Abstract
Background
Widespread respiratory infections with high morbidity rates caused by respiratory viruses represent a significant global public health problem. Our objective was to describe cases and deaths from severe acute respiratory infection (SARI) in Brazil over the past 8 y as well as changes in the distribution and risk of illness and death from SARI before and in the first year of the coronavirus disease 2019 (COVID-19) pandemic (FYP).
Methods
We performed a descriptive epidemiological study of hospitalized SARI cases and deaths between 2013 and 2020 in Brazil, separated into pre-pandemic (2013 to 2019) and FYP (2020). We estimate the increase in SARI cases and deaths in the FYP as well as the mortality and infection risks attributable to the FYP (MRAP and IRAP, respectively).
Results
In 2020, an excess of 425 054 cases and 109 682 deaths was observed, with a significant increase in the risk of falling ill and dying from SARI, with an IRAP of 200.06 and an MRAP of 51.68 cases per 100 000 inhabitants. The increase in SARI cases and deaths was particularly prominent among patients with COVID-19, the elderly, males, those self-identifying as mixed race and patients with heart disease and diabetes. We conclude that an important increase in morbidity and mortality due to SARI was observed in the FYP. More vulnerable groups and those living in the Southeast, North and Center-West regions of the country suffered the most.
Keywords: COVID-19, hospitalizations, influenza, respiratory syncytial virus, SARI
Introduction
Respiratory viral infections often evolve from mild to moderate or severe cases and can become critical global health problems due to their high rates of spread and morbidity, especially in children, the elderly and immunocompromised people.1 In 2016 there were an estimated 336 million lower respiratory tract infections worldwide, with approximately 2.3 million deaths.2,3 The estimated annual global mortality from lower respiratory tract infections includes 134 000 deaths from influenza (43%) and respiratory syncytial virus (RSV; 57%), excluding other aetiological agents.2 These figures are dwarfed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with 240 million cases and 4.8 million deaths reported by mid-October 2021.4
In Brazil, since the influenza A(H1N1)pdm09 pandemic of 2009, the epidemiological surveillance system has reported hospitalizations due to severe acute respiratory infection (SARI) influenza-related cases and deaths.5 The initial SARI surveillance protocol aimed to identify the influenza A and B viruses but expanded in 2012 to include other respiratory viruses such as RSV, adenovirus and parainfluenza 1, 2 and 3.6,7 Since then, the Ministry of Health has improved and strengthened the surveillance, recently incorporating coronavirus disease 2019 (COVID-19) into the scope of monitoring after identifying the first case in Brazil.5
The COVID-19 pandemic represents the world's greatest public health challenge of the century and caused a severe public health crisis in Brazil, which deepened and highlighted the social problems already present in the country.8–10 This reinforces the importance and challenges of public health surveillance, as most diseases related to public health emergencies are caused by viruses and are identified as zoonoses and/or depend on vectors to be transmitted.11
In this context, considering the paramount importance of SARI surveillance in our country and globally, we conducted an unprecedented evaluation of epidemiological profile variations in terms of morbidity and mortality due to SARI, independent of aetiology. Our study summarizes cases and deaths from SARI in Brazil over 8 y, exploring changes in the distribution and risk of illness and death from SARI before and during the first year of the COVID-19 pandemic (FYP) in Brazil.
Methods
We performed a descriptive epidemiological study from records of hospitalizations and deaths from SARI identified between 2013 and 2020. Data from 2013 to 2018 were obtained from the Notifiable Disease Information System (SINAN) Influenza Web and data from 2019 and 2020 were obtained from the Influenza Epidemiological Surveillance Information System (SIVEP-Gripe). These information systems were designed to support epidemiological surveillance, ensuring that the epidemiological and laboratory profiles of hospitalized cases and deaths from SARI could be recorded and monitored. It is noteworthy that SIVEP-Gripe replaced the SINAN Influenza Web in 2019.
The databases containing anonymous information were obtained through the Open Data Portal of the Brazilian healthcare system (OpenDATASUS) following the General Data Protection Law (LGPD). In order to homogenize the definition of SARI cases throughout the study period, given changes introduced by the COVID-19 pandemic, we considered all cases that met the definition of SARI of the Brazilian Ministry of Health (MoH) in a non-pandemic context12 (Chart 1).
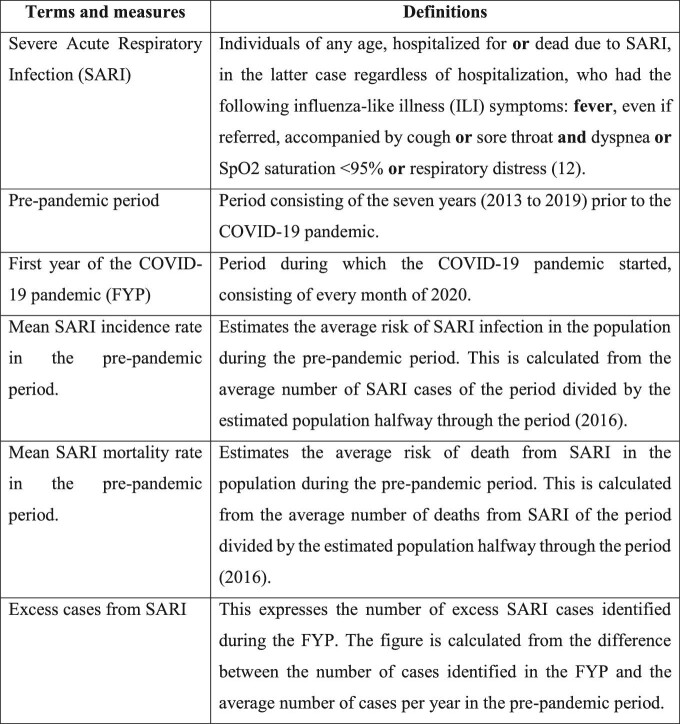
Chart 1.
Description of terms, measures and definitions used in the present study.
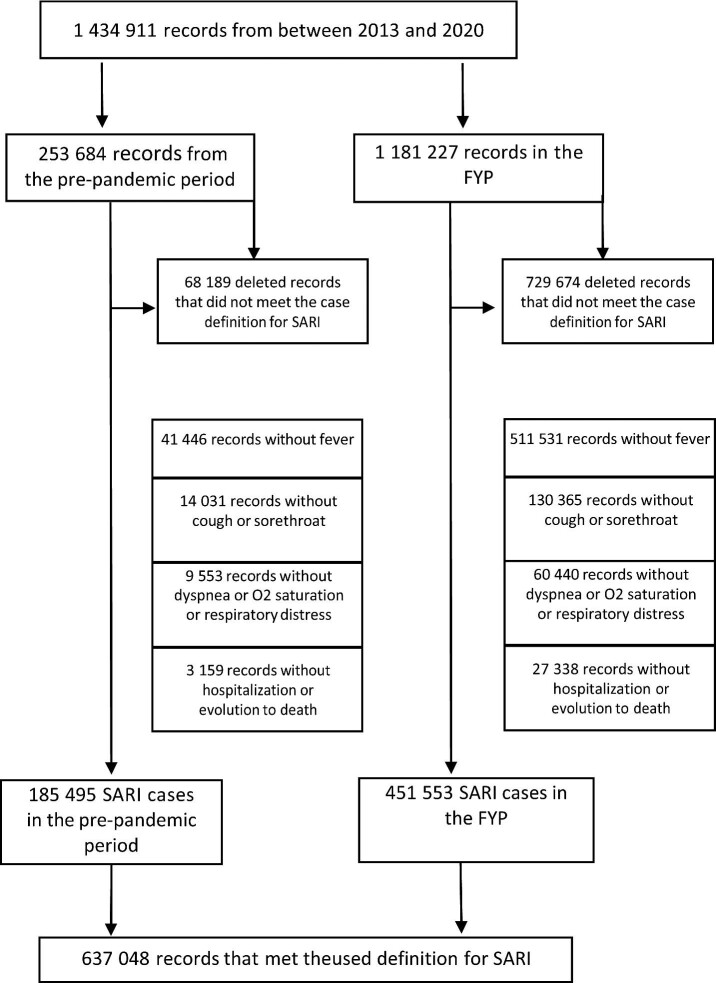
We excluded cases that did not meet the established definition using a four-stage protocol. As a first step, cases without fever were excluded. In the second stage, cases involving a fever were excluded when no hospitalization or death occurred. The third stage excluded cases with fever and hospitalization or death but no cough or sore throat. In the fourth stage, cases without dyspnea, oxygen saturation or respiratory discomfort were excluded from the group of patients with a fever and a cough or sore throat whose condition required hospitalization or resulted in death (Figure 1).
Figure 1.
Flow chart for identifying cases of SARI using the adopted definition for the full study period and separately for the pre-pandemic and the FYP.
After organizing the database, SARI cases and deaths were estimated and described according to their sociodemographic and clinical characteristics. Incidence and mortality rates were estimated for pre-pandemic (2013–2019) and 2020, the FYP in Brazil. It is important to note that the pandemic quickly increased in Brazil from the first case of COVID-19, which was reported in February 2020, and that no vaccines were available in the country that year. The national vaccination campaign against COVID-19 in Brazil began on January 2021, after the Brazil National Health Surveillance Agency (ANVISA) authorized the emergency use of CoronaVac (Sinovac Life Sciences, Beijing, China).13
The average number of annual cases and deaths, as well as incidence and mortality rates, were calculated for the pre-pandemic period. For the FYP, these measures were described and calculated considering all months of 2020 (Table 1). Based on both periods, to estimate the impact of all factors present in 2020, whether directly linked to COVID-19 or not, the following measures were estimated: excess cases, excess deaths, mortality risk attributable to the FYP (MRAP) and infection risk attributable to the FYP (IRAP; Table 1). The MRAP and IRAP figures were only calculated for the variables with population data available from the Brazilian Institute of Geography and Statistics (IBGE). These included the state of residence, sex and age group, which enabled the calculation of incidence and mortality rates for different groups.14 Chart 1 presents details about the calculation of these measures.
Table 1.
Risks attributable to the FYP and excess SARI cases and deaths by aetiological classification, sex and age group, Brazil 2013–2020
| Cases | Deaths | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pandemic | FYPa | Pre-pandemic | FYPa | |||||||||
| SARI | Cases (mean) | Mean incidence rate | Cases | Incidence rateb | IRAP (95% CI)b | Excess cases | Deaths (mean) | Mean mortality rateb | Deaths | Mortality rateb | MRAP (95% CI)b | Excess deaths |
| General | 26 499 | 12.86 | 451 553 | 212.92 | 200.06 (199.4 to 200.7) | 425 054 | 2795 | 1.36 | 112 477 | 53.04 | 51.68 (51.37 to 51.99) | 109 682 |
| According to aetiologyc | ||||||||||||
| Influenza | 4499 | 2.18 | 1756 | 0.83 | −1.36 (−1.43 to −1.28) | −2743 | 707 | 0.34 | 192 | 0.09 | −0.25 (−0.28 to −0.22) | −515 |
| Other respiratory viruses | 4000 | 1.94 | 2531 | 1.19 | −0.75 (−0.82 to −0.67) | −1469 | 201 | 0.10 | 143 | 0.07 | 0.03 (−0.05 to −0.01) | −58 |
| Other aetiological agents | 185 | 0.09 | 1072 | 0.51 | 0.42 (0.38 to 0.45) | 887 | 40 | 0.02 | 234 | 0.11 | 0.09 (0.08 to 0.11) | 194 |
| Unspecified | 17 302 | 8.40 | 130 708 | 61.63 | 53.24 (52.88 to 53.59) | 113 406 | 1823 | 0.88 | 22 419 | 10.57 | 9.69 (9.54 to 9.83) | 20 596 |
| COVID-19 | – | 0.00 | 287 966 | 135.78 | 135.78 (135.29 to 136.28) | 287 966 | – | 0.00 | 88 992 | 41.96 | 41.96 (41.69 to 42.24) | 88 992 |
| Under investigation | 513 | 0.25 | 27 520 | 12.98 | 12.73 (12.57 to 12.88) | 27 007 | 24 | 0.01 | 497 | 0.23 | 0.22 (0.20 to 0.24) | 473 |
| Sexc,d | ||||||||||||
| Female | 12 976 | 12.43 | 191 455 | 178.05 | 165.61 (164.79 to 166.44) | 178 479 | 1301 | 1.25 | 44 863 | 41.72 | 40.47 (40.08 to 40.87) | 43 562 |
| Male | 13 518 | 13.29 | 260 000 | 248.69 | 235.40 (234.42 to 236.38 | 246 482 | 1494 | 1.47 | 67 594 | 64.65 | 63.19 (62.69 to 63.68) | 66 100 |
| Age group (years)c,d | ||||||||||||
| ≤4 | 12 448 | 85.58 | 20 282 | 146.49 | 60.91 (58.40 to 63.43) | 7834 | 369 | 2.53 | 634 | 4.58 | 2.04 (1.60 to 2.48) | 265 |
| 5–9 | 1399 | 8.99 | 6022 | 40.94 | 31.95 (30.81 to 33.09) | 4623 | 49 | 0.31 | 100 | 0.68 | 0.37 (0.21 to 0.53) | 51 |
| 10–19 | 1222 | 3.61 | 6782 | 20.80 | 17.19 (16.66 to 17.73) | 5560 | 104 | 0.31 | 452 | 1.39 | 1.08 (0.94 to 1.22) | 348 |
| 20–39 | 3502 | 5.15 | 64 930 | 94.80 | 89.65 (88.90 to 90.40) | 61 428 | 425 | 0.62 | 5845 | 8.53 | 7.91 (7.68 to 8.14) | 5420 |
| 40–59 | 3825 | 7.78 | 142 560 | 268.29 | 260.51 (259.10 to 261.92) | 138 735 | 832 | 1.69 | 24 252 | 45.64 | 43.95 (43.36 to 44.53) | 23 420 |
| ≥60 | 4103 | 16.46 | 210 977 | 720.29 | 703.83 (700.73 to 706.93) | 206 874 | 1017 | 4.08 | 81 194 | 277.20 | 273.12 (271.10 to 275.04) | 80 177 |
aFirst year of the COVID-19 pandemic.
bPer 100 000 inhabitants.
The differences expressed for case and death increments were statistically significant (p<0.001).
dAn average of 5 cases in the pre-pandemic period and 98 cases in the FYP were without sex information. In the FYP period, 20 deaths were without sex information.
We present crude incidence rates, crude mortality rates and age-standardized mortality rates for all of Brazil and by state for both parts of the study period. We use the direct method to calculate the age-standardized mortality rate, adopting the estimated Brazilian population for 2020 as the base population.
RStudio software (RStudio, Boston, MA, USA) was used to handle the database, which consisted of more than a million reports. For the statistical analysis we used the Statistical Packages for Social Sciences version 22 (IBM, Armonk, NY, USA) and Excel 2016 (Microsoft, Redmond, WA, USA) was used to produce graphs and tables. Pearson's χ2 test was used to verify the association between independent categorical variables using a significance level of 5% (p<0.05). Confidence intervals (CIs) were calculated for risks attributed to the FYP with a 95% confidence level. Maps depicting the spatiotemporal distribution of SARI cases were made using QGIS version 3.18.2.
Results
During the study period, 1 434 911 reports were logged, 17.67% (253 684) of which were from the pre-pandemic period (2013–2019). Of the total notifications, 26.87% (68 189) of the pre-pandemic reports and 61.77% (729 674) of the FYP reports were excluded because they did not meet the criteria defining SARI cases used in this study. As such, 637 048 reported cases were considered, with 29.11% belonging to the pre-pandemic period and 70.88% reported during the FYP (Figure 1).
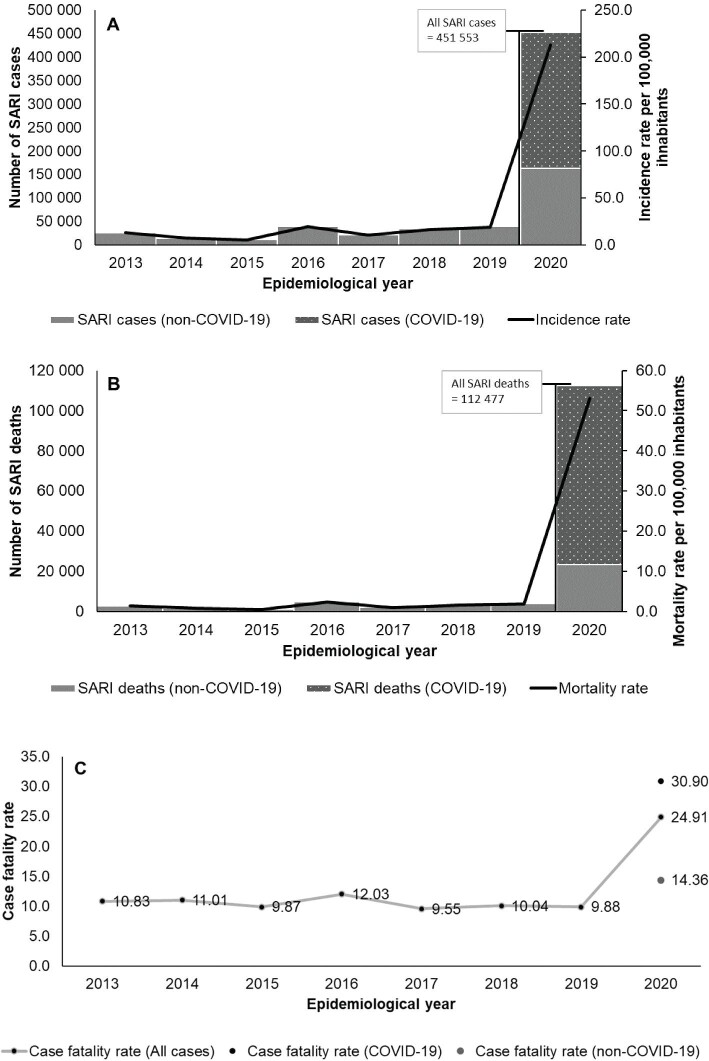
Figure 2 shows the yearly figures of SARI cases, mortality and the case fatality rate. SARI incidence rates were lowest in 2014 and 2015 (7.03 and 5.28 per 100 000 inhabitants, respectively), with the pre-pandemic peak of 19.28 cases per 100 000 inhabitants occurring in 2016. In 2020, the SARI incidence rate jumped to 212.92 per 100 000 inhabitants. The highest pre-pandemic mortality rate was also seen in 2016 (2.32 per 100 000 inhabitants), followed by 2018 and 2019 (1.64 and 1.84 per 100 000 inhabitants, respectively). Meanwhile, the mortality rate during the FYP was 53.04 per 100 000 inhabitants. A similar trend was observed for the SARI case fatality rate, with the pre-pandemic rate growing no higher than 12.03% in 2016 but increasing to 24.91% in the FYP. The case fatality rate was 30.90% for separate cases due to COVID-19 and 14.36% for non-COVID-19 cases (cases associated with other aetiological agents, an unspecified aetiological agent and under investigation). Of all SARI cases and deaths, SARI due to COVID-19 predominated in 2020, accounting for 63.77% and 79.12%, respectively (Figure 2).
Figure 2.
SARI incidence, mortality and case fatality rates, Brazil 2013–2020: (A) absolute number of cases and incidence rate, (B) absolute number of deaths and mortality rate and (C) case fatality rate.
During the pre-pandemic period in Brazil, the average annual number of SARI cases was 26 499, corresponding to an average incidence rate of 12.86 cases per 100 000 inhabitants. Unspecified SARI cases had the highest mean incidence rate in this period, at 8.40 cases per 100 000 inhabitants, followed by SARI caused by influenza (2.18 per 100 000 inhabitants) and other respiratory viruses (1.94 per 100 000 inhabitants). In the same period, the average number of cases per 100 000 inhabitants was 13.29 for men and 12.43 for women. Children ≤4 y of age were the most frequently infected, with 85.58 cases per 100 000 inhabitants, followed by elderly individuals ≥60 y of age (16.46 per 100 000 inhabitants) (Table 1).
In the FYP, the number of SARI cases increased significantly, as shown by an excess of 425 054 cases and an IRAP of 200.06 cases per 100 000 inhabitants. As expected, the highest incidence was that of SARI cases caused by COVID-19 (135.78 per 100 000 inhabitants), followed by cases of unspecified SARI (61.63 per 100 000 inhabitants). During the FYP, the incidence rate was 248.69 cases per 100 000 inhabitants among men and 178.05 per 100 000 for women. Findings of interest regarding the impact of the FYP include the higher IRAP among males (235.40 per 100 000 inhabitants) and a considerable reduction in SARI cases due to influenza (−2743) and other respiratory viruses (−1469) relative to the pre-pandemic period (Table 1).
During the FYP, the SARI incidence rate was highest among the elderly (720.29 cases per 100 000 inhabitants), followed by individuals 40–59 y of age (268.29 per 100 000 inhabitants). These data represent an increase of 206 874 cases and an IRAP of 703.83 per 100 000 inhabitants for the elderly, whom we define as those ≥60 y of age.
The average mortality rate for SARI cases prior to the pandemic was 1.36 per 100 000 inhabitants, increasing to 53.04 per 100 000 during the FYP, representing an excess of 109 682 deaths and an MRAP of 51.68 per 100 000 inhabitants. During the pre-pandemic, the highest mean mortality rate was for unspecified SARI (0.88 per 100 000 inhabitants), followed by SARI from influenza (0.34 per 100 000 inhabitants). In 2020, deaths from SARI by COVID-19 were the largest group, with a mortality rate of 41.96 per 100 000 inhabitants. The pandemic also saw a reduction in deaths from SARI due to influenza (−515) and other respiratory viruses (−58) relative to the pre-pandemic period (Table 1).
The mortality rate among males grew from 1.47 to 64.65 deaths per 100 000 inhabitants, reflecting an MRAP of 63.19 deaths per 100 000 inhabitants. Males had a higher mortality rate than females during both periods (Table 1).
In contrast to the trends observed for incidence rates, the average mortality rate in the pre-pandemic period was higher in the elderly, at 4.08 deaths per 100 000 inhabitants, followed by those ≤4 y of age, who accounted for 2.53 deaths per 100 000 inhabitants. In 2020, it was observed that mortality was highest among those ≥40 y of age: 45.64 per 100 000 among those 40–59 y of age and 277.20 per 100 000 for those ≥60 y of age. The MRAP was highest for those ≥60 y of age (273.12 per 100 000 inhabitants), followed by the population 40–59 y of age (43.95 per 100 000). It is also noteworthy that the MRAP among children ≤4 y of age was 2.04 per 100 000 inhabitants (Table 1).
Table 2 shows the proportions and excess cases and deaths from SARI according to sociodemographic characteristics and risk factors/comorbidities over the study period. The differences in proportions between the pre-pandemic and FYP were significant for all groups analysed, with particularly large differences observed due to race/skin colour, risk factor/comorbidity and gestational age.
Table 2.
Increases in cases and deaths from SARI by race/skin colour, level of education, comorbidities and the gestational and post-partum periods, Brazil 2013–2020
| Cases | Deaths | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pandemic | FYPa | Pre-pandemic | FYPa | |||||||
| SARI | Cases (mean) | % | Cases | % | Excess cases | Deaths (mean) | % | Deaths | % | Excess deaths |
| General | 26 499 | 100.00 | 451 553 | 100.00 | 425 054 | 2795 | 100.00 | 112 477 | 100.00 | 109 682 |
| Declared race/colourb | ||||||||||
| White | 13 623 | 51.41 | 166 358 | 36.84 | 152 735 | 1486 | 53.14 | 39 937 | 35.51 | 38 451 |
| Black | 1043 | 3.94 | 21 536 | 4.77 | 20 493 | 130 | 4.67 | 5801 | 5.16 | 5671 |
| Yellow | 163 | 0.62 | 4855 | 1.08 | 4692 | 22 | 0.80 | 1324 | 1.18 | 1302 |
| Mixed race (pardo) | 7268 | 27.43 | 164 469 | 36.42 | 157 201 | 787 | 28.15 | 44 030 | 39.15 | 43 243 |
| Indigenous | 186 | 0.70 | 1595 | 0.35 | 1409 | 16 | 0.57 | 504 | 0.45 | 488 |
| Ignored/blank | 4216 | 15.91 | 92 740 | 20.54 | 88 524 | 354 | 12.67 | 20 881 | 18.56 | 20 527 |
| Educationb | ||||||||||
| Uneducated/illiterate | 561 | 2.12 | 14 474 | 3.21 | 13 913 | 97 | 3.48 | 5270 | 4.69 | 5173 |
| Elementary or fundamental 1 | 3237 | 12.21 | 46 124 | 10.21 | 42 887 | 577 | 20.64 | 14 918 | 13.26 | 14 341 |
| Elementary 2 | 280 | 1.06 | 30 497 | 6.75 | 30 217 | 53 | 1.90 | 8584 | 7.63 | 8531 |
| High school | 2440 | 9.21 | 50 950 | 11.28 | 48 510 | 336 | 12.02 | 10 417 | 9.26 | 10 081 |
| Higher education | 1084 | 4.09 | 23 664 | 5.24 | 22 580 | 116 | 4.15 | 3952 | 3.51 | 3836 |
| Not applicable | 12 748 | 48.11 | 14 112 | 3.13 | 1364 | 380 | 13.60 | 504 | 0.45 | 124 |
| Ignored/blank | 6149 | 23.21 | 271 732 | 60.18 | 265 583 | 1236 | 44.20 | 68 832 | 61.20 | 67 596 |
| Presenting at least one comorbidityb | ||||||||||
| Hearth disease | 2587 | 32.75 | 141 107 | 59.60 | 138 520 | 591 | 41.24 | 47 071 | 63.70 | 46 480 |
| Chronic hematologic disease | 55 | 0.69 | 3810 | 1.61 | 3755 | 11 | 0.76 | 1245 | 1.68 | 1234 |
| Down syndrome | 332 | 4.20 | 1582 | 0.67 | 1250 | 46 | 3.19 | 447 | 0.60 | 401 |
| Chronic liver disease | 194 | 2.45 | 3902 | 1.65 | 3708 | 55 | 3.85 | 1514 | 2.05 | 1459 |
| Asthma | 322 | 4.08 | 17 921 | 7.57 | 17 599 | 23 | 1.61 | 2824 | 3.82 | 2801 |
| Diabetics | 1626 | 20.58 | 101 571 | 42.90 | 99 945 | 406 | 28.32 | 35 292 | 47.76 | 34 886 |
| Chronic neurological disease | 1224 | 15.50 | 19 567 | 8.26 | 18 343 | 209 | 14.60 | 7733 | 10.46 | 7524 |
| Lung disease | 3170 | 40.12 | 20 953 | 8.85 | 17 783 | 459 | 32.05 | 7400 | 10.01 | 6941 |
| Immunosuppression | 1128 | 14.28 | 13 787 | 5.82 | 12 659 | 277 | 19.36 | 4603 | 6.23 | 4326 |
| Chronic kidney disease | 576 | 7.29 | 15 913 | 6.72 | 15 337 | 139 | 9.69 | 6928 | 9.37 | 6789 |
| Obesity | 735 | 9.30 | 24 845 | 10.49 | 24 110 | 194 | 13.52 | 7199 | 9.74 | 7005 |
| Pregnancyb | ||||||||||
| 1st gestational trimester | 114 | 17.26 | 404 | 11.98 | 290 | 6 | 17.87 | 9 | 5.20 | 3 |
| 2nd gestational trimester | 263 | 39.74 | 1079 | 31.99 | 816 | 13 | 39.15 | 59 | 34.10 | 46 |
| 3rd gestational trimester | 270 | 40.80 | 1656 | 49.10 | 1386 | 13 | 39.15 | 96 | 55.49 | 83 |
| Unknown gestational age | 15 | 2.20 | 234 | 6.94 | 219 | 1 | 3.83 | 9 | 5.20 | 8 |
| Post-partumb | ||||||||||
| Yes | 123 | 0.47 | 1110 | 0.25 | 987 | 14 | 0.51 | 164 | 0.15 | 150 |
| No | 22 021 | 83.10 | 177 612 | 39.33 | 155 591 | 2423 | 86.67 | 52 739 | 46.89 | 50 316 |
| Ignored/blank | 4355 | 16.43 | 272 831 | 60.42 | 268 476 | 358 | 12.82 | 59 574 | 52.97 | 59 216 |
aFirst year of the COVID-19 pandemic.
The differences expressed for case and death increments were statistically significant (p<0.001).
Comparing the studied periods, we observed that people who self-identified as white had a decrease in the proportion of cases and deaths compared with those self-identifying as mixed race (pardo). The latter group had a total of 157 201 excess cases and 43 243 excess deaths (Table 2).
Among individuals with one or more risk factors and/or comorbidities, there was a particularly steep increase in SARI cases and deaths in 2020 among cardiac and diabetic patients, while these figures were lower among patients with pneumopathies. The increase in cases and deaths were 138 520 cases and 46 480 deaths among cardiac patients, followed 99 945 cases and 34 886 deaths among diabetics (Table 2).
There was an increase in the proportion of cases and deaths from SARI among pregnant women in the third trimester of pregnancy, with 1386 excess cases and 83 excess deaths (Table 2). In contrast, we observed a reduction in cases for women in the second trimester.
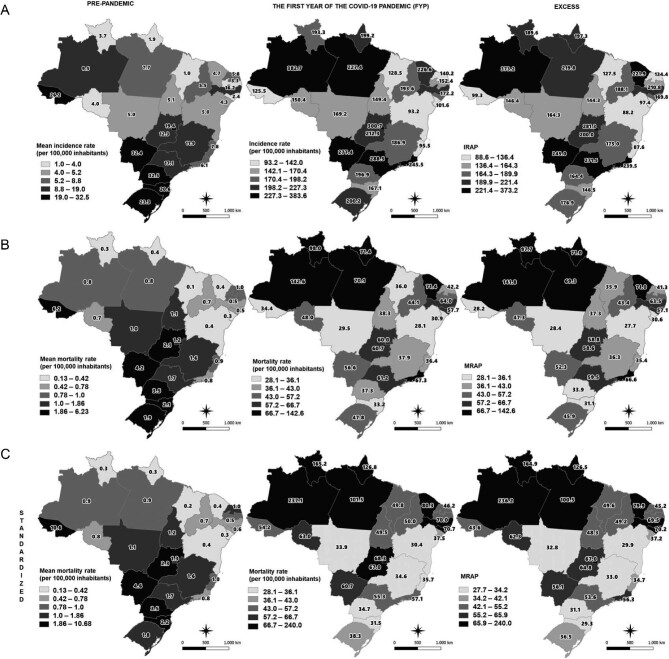
The Southern Region of Brazil had the highest mean SARI incidence rate prior to the pandemic (26.2 per 100 000 inhabitants), especially in the states of Paraná (32.5 per 100 000 inhabitants) and Rio Grande do Sul (23.3 per 100 000 inhabitants), followed by the Center-West Region (15.6 per 100 000 inhabitants). In the Center-West Region, Mato Grosso do Sul state had the highest average incidence rate (32.4 per 100 000 inhabitants), followed by the Federal District (19.4 per 100 000 inhabitants) and Goiás (12.3 per 100 000 inhabitants). However, during the FYP, the highest SARI incidence rate was observed in the Southeastern Region (246.7 per 100 000 inhabitants), especially São Paulo (288.5 per 100 000 inhabitants) and Rio de Janeiro (245.5 per 100 000 inhabitants), followed by the Northern Region (241.4 per 100 000 inhabitants), with particular increases in the states of Amazonas (328.7 per 100 000 inhabitants) and Pará (277.4 per 100 000 inhabitants). The IRAP in the Northern Region was particularly high (233.4 per 100 000 inhabitants), especially in Amazonas (373.2 per 100 000 inhabitants) and Pará (219.8 per 100 000 inhabitants), with considerable increases observed in the southeastern states of São Paulo (271.5 per 100 000 inhabitants) and Rio de Janeiro (239.5 per 100 000 inhabitants) (Figure 3).
Figure 3.
Distribution of (A) SARI incidence rates and IRAP, (B) mortality rates and MRAP and (C) standardized mortality rates and MRAP. Figures calculated by state of residence for pre-pandemic and FYP in Brazil.
In the pre-pandemic period, a higher average mortality rate from SARI was observed in the southern region (2.5 per 100 000 inhabitants), with the highest mortality rate in the state of Paraná (3.5 per 100 000 inhabitants), followed by the Central-West Region (2.1 per 100 000 inhabitants), while Mato Grosso do Sul (4.2 per 100 000 inhabitants) and Goiás (2.1 per 100 000 inhabitants) had the highest average mortality rates. Meanwhile, the highest MRAP figures were observed in the Northern Region, with 79.9 per 100 000 inhabitants, with the highest rates found once again in Amazonas (141.8 per 100 000 inhabitants). The MRAP figure was 54.1 per 100 000 inhabitants in southeastern Brazil, with high values in Rio de Janeiro (66.6 per 100 000 inhabitants) and São Paulo (59.5 per 100 000 inhabitants) (Figure 3). When the mortality rate is standardized by age group and state of residence, the state of Acre had the highest average pre-pandemic rate (10.6 per 100 000 inhabitants), followed by Mato Grosso do Sul and Paraná, with 4.6 and 3.5 per 100 000 inhabitants, respectively. In 2020, the highest standardized mortality rate was observed in the northern states of Amazonas (237.1 per 100 000 inhabitants), Roraima (165.2 per 100 000 inhabitants), Pará (101.5 per 100 000 inhabitants) and Amapá (126.8 per 100 000 inhabitants), with standardized MRAP rates of 236.2 per 100 000 inhabitants in Amazonas, followed by 164.9 per 100 000 inhabitants for Roraima (Figure 3).
Table 3 shows a breakdown of SARI cases and deaths by treatment characteristics during hospitalization, medical imaging, type of biological sample and diagnostic criteria. The proportions of cases and deaths for each variable changed significantly during the FYP, highlighting the importance of some of the categories discussed below.
Table 3.
Increase in cases and deaths from SARI by ICU treatment, mechanical ventilation use and diagnostic criteria, Brazil 2013–2020
| Cases | Deaths | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pandemic | FYPa | Pre-pandemic | FYPa | |||||||
| SARI | Cases (mean) | % | Cases | % | Excess cases | Deaths (mean) | % | Deaths | % | Excess deaths |
| General | 26 499 | 100.00 | 451 553 | 100.00 | 425 054 | 2795 | 100.00 | 112 477 | 100.00 | 109 682 |
| ICUb | ||||||||||
| Yes | 8483 | 32.01 | 129 715 | 28.73 | 121 232 | 1908 | 68.26 | 57 864 | 51.45 | 55 956 |
| No | 17 153 | 64.73 | 267 072 | 59.15 | 249 919 | 790 | 28.27 | 38 095 | 33.87 | 37 305 |
| Unknown/blank | 864 | 3.26 | 54 766 | 12.13 | 53 902 | 97 | 3.48 | 16 518 | 14.69 | 16 421 |
| Mechanical ventilationb | ||||||||||
| Invasive | 7796 | 29.42 | 67 421 | 14.93 | 59 625 | 1698 | 60.74 | 44 841 | 39.87 | 43 143 |
| Non-invasive | 6859 | 25.88 | 214 901 | 47.59 | 208 042 | 677 | 24.20 | 39 174 | 34.83 | 38 497 |
| No | 10 766 | 40.63 | 102 812 | 22.77 | 92 046 | 324 | 11.59 | 11 111 | 9.88 | 10 787 |
| Unknown/blank | 1077 | 4.07 | 66 419 | 14.71 | 65 342 | 97 | 3.46 | 17 351 | 15.43 | 17 254 |
| Diagnostic criteriab | ||||||||||
| Laboratory | 22 092 | 83.37 | 384 014 | 85.04 | 361 922 | 2371 | 84.83 | 101 918 | 90.61 | 99 547 |
| Epidemiological | 593 | 2.24 | 3788 | 0.84 | 3195 | 71 | 2.52 | 1211 | 1.08 | 1140 |
| Clinical | 3104 | 11.71 | 15 727 | 3.48 | 12 623 | 308 | 11.02 | 4406 | 3.92 | 4098 |
| Medical imaging | 0 | 0.00 | 9321 | 2.06 | 9321 | 0 | 0.00 | 2548 | 2.27 | 2548 |
| Unknown/blank | 710 | 2.68 | 38 703 | 8.57 | 37 993 | 45 | 1.62 | 2394 | 2.13 | 2349 |
aFirst year of the COVID-19 pandemic.
The differences expressed for case and death increments were statistically significant (p<0.001).
A considerable proportion of patients with SARI required hospitalization in an intensive care unit (ICU) both before and in 2020: 32.01% in the pre-pandemic period and 28.73% in 2020. There was also a large increase in SARI cases requiring ICU treatment attributed to the FYP (121 232 cases), with 55 956 excess deaths among those admitted to an ICU (Table 3).
We observed an increase in the proportions of SARI cases and deaths during the FYP among patients requiring non-invasive mechanical ventilation techniques, with a corresponding decrease among those requiring invasive ventilation relative to the pre-pandemic period. In absolute numbers, an additional 267 667 patients required some form of mechanical ventilation during the FYP.
Table 3 shows an increase in the proportion of SARI cases and deaths among patients with laboratory diagnostics and a reduction in all other groups during the FYP, except for the medical imaging group. The increase in cases and deaths for patients with laboratory diagnostics amounted to 361 922 cases and 99 547 deaths (Table 3).
Discussion
The pandemic caused by SARS-CoV-2 triggered catastrophic social and economic impacts all over the world, constituting one of the biggest health challenges ever faced.8 In Brazil, the exponential growth of COVID-19 cases in 2020 increased the number of SARI cases, substantially surpassing the average number of cases observed in the last 10 y, as evidenced by the increased number of hospitalizations in 2020.15
Our study showed a large increase in cases and deaths due to SARI, with most of them associated with COVID-19, followed by cases and deaths with no identification of the aetiological agent. Kupek16 found similar results in Brazil when adjusting underreported data (21.62%) for 2020. He estimated that approximately three of four SARI deaths were caused by COVID-19, corresponding to a mortality rate of 115 deaths per 100 000 inhabitants. Another study analysing 500 patients hospitalized due to SARI in India showed a higher mortality rate among cases associated with COVID-19 (34.1%) compared with those not associated with COVID-19 (20.6%).17
According to Bastos et al.,15 before the introduction of SARS-CoV-2 in Brazil, the highest frequency of hospitalizations for SARI was associated with the influenza A virus until 2018, with hospitalizations peaking in 2016 (34.5% of all SARI hospitalizations in 2016). Between 2018 and 2019 there was an increase in SARI associated with RSV. In 2019, RSV was detected in 23.3% of cases and influenza A in only 4.9%. Influenza B, parainfluenza and adenovirus did not exceed frequencies of 3%, 2% and 1%, respectively, during the 10 y preceding the pandemic.
Considering the cases in which an aetiological agent was identified, we found a predominance of cases and deaths of SARI due to influenza followed by those caused by other respiratory viruses in the pre-pandemic period. This situation was modified in 2020, with a decrease in cases and deaths of SARI due to the referred causes. A study on excess mortality associated with COVID-19 in Brazil also detected reduced mortality due to influenza.18
There are high incidence and mortality rates due to unspecified SARI in the study period, highlighting their growth during the FYP. The lack of aetiological characterization can result from problems with the samples, such as low quality, inadequate handling, improper storage, inadequate transport and delays in updating processes, impairing the diagnosis.7 The increase in the rate of unspecified SARI in 2020 is probably due to the increased demand for diagnosis during the COVID-19 pandemic.
The Health Surveillance Office19 highlights the high proportion of cases classed as unspecified SARI in Brazil. In 2019, 59.7% of the year's 40 294 SARI cases were classified as unspecified. This figure represented 66% of cases in 2017 and 56% in 2018.20,21
In the pre-pandemic period, children up to 4 y of age were the most susceptible to SARI, while elderly patients (those ≥60 y of age) had the highest risk of progression to death. In 2020, the elderly had the highest mortality and incidence rates. This echoes the findings of Bastos et al.,15 who noted that SARI was more frequent in children up to 2 y of age before the COVID-19 pandemic and in the elderly during the FYP, with the elderly having the highest risk of dying from SARI in both periods.
The increased risk of dying from SARI among the elderly population in Brazil may be due to the deleterious consequences of the pandemic’s impacts on society, the economy and health services, in addition to the characteristics of the novel aetiological agent itself. Several studies indicate advanced age as an important prognostic factor associated with death from COVID-19. According to Barbosa et al.,22 the highest proportion of deaths due to COVID-19 have been observed among the elderly, who also have higher mortality rates than those of the general population. Studies report high case fatality rates for COVID-19 among elderly patients or those with comorbidities,23–25 with more than one-fifth of deaths occurring among those >80 y of age in China (21.9%) and Italy (20.2%).25 Barbosa et al.22 also noted the influence of sociodemographic factors related to race/colour and income on mortality rates among the elderly.
The increased risk due to COVID-19 among the elderly amounts to a risk of mortality 2.87 times greater among those ages 60–79 y relative to those ≤59 y, while the risk is 7.06 times among those ≥80 y. Several clinical studies have found an association between older age and severe COVID-19 symptoms.26–28 Liu et al.28 compared patients with SARI due to COVID-19 with patients without SARI and reported that elderly patients developed the most severe symptoms, with most of these patients also exhibiting one or more comorbidities. They also reported high mortality rates among patients who had previously undergone moderate or severe SARI.
It is also noteworthy that although the increased risk of contracting or dying from SARI was significant among the elderly, we detected an increased risk during the pandemic in all age groups. This result is consistent with evidence from Quast and Andel,29 who also identified high mortality in patients 20–49 y of age in the pandemic period, while Vestergaard et al.30 observed a significant increase in mortality from SARI among those 15–64 y of age during the pandemic. These findings indicate that the COVID-19 pandemic has changed the age profile of SARI cases in 2020, a fact also evidenced by Pannu et al.31 in India. The authors found that COVID-19 patients were, on average, 6 y older than non-COVID-19 patients.
In addition to age, comorbidities such as hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease and chronic kidney disease are also considered factors strongly associated with the worsening of the clinical picture of patients with COVID-19,32 which is the predominant aetiological cause in the pandemic. In our study, we found that heart disease and diabetes were the comorbidities with the greatest increases in cases and deaths from SARI between the pre-pandemic period and FYP.
A recent meta-analysis combining data from studies on the association between diabetes and the severity and mortality of COVID-19 indicates that diabetic COVID-19 patients have a significant probability of developing severe symptoms (pooled odds ratio [OR] 2.75 [95% CI 2.09 to 3.62]; I2=63%) or death (pooled OR 1.90 [95% CI 1.37 to 2.64]; I2=32%).33
Uncontrolled blood pressure is associated with COVID-19 infection and a high case fatality rate. A study carried out in China found a case fatality rate of 6% among hypertensive patients with COVID-19.34 According to the meta-analysis carried out by Gold et al.,35 there is evidence of a disproportionate prevalence of comorbidities among patients who die from COVID-19. Hypertension was significantly more prevalent in patients with severe symptoms (47.65% [95% CI 35.04 to 60.26]) as well as among fatalities (47.90% [95% CI 40.33 to 55.48]). Diabetes and respiratory diseases also had a high frequency among severe cases and deaths.
Our study corroborates the findings of Onder et al.,36 who point out that men are more susceptible to severe COVID-19 cases and death from the disease. In Brazil, men were more frequently hospitalized than women for COVID-19,37 a trend also observed in studies from China and the USA.38,39 A greater excess of deaths from natural causes among men was also identified in Brazil in 2020.40 Hunt et al.41 and Krieger et al.42 associate these results with the lower demand from men for health services and their low adherence to healthy habits. For example, compared with women, men smoke more and do not regularly wash their hands. In contrast, no differences were found in mortality between males and females in a study conducted in the USA. According to Kim,43 the risk of death from COVID-19 in South Korea may be higher in females, a phenomenon linked to issues of gender inequality.
We did not find conclusive published evidence to justify, from a biological perspective, a higher risk of hospitalization and death for SARI related to race or skin colour.44 However, we did observe a greater excess of cases and deaths from SARI during the FYP among those self-identifying as mixed race (pardo). These outcomes may be due to the vulnerability brought about by historical racial inequality in Brazil. However, as hypothesized by Chaudhary et al.,45 the differences in mortality and thromboembolic events in COVID-19 may be partially explained by race-related disparities in intrinsic thrombogenicity.
In this regard, Teixeira et al.40 analysed the excess mortality due to natural causes according to race/skin colour during the COVID-19 pandemic in Brazil. They found that the black population (considering the sum of black and mixed race people) had higher rates of deaths above the expected (27.8%; n=153 284) than the white population (17.6%; n=117 037).
Certain factors have been recognized as increasing susceptibility to infection and death, including socio-economic, ethnic and racial determinants, a country's level of economic development and demographics. These have exacerbated health inequities in the pandemic context, regardless of the country and region, resulting in disproportionate impacts on the most economically vulnerable populations.46–48
The present study identified an excess of SARI cases and deaths among women in advanced stages of pregnancy, corroborating results presented by Mosby et al.,49 who report that pregnancy was significantly associated with the severity of infection during the influenza A(H1N1)pdm09 pandemic. According to findings by Novoa et al.,50 a considerable proportion of women required ICU hospitalization due to complications related to SARS-CoV-2 infection and reported high levels of adverse obstetric outcomes related to iatrogenic preterm birth, labour induction or caesarean delivery due to worsening of the patients’ clinical or obstetric condition.
All regions of the country showed a significant increase in SARI-related incidence and mortality rates in 2020, with the highest IRAP and MRAP found in the Southeast, North and Center-West Regions. The large increase in cases and regional differences need to be studied in greater depth. However, future studies may identify several causes, including health system overload, deepening social inequalities, demographics and the adoption of different strategies against the COVID-19 pandemic.
The health system collapse in Manaus, the capital of the northern state of Amazonas, is a typical example of the regional impact of the pandemic on the health system. Manaus had high COVID-19 incidence and mortality rates in May 2020, causing an overload of the health system and a collapse of the funeral system.51 A second wave of cases at the end of 2020 deepened the crisis even further, this time with the collapse of the municipal health system, marked by a lack of nursing and ICU beds and a limited oxygen supply.51
It is also emphasized that the increase in the mortality rate is not restricted to underdeveloped countries, as it has also been observed in places with a high economic and social development, such as New York, USA, and the provinces of Bergamo and Brescia in northern Italy.36,52,53 However, this increase was probably influenced by different socio-economic and behavioural factors of each different locality.
This study shows that people in the Southeast Region have the most significant increase in the risk of contracting and dying from SARI. The results also indicate that the Southern Region had the highest SARI incidence and mortality rates in the pre-pandemic, however, in spite of a substantial increase in cases and deaths during the pandemic, it had the fourth-highest number of cases and the lowest mortality rates of any region. Niquini et al.54 reported that the Southern Region had the highest percentage of influenza-related SARI records between 2019 and 2020. The predominance of these records may be related to the region's subtropical climate, which leads to a higher incidence and circulation of respiratory viruses such as influenza,55 while the region also has a greater proportion of elderly inhabitants.14
The standardized MRAP values are highest in the Amazonian states of Amazonas, Roraima, Pará and Amapá and the northeastern states of Ceará, Alagoas and Pernambuco. This reinforces the hypothesis that the most socio-economically vulnerable regions were most impacted by the pandemic. According to a report by the Pan-American Health Organization, the states of the country's North and Northeast Regions are among those with the lowest human development levels in Brazil, in addition to experiencing greater difficulties in accessing healthcare, such as having a number of ventilators per inhabitant below the national average.56
Regarding the case fatality rate due to SARI in Brazil, there was a substantial increase in 2020 (21.90%), surpassing the highest case fatality rate recorded in the pre-pandemic period in 2016 (12.00%). Although case fatality rates should be interpreted carefully, as they reflect hospitalized cases and deaths captured by the SARI surveillance system, which is continuously improving its sensitivity (unpublished information), the increased case fatality rate may be due to factors associated with the pandemic.
When comparing the periods, the increase in the proportions of cases and deaths during the pandemic was demonstrated by occurrences diagnosed using laboratory methods, demonstrating the significance of laboratory surveillance in identifying the aetiological agent, especially SARS-CoV-2. This fact is also shown in a study by Ribeiro et al.57 that reports the SARI surveillance system's capacity to fulfil the function of monitoring respiratory viruses circulating in the country.
Our study has limitations regarding the change in the case definition over the analysed period, which highlights the need to adjust the data bank's analysis, which adopts a single case definition to maintain comparability throughout the study period. Therefore we adopted the definition of SARI used in Brazil in a non-pandemic context and kept only the records that met that definition (Figure 1), leading to a high number of exclusions. In addition, the possibility of notification delays is a particular concern during the pandemic, mainly due to the high healthcare demand, despite improvement of the Brazilian SARI surveillance system's sensitivity over time. Even considering these limitations, the magnitude of our results strongly indicates that the pandemic period showed a significant increase in illness risk, cases and deaths related to SARI in Brazil.
Conclusions
We concluded that the substantial increase in SARI cases and deaths observed between 2013 to 2019 and 2020 in Brazil is attributable to the FYP, as evidenced by the increase in cases and deaths and the high risk during the pandemic period of falling ill and dying from SARI. The most vulnerable groups, such as the elderly, patients with comorbidities, pregnant women and people who declare themselves as mixed race, in addition to those living in the country's Southeast, North and Center-West Regions, suffered the greatest impact. We suggest that the factors potentially associated with that impact must be addressed by a research program that considers potential factors such as overload of the health system, social inequities and regional differences regarding the adoption of coping strategies of the COVID-19 pandemic in addition to characteristics of the aetiological agent itself. Therefore we highlight the need for public policies that strengthen the Unified Health System, its assistance network and health surveillance, particularly the surveillance of respiratory viruses.
Contributor Information
Felipe Cotrim de Carvalho, Center of Tropical Medicine, Faculty of Medicine, University of Brasilia, Brasilia, Brazil; Surveillance Secretariat in Health, Ministry of Health, Brasília, Federal District, Brazil.
Erica Tatiane da Silva, Evidence Program for Health Policies and Technologies, Oswaldo Cruz Foundation, Brasilia, Federal District, Brazil.
Walquiria Aparecida Ferreira de Almeida, Surveillance Secretariat in Health, Ministry of Health, Brasília, Federal District, Brazil.
Matheus Almeida Maroneze, Surveillance Secretariat in Health, Ministry of Health, Brasília, Federal District, Brazil.
Jaqueline de Araujo Schwartz, Surveillance Secretariat in Health, Ministry of Health, Brasília, Federal District, Brazil.
João Pedro Vieira Jardim, Center of Tropical Medicine, Faculty of Medicine, University of Brasilia, Brasilia, Brazil.
Henry Maia Peixoto, Center of Tropical Medicine, Faculty of Medicine, University of Brasilia, Brasilia, Brazil; National Institute of Science and Technology for Health Technology Assessment, Porto Alegre, Brazil.
Authors’ contributions
FCC, ETS and HMP were responsible for designing and implementing the study and wrote the manuscript. FCC and HMP were responsible for the analysis and interpretation of data. WAFA, MAM, JAS and JPVJ helped in writing and data review. All the authors reviewed the manuscript and read and approved the final version.
Acknowledgments
The authors are grateful for the support of the National Council for Scientific and Technological Development for funding the research.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Chamada CNPq/Gerência Regional de Brasília–FIOCRUZ, no. 41/2018).
Competing interests
None declared.
Ethical approval
The Ethics Committee of the Faculty of Medicine of the University of Brasília approved this study under opinion 4.112.196.
Data availability
All data generated or analysed during this study can be shared on reasonable request to the corresponding author.
References
- 1. Williams BG, Gouws E, Boschi-Pinto Cet al. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2(1):25–32. [DOI] [PubMed] [Google Scholar]
- 2. Troeger C, Blacker B, Khalil IAet al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naghavi M, Abajobir AA, Abbafati Cet al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Weekly epidemiological update on COVID-19 - 19 October 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 5. Brasil Ministério da Saúde . Guia de vigilância epidemiológica - Emergência de súde pública de importância nacional pela doença pelo coronavírus 2019. Brasília: Ministério da Saúde; 2020. [Google Scholar]
- 6. Secretaria de Vigilância em Saúde, Ministério da Saúde . Vigilância sentinela de síndrome respiratória aguda grave (SRAG) em unidade de terapia intensiva. Brasília: Ministério da Saúde; 2015. [Google Scholar]
- 7. Brasil Ministério da Saúde . Guia para a rede laboratorial de vigilância de influenza no brasil guia para a rede laboratorial de vigilância. Brasília: Ministério da Saúde; 2016. [Google Scholar]
- 8. Werneck GL, Carvalho MS.. A pandemia de COVID-19 no Brasil: crônica de uma crise sanitária anunciada. Cad Saude Publica. 2020;36(5):e00068820. [DOI] [PubMed] [Google Scholar]
- 9. Ribeiro-Silva R de C, Pereira M, Campello Tet al. Covid-19 pandemic implications for food and nutrition security in Brazil. Cien Saude Colet. 2020;25(9):3421–30. [DOI] [PubMed] [Google Scholar]
- 10. Costa S da S. Pandemia e desemprego no Brasil. Rev Adm Pública. 2020;54(4):969–78. [Google Scholar]
- 11. Graham BS, Sullivan NJ.. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat Immunol. 2018;19(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brasil Ministério da Saúde . Guia de vigilância em saúde. Brasília: Ministério da Saúde; 2019. [Google Scholar]
- 13. Brasil Ministério da Saúde . Plano nacional de operacionalização da vacinação contra a COVID-19, 12aedição. Brasília: Ministério da Saúde; 2022. [Google Scholar]
- 14. Instituto Brasileiro de Geografia e Estatística . Projeção da população do Brasil e Unidades da Federação por sexo e idade para o período 2000–2030. Brasília: Instituto Brasileiro de Geografia e Estatística; 2021. [Google Scholar]
- 15. Bastos LS, Niquini RP, Lana RMet al. COVID-19 e hospitalizações por SRAG no Brasil: uma comparação até a 12a semana epidemiológica de 2020. Cad Saude Publica. 2020;36(4):1–8. [DOI] [PubMed] [Google Scholar]
- 16. Kupek E. How many more ? Under-reporting of the COVID-19 deaths in Brazil in 2020. Trop Med Int Heal. 2021;26(9):1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma A, Kothari N, Goel Aet al. Clinical features and mortality in COVID-19 SARI versus non COVID-19 SARI cases from Western Rajasthan, India. J Fam Med Prim Care. 2021;10(9):3240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nucci LB, Enes CC, Ferraz FRet al. Excess mortality associated with COVID-19 in Brazil : 2020–2021. J Public Health (Bangkok). 2021;doi:10.1093/pubmed/fdab398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Secretaria de Vigilância em Saúde . Influenza: monitoramento até a semana epidemiológica 52 de 2019. Bol Epidemiol. 2019;47:1–10. [Google Scholar]
- 20. Brasil Ministério da Saúde . Informe epidemiológico Secretaria de Vigilância em Saúde influenza: monitoramente até a semana epidemiológica 52 de 2017. Brasília: Ministério da Saúde; 2017. [Google Scholar]
- 21. Brasil Ministério da Saúde . Informe epidemiológico secretaria de vigilância em saúde influenza: monitoramente até a semana epidemiológica 52 de 2018. Brasília: Ministério da Saúde; 2018. [Google Scholar]
- 22. Barbosa IR, Galvão MHR, de Souza TAet al. Incidence of and mortality from COVID-19 in the older Brazilian population and its relationship with contextual indicators: an ecological study. Rev Bras Geriatr Gerontol. 2020;23(1):e200171. [Google Scholar]
- 23. Barra RP, De Moraes EN, Jardim AAet al. A importância da gestão correta da condição crônica na Atenção Primária à Saúde para o enfrentamento da COVID-19 em Uberlândia, Minas Gerais. APS Rev. 2020;2(1):38–43. [Google Scholar]
- 24. Lloyd-Sherlock P, Ebrahim S, Geffen Let al. Bearing the brunt of covid-19: older people in low and middle income countries. BMJ. 2020;368:m1052. [DOI] [PubMed] [Google Scholar]
- 25. Lai C-C, Wang J-H, Ko W-Cet al. COVID-19 in long-term care facilities: an upcoming threat that cannot be ignored. J Microbiol Immunol Infect. 2020;53(3):444–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lai C-C, Liu YH, Wang C-Yet al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li LQ, Huang T, Wang YQet al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Sun W, Li Jet al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv. 2020;doi: 10.1101/2020.02.17.20024166. [Google Scholar]
- 29. Quast T, Andel R.. Excess mortality and potential undercounting of COVID-19 deaths by demographic group in Ohio. medRxiv. 2020; doi:https://doi.org/10.1101/2020.06.28.20141655. [Google Scholar]
- 30. Vestergaard LS, Nielsen J, Richter Let al. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pannu AK, Kumar M, Singh Pet al. Severe acute respiratory infection surveillance during the initial phase of the COVID-19 outbreak in north India: a comparison of COVID-19 to other SARI causes. Indian J Crit Care Med. 2021:25(7):761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang X, Li S, Yu Het al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY). 2020;12(13):12493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar A, Arora A, Sharma Pet al. Is diabetes mellitus associated with mortality and severity of COVID- 19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma L-Y, Chen W-W, Gao R-Let al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gold MS, Sehayek D, Gabrielli Set al. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020;132(8):749–55. [DOI] [PubMed] [Google Scholar]
- 36. Onder G, Rezza G, Brusaferro S.. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–6. [DOI] [PubMed] [Google Scholar]
- 37. Brasil Ministério da Saúde . Boletim epidemiológico especial - Doença pelo novo coronavírus - COVID-19 semanda epidemiológica 38 de 2021. Brasília: Ministério da Saúde; 2021. [Google Scholar]
- 38. Zhou F, Yu T, Du Ret al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China : a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richardson S, Hirsch JS, Narasimhan Met al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teixeira RA, Maria A, Vasconcelos Net al. Excess mortality due to natural causes among whites and blacks during the COVID-19 pandemic in Brazil. Rev Soc Bras Med Trop. 2022;55(Suppl 1):e0283–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hunt K, Adamson J, Hewitt Cet al. Do women consult more than men? A review of gender and consultation for back pain and headache. J Health Serv Res Policy. 2011;16(2):108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krieger N, Chen JT, Waterman PD.. Excess mortality in men and women in Massachusetts during the COVID-19 pandemic. Lancet. 2020;395(10240):1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim D. Women's health during the COVID-19 pandemic. Korean J Women Health Nurs. 2020;26(2):106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mertz D, Kim TH, Johnstone Jet al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaudhary R, Bliden KP, Kreutz RPet al. Race-related disparities in COVID-19 thrombotic outcomes: beyond social and economic explanations. EClinicalMedicine. 2020;29–30:100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Devakumar D, Bhopal SS, Shannon G.. COVID-19: the great unequaliser. J R Soc Med. 2020;113(6):234–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nepomuceno MR, Acosta E, Alburez-Gutierrez Det al. Besides population age structure, health and other demographic factors can contribute to understanding the COVID-19 burden. Proc Natl Acad Sci USA. 2020;117(25):13881–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marmot M, Allen J.. COVID-19: exposing and amplifying inequalities. J Epidemiol Community Health. 2020;74(9):681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosby LG, Rasmussen SA, Jamieson, DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–8. [DOI] [PubMed] [Google Scholar]
- 50. Novoa RH, Quintana W, Llancarí Pet al. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med Infect Dis. 2021;39:101919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barreto ICdHC, Filho RVC, Ramos RFet al. Colapso na saúde em Manaus: o fardo de não aderir às medidas não farmacológicas de redução da transmissão da COVID-19. Saude Debate. 2021;45(131):1126–39. [Google Scholar]
- 52. Weinberger DM, Cohen T, Crawford FWet al. Estimating the early death toll of COVID-19 in the United States. medRxiv. 2020;doi: 10.1101/2020.04.15.20066431. [Google Scholar]
- 53. Shi S, Qin M, Shen Bet al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niquini RP, Lana RM, Pacheco AGet al. SRAG por COVID-19 no Brasil: descrição e comparação de características demográficas e comorbidades com SRAG por influenza e com a população geral. Cad Saúde Pública. 2020;36(7):e00149420. [DOI] [PubMed] [Google Scholar]
- 55. Almeida A, Codeço C, Luz PM.. Seasonal dynamics of influenza in Brazil : the latitude effect. BMC Infect Dis. 2018:18;695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Programa das Nações Unidas para o Desenvolvimento (PNUD), Fundo das Nações Unidas para a Infância (UNICEF), Organização das Nações Unidas para a Educação a Ciância e a Cultura (UNESCO), Organização Pan-americana da Saúde (OPAS) . COVID-19 e desenvolvimento sustentável [livro eletrônico] : avaliando a crise de olho na recuperação : sumário executivo, ed. Brasília, DF; 2021.
- 57. Ribeiro IG, Sanchez MN.. Avaliação do sistema de vigilância da síndrome respiratória aguda grave (SRAG) com ênfase em influenza, no Brasil, 2014 a 2016. Epidemiol Serv Saude Bras. 2020;29(3):e2020066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study can be shared on reasonable request to the corresponding author.