs asymptomatic: aOR, 0.71; 95% CI, 0.19–2.47), but a higher proportion of primary case adults (85.3%) reported symptoms than adolescents (60.0%) or children (29.4%) (Supplementary Table 6). Participants infected with the Delta variant (23.1%) compared with another lineage (76.9%) of SARS-CoV-2 were not associated with odds of SARS-CoV-2 transmission to another household member (aOR, 1.23; 95% CI, 0.27–5.88); results remained similar when also adjusting for age (aOR, 1.46; 95% CI, 0.28–8.74).
The only mitigation measure significantly associated with reduced odds of SARS-CoV-2 transmission was staying in a separate room from other household members (aOR, 0.17; 95% CI, 0.02–0.97) (Table 3). Among a subset of only symptomatic primary cases, mitigation measure results were the same (Supplementary Table 7). Across demographic and infection risk factors, results were similar using the proportion of susceptible household contacts who were infected as an alternative measure of transmission (Supplementary Table 5), log-risk regression to estimate an adjusted risk ratio of transmission (Supplementary Table 8), and a probabilistic modeling approach (Supplementary Results, Supplementary Tables 9–11).
DISCUSSION
Within large, multisite prospective cohorts of households with children who were under active symptom surveillance and tested weekly, we found that 1 in 4 households experienced onward spread of SARS-CoV-2 among household contacts. We examined factors associated with odds of acquiring or transmitting SARS-CoV-2 in the household and observed that individuals of all ages and symptomatic status had similar odds of transmitting SARS-CoV-2. Fully vaccinated individuals had reduced odds of SARS-CoV-2 infection. Adults also had lower odds of infection, possibly due to higher rates of COVID-19 vaccination. Infected individuals who stayed in a separate room had lower odds of transmitting SARS-CoV-2 to another household member. These findings underscore the importance of COVID-19 vaccination and following recommendations to prevent infection and control transmission within the household regardless of the age or symptoms of the primary case [40].
In our study, people of all ages acquired and transmitted SARS-CoV-2 within their households, but the odds of viral acquisition were lower in adults. Other studies had variable results concerning the association between age and SARS-CoV-2 infection, with some studies finding that young children had lower infection rates than adults [7, 8, 11, 14, 17] and others no clear association [12, 15]. Meta-analyses have described comparable SARS-CoV-2 transmission by all age groups (as we found) [2] but higher SARS-CoV-2 secondary acquisition in adults [2, 41], which differs from our results. We observed roughly 70% lower odds of household acquisition of SARS-CoV-2 among adults compared with children age <12 years, but the apparent protective effect of age on SARS-CoV-2 acquisition may be related to vaccination status, as adults had earlier access to COVID-19 vaccination during the study period than children or adolescents. When we compared SARS-CoV-2 acquisition in unvaccinated adults with unvaccinated children, the protective association with age was greatly reduced and no longer significant, suggesting that vaccination status played a role in the association between age and SARS-CoV-2 infection; however, results were limited by small sample size. Additional larger studies may be able to better disentangle the effect of age and COVID-19 vaccination on risk of household SARS-CoV-2 acquisition.
COVID-19 vaccination was associated with reduced odds of SARS-CoV-2 infection, but there was not a significant reduction in the likelihood of vaccinated individuals transmitting the virus. Previous results from data collected early in COVID-19 vaccine availability suggested that vaccination lessened the likelihood of transmission to household members [42]. Other work found that this protective effect was reduced but still present with the emergence of the Delta variant [43]. Similar to our findings, recent household cohort studies restricting analyses to Delta variant circulation indicated that COVID-19 vaccination reduced household SARS-CoV-2 acquisition but was not significantly protective against transmission [18, 19]. Because the effect of COVID-19 vaccination at preventing household transmission may differ with new SARS-CoV-2 variants, it is important to follow recommendations to mitigate transmission in the household even when the primary case has been vaccinated.
A strength of our study, with weekly SARS-CoV-2 testing, was that primary cases in households were captured prospectively and irrespective of symptoms, enabling us to compare transmission risk from symptomatic and asymptomatic primary cases. We found no association between a person having an asymptomatic or symptomatic SARS-CoV-2 infection and odds of transmission to another household member. This aligns with previous work that found no effect of symptom development on transmissibility [11, 15]; however, 2 meta-analyses observed that symptomatic compared with asymptomatic infections were associated with increased transmission to household members [2, 41]. Due to the cross-sectional [16] or retrospective nature [4, 7, 8, 11, 13, 17] of many household cohorts, some studies may have underascertained asymptomatic infections, which could bias findings toward a positive association between the presence of symptoms during infection and transmission; our study design was less likely to be impacted by this bias.
Our study was subject to a few limitations. Due to use of a nonprobabilistic modeling approach, households with multiple primary (co-primary) cases were excluded from the transmission analysis, decreasing our sample size and power to detect small effects. Our approach assumed that all household members were infected from the household’s primary case, possibly misclassifying some infections as stemming from the household rather than the community. Because transmission results were generally similar in sensitivity analyses using a probabilistic approach that included households with co-primary cases and accounted for community transmission and because SARS-CoV-2 sequences were similar within households, we anticipate that these limitations had minimal effect on study findings. Comparison of mitigation measures associated with the odds of SARS-CoV-2 transmission were limited by the large number of unknown observations (range, 55.7% to 57.4% depending on the questionnaire item). Finally, this analysis was likely underpowered to detect differences in susceptibility while accounting for COVID-19 vaccination across age groups; to mitigate this, a sensitivity analysis assessed how odds of acquisition changed in vaccinated adults, unvaccinated adults, and unvaccinated children and found that vaccination played a role in the association between age and acquisition.
In summary, we found that in a household setting people of all ages transmit and acquire SARS-CoV-2, those with asymptomatic infection were as likely to transmit SARS-CoV-2 as those with symptomatic infection, and those who had completed a primary COVID-19 vaccine series were less likely to be infected. To reduce the likelihood of ongoing transmission, households with a SARS-CoV-2-infected member should follow recommended prevention measures [40], irrespective of the presence or absence of symptoms, COVID-19 vaccination status, or age of the affected household member. To reduce transmission potential, nonpharmaceutical interventions could be adopted by all age-eligible household members when possible, including children with asymptomatic infection and caregivers of those children. Future studies could investigate how these associations change with the emergence of new SARS-CoV-2 variants or vaccine approval for younger children.
Supplementary Material
Acknowledgments
The authors thank the families who participated in the C-HEaRT and SEARCh cohorts and Annapolis Pediatrics, Columbia Medical Practice, Dundalk Pediatrics, and Johns Hopkins Community Physicians for allowing households to be recruited from their practices. The authors also acknowledge the CDC Laboratory and Testing Task Force Strain Surveillance and Emerging Variants Team for their support in the processing of the sequence data included in this study: Dhwani Batra, Jason Caravas, Peter Cook, Dakota Howard, Kristine Lacek, Clint Paden, Ben Rambo-Martin, and Samuel Shepard.
CDC Genomic Sequencing Laboratory. Meghan L. Bentz, Alex Burgin, Mark Burroughs, Morgan L. Davis, Joseph C. Madden Jr., Sarah Nobles, Jasmine Padilla, and Mili Sheth from the Centers for Disease Control and Prevention.
C-HEaRT Study Team. Michael Daugherty, Yan Li, Anna Kelleher, Ying Tao, Jing Zhang, Brian Lynch, Adam Retchless, Anna Uehara, and Han Jia Ng from the Centers for Disease Control and Prevention.
SEARCh Study Team. Christine Council-DiBitetto, Tina Ghasri, Amanda Gormley, Milena Gatto, Maria Jordan, Karen Loehr, Jason Morsell, Jennifer Oliva, Jocelyn San Mateo, Kristi Herbert, Khadija Smith, Kimberli Wanionek, Cathleen Weadon, and Suzanne Woods from Johns Hopkins University.
Financial support. The C-HEaRT cohort study was funded by the US Centers for Disease Control and Prevention (Contract 75D30120C08150 with Abt Associates), and data collected were managed using REDCap electronic data capture tools hosted at Vanderbilt University Medical Center (UL1TR000445 from National Institutes of Health—National Center for Advancing Translational Sciences). The SEARCh cohort study was funded by the US Centers for Disease Control and Prevention (Contract 75D30120C08737), and data collected were managed using REDCap electronic data capture tools hosted at Johns Hopkins University.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Patient consent. For C-HEaRT and SEARCh, written informed consent was obtained from adults >18 years of age; for children <18 years of age, parents or legal guardians provided written informed consent on behalf of their children. For C-HEaRT, children 12–17 years of age also provided assent. For SEARCh, children 7–17 years of age provided additional assent. For C-HEaRT, participating households also received small monetary incentives for study activities. This study was reviewed and approved by the Institutional Review Boards of the University of Utah and Columbia University (C-HEaRT) and of Johns Hopkins University (SEARCh) in the United States (See 45 C.F.R. part 46; 21 C.F.R. part 56).
Contributor Information
Kelsey M Sumner, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA; Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Ruth A Karron, Center for Immunization Research, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Melissa S Stockwell, Division of Child and Adolescent Health, Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, New York, USA; Department of Population and Family Health, Mailman School of Public Health, Columbia University Irving Medical Center, New York, New York, USA.
Fatimah S Dawood, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Joseph B Stanford, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Alexandra Mellis, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Emily Hacker, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Priyam Thind, Division of Child and Adolescent Health, Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, New York, USA.
Maria Julia E Castro, Division of Child and Adolescent Health, Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, New York, USA.
John Paul Harris, Division of Child and Adolescent Health, Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, New York, USA.
Maria Deloria Knoll, International Vaccine Access Center, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Elizabeth Schappell, Center for Immunization Research, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Marissa K Hetrich, International Vaccine Access Center, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Jazmin Duque, Abt Associates, Cambridge, Massachusetts, USA.
Zuha Jeddy, Abt Associates, Cambridge, Massachusetts, USA.
Kim Altunkaynak, Abt Associates, Cambridge, Massachusetts, USA.
Brandon Poe, Abt Associates, Cambridge, Massachusetts, USA.
Jennifer Meece, Marshfield Clinic Research Institute, Marshfield, Wisconsin, USA.
Elisha Stefanski, Marshfield Clinic Research Institute, Marshfield, Wisconsin, USA.
Suxiang Tong, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Justin S Lee, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Ashton Dixon, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Vic Veguilla, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Melissa A Rolfes, Centers for Disease Control and Prevention COVID-19 Response, Atlanta, Georgia, USA.
Christina A Porucznik, Division of Public Health, Department of Family and Preventive Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
CDC Genomic Sequencing Laboratory, SEARCh and C-HEaRT Study Teams:
Meghan L Bentz, Alex Burgin, Mark Burroughs, Morgan L Davis, Madden Joseph C, Jr, Sarah Nobles, Jasmine Padilla, Mili Sheth, Michael Daugherty, Yan Li, Anna Kelleher, Ying Tao, Jing Zhang, Brian Lynch, Adam Retchless, Anna Uehara, Han Jia Ng, Christine Council-DiBitetto, Tina Ghasri, Amanda Gormley, Milena Gatto, Maria Jordan, Karen Loehr, Jason Morsell, Jennifer Oliva, Jocelyn San Mateo, Kristi Herbert, Khadija Smith, Kimberli Wanionek, Cathleen Weadon, and Suzanne Woods
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . COVID data tracker. Available at: https://covid.cdc.gov/covid-data-tracker. Accessed 18 February, 2022.
- 2. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr 2021; 175:1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis 2021; 21:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lyngse FP, Molbak K, Skov RL, et al. Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load. Nat Commun 2021; 12:7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soriano-Arandes A, Gatell A, Serrano P, et al. Household SARS-CoV-2 transmission and children: a network prospective study. Clin Infect Dis 2021; 73:e1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metlay JP, Haas JS, Soltoff AE, Armstrong KA. Household transmission of SARS-CoV-2. JAMA Netw Open 2021; 4:e210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng DC, Tan KK, Chin L, et al. Risk factors associated with household transmission of SARS-CoV-2 in Negeri Sembilan, Malaysia. J Paediatr Child Health 2022; 58:769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez DA, Klein EY, Parent C, et al. Latino household transmission of SARS-CoV-2. Clin Infect Dis 2022;74:1675–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis 2020; 71:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Zhang B, Lu J, et al. Characteristics of household transmission of COVID-19. Clin Infect Dis 2020; 71:1943–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis NM, Chu VT, Ye D, et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis 2021; 73:1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu VT, Yousaf AR, Chang K, et al. Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med 2021; 385:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musa S, Kissling E, Valenciano M, et al. Household transmission of SARS-CoV-2: a prospective observational study in Bosnia and Herzegovina, August–December 2020. Int J Infect Dis 2021; 112:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Julin CH, Robertson AH, Hungnes O, et al. Household transmission of SARS-CoV-2: a prospective longitudinal study showing higher viral load and increased transmissibility of the alpha variant compared to previous strains. Microorganisms 2021; 9:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stich M, Elling R, Renk H, et al. Transmission of severe acute respiratory syndrome coronavirus 2 in households with children, Southwest Germany, May-August 2020. Emerg Infect Dis 2021; 27:3009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jing QL, Liu MJ, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng OT, Koh V, Chiew CJ, et al. Impact of Delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Lancet Reg Health West Pac 2021; 17:100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawood FS, Porucznik CA, Veguilla V, et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. JAMA Pediatr 2022; 176:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karron RA, Quesada MG, Schappell EA, et al. Binding and neutralizing antibody responses to SARS-CoV-2 in infants and young children exceed those in adults. medRxiv 21268034 [Preprint]. December 21, 2021.
- 22. Stockwell MS, Reed C, Vargas CY, et al. MoSAIC: mobile surveillance for acute respiratory infections and influenza-like illness in the community. Am J Epidemiol 2014; 180:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. University of Utah School of Medicine . Utah Children’s Project. Available at: https://admin.pediatrics.medicine.utah.edu/pediatrics/research/programs/utah-childrens-project. Accessed 30 June, 2022.
- 24. Quidel . Lyra SARS-CoV-2 Assay. Available at: https://www.quidel.com/molecular-diagnostics/lyra-sars-cov-2-assay. Accessed 1 March, 2022.
- 25. ThermoFisher . TaqPath COVID-19 Combo Kit. Available at: https://www.thermofisher.com/order/catalog/product/A47814. Accessed 1 March, 2022.
- 26. Paden CR, Tao Y, Queen K, et al. Rapid, sensitive, full-genome sequencing of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 2020; 26: 2401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rambaut A, Holmes EC, O’Toole A, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020; 5:1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Integrated DNA Technologies Inc . IDT xGen SARS-CoV-2 library prep kit. Available at: https://www.idtdna.com/pages. Accessed 1 March, 2022.
- 29. Bushnell B. BBMap. 38.87 ed. 2022. Available at: https://sourceforge.net/projects/bbmap/. Accessed 1 March, 2022.
- 30. Shepard SS, Meno S, Bahl J, Wilson MM, Barnes J, Neuhaus E. Viral deep sequencing needs an adaptive approach: iRMA, the iterative refinement meta-assembler. BMC Genomics 2016; 17:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aksamentov I, Roemer C, Hodcroft E, Neher R. Nextclade: clade assignment, mutation calling and quality control for viral genomes. J Open Source Softw 2021; 6:3773. [Google Scholar]
- 32. O’Toole Á, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the Pangolin tool. Virus Evol 2021; 7:veab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rolfes MA, Grijalva CG, Zhu Y, et al. Implications of shortened quarantine among household contacts of index patients with confirmed SARS-CoV-2 infection–Tennessee and Wisconsin, April–September 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roche . Roche Eclecsys Anti-SARS-CoV-2 Assay. Available at: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html. Accessed 5 March, 2022.
- 35. Luminex . Luminex xMAP SARS-CoV-2 Multiantigen Assay. Available at: https://www.luminexcorp.com/xmap-sars-cov-2-antibody-testing/. Accessed 5 March, 2022.
- 36. Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol 2004; 160:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R: A Language and Environment for Statistical Computing. 4.0.4 ed. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 38. Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw 2019; 4:1686. [Google Scholar]
- 39. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 26:48. [Google Scholar]
- 40. Centers for Disease Control and Prevention . What to do if you are sick. Available at: https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html. Accessed 18 February, 2022.
- 41. Koh WC, Naing L, Chaw L, et al. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLoS One 2020; 15:e0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of SARS-CoV-2. N Engl J Med 2021; 385:1718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Gier B, Andeweg S, Backer JA, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Euro Surveill 2021; 26:2100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.