Abstract
Objectives
The effectiveness of the BNT162b2 mRNA COVID-19 vaccine for adolescents with juvenile-onset inflammatory or immune rheumatic diseases (IRDs) is unknown. Several studies have suggested attenuated immunogenicity in patients with IRD. This study evaluated the effectiveness of the BNT162b2 mRNA COVID-19 vaccine in preventing COVID-19 infection in adolescents with juvenile-onset IRD compared with controls without immune rheumatic disease.
Methods
We used data from Clalit Health Services, the largest health-care organization in Israel, to conduct an observational cohort study from February to December 2021, involving 12–18 year-old adolescents diagnosed with IRD. Study outcomes included documented COVID-19 infection in relation to vaccination status and immunomodulatory therapy. We estimated vaccine effectiveness as one minus the risk ratio. Adolescents aged 12–18 years without immune rheumatic disease served as controls.
Results
A total of 1639 adolescents with IRD (juvenile idiopathic arthritis, SLE, or familial Mediterranean fever) were included and compared with 524 471 adolescents in the same age range without IRD. There was no difference in COVID-19 infection rates after the second dose of vaccine between those with IRD and controls (2.1% vs 2.1% respectively, P = 0.99). The estimated vaccine effectiveness for adolescents with IRD was 76.3% after the first dose, 94.8% after the second and 99.2% after the third dose.
Conclusion
We found that the BNT162b2 mRNA vaccine was similarly effective against COVID-19 infection in adolescents with and without IRD. Immunomodulatory therapy did not affect its effectiveness. These results can encourage adolescents with IRD to get vaccinated against COVID-19.
Keywords: adolescent rheumatology, COVID-19 vaccine, effectiveness, immunomodulatory therapy
Rheumatology key messages.
Estimated mRNA COVID-19 vaccine effectiveness for adolescents with IRD was similar to controls without immune rheumatic disease.
Immunomodulatory therapy did not affect the effectiveness of the vaccine.
The results presented here can encourage adolescents with IRD to get vaccinated against COVID-19
Introduction
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has led to significant morbidity and mortality worldwide [1]. Children and adolescents typically have milder COVID-19 infections compared with adults [2]. Nevertheless, severe illness can occur in these age groups [3–4]. Liguoro et al. reported that among 1475 cases of children hospitalized with COVID-19, 615 (42%) had moderate or severe disease [5]. In a recent prospective study that included 20 hospitals in Israel, 17.8% of children hospitalized due to COVID-19 or multisystem inflammatory syndrome in children (MIS-C) had moderate or severe disease [6]. Furthermore, long COVID or post-COVID syndrome has been described in both children and adults [7]. The incidence of this condition in children varies from 0 to 40% [7, 8]. Young adolescents and children are significant vectors in SARS-CoV-2 transmission and can infect the elderly, who are susceptible to severe disease [9].
Due to the above, preventing COVID-19 transmission is of paramount importance. The BNT162b2 (Pfizer–BioNTech, New York, NY, USA) vaccine, an mRNA-based vaccine, was shown to have a favourable safety profile, with 95% efficacy in preventing COVID-19 infection in healthy participants over 16 years of age [10] and in healthy adolescents aged 12–15 years [11], leading to FDA approval in these age groups. The Centers for Disease Control have issued guidelines for use in children aged 5–11 [12]. In June 2021, the B.1.617.2 (delta) variant of SARS-CoV-2 became the dominant strain in Israel and went on to cause a major resurgence of COVID-19 infection [13]. Studies conducted during this period found vaccine effectiveness against SARS-CoV-2severe infection to be around 96% and 92% after the second vaccine dose for persons 16–39 years of age and for adolescents 12–15 years of age, respectively [14–15].
Children with autoimmune inflammatory rheumatic diseases (IRDs) may have an increased risk of infections due to the disease itself or due to treatment with immunomodulating or immunosuppressive drugs [16–17]. Patients with paediatric-onset IRD require a personalized vaccination schedule that considers many factors, including disease activity, treatment, infection risk, and vaccine safety and efficacy [18]. Most studies have evaluated the immunogenicity (ability to induce humoral and cellular immune responses) of vaccines, rather than the effect of vaccination on infection rates (i.e. vaccine efficacy or effectiveness) [19]. The ongoing COVID-19 pandemic is an opportunity to examine the immunogenicity and the efficacy of the COVID-19 vaccine among different populations, including adolescents with IRD.
This study estimated the effectiveness of the BNT162b2 mRNA vaccine in adolescents with IRD compared with controls without immune rheumatic disease.
Methods
Study design and population
This observational cohort study was designed to estimate the effectiveness of the BNT162b2 mRNA COVID-19 vaccine in adolescents with IRD. Eligibility criteria were adolescents aged 12–18 years, who were members of Clalit Health Services (CHS) and had a documented diagnosis of JIA, SLE or FMF [according to International Classification of Diseases, ninth edition (ICD-9) codes or Clalit diagnostic criteria] during the 6 months preceding data extraction.
We extracted data regarding vaccination status and current treatment, including treatment with conventional DMARDs (c-DMARDs) and biologic DMARDs (b-DMARDS). The pharmaceutical data was based on the CHS prescription system if the regimen was issued during the 3 months before the study period. The control group consisted of adolescents without IRD of the same ages who were members of CHS.
The primary outcome was documented SARS-CoV-2 infection based on a positive PCR test. The secondary outcome was hospitalization due to COVID-19.
Data source
CHS is the largest health-care organization in Israel and insures 4.7 million patients (53% of the population). The CHS membership is generally representative of the Israeli population. The present study was based on CHS data covering adolescent patients vaccinated between February 2021 and December 2021. CHS data are integrated daily with data collected centrally by the Israeli Ministry of Health regarding COVID-19 vaccines, SARS-CoV-2 tests, and COVID-19–related hospitalizations and deaths.
Patient and public involvement
Patients took part in the data research analysis, broadening the scope of the research question, and providing information that would help dispel parents’ fears of the vaccination, and encourage them to vaccinate their children. Patients also reviewed and commented on the research paper and will take an important and active part in disseminating the outcomes of the study through European Network for Children with Arthritis (ENCA) and other parents’ organizations, locally and abroad.
Ethics
This study was approved by the CHS Community Institutional Review Board on 21 September 2021, (no. COM2-0139–21). Since the study was an observational, non-interventional study, it was exempt from the requirement for informed consent.
Data analysis
Data were analysed using SPSS, version 27. Descriptive statistics were produced using frequencies (n and %) for all variables. The rates of adolescents who were infected by COVID-19 in both cohorts were compared using χ2 tests. The level of significance (P-value) was set at <5%.
We calculated risk ratios for vaccination, compared with no vaccination, and estimated the vaccine effectiveness as one minus the risk ratio. We validated the results using the formula: VE = (NVAC – VAC)/NVAC, where VE is vaccine effectiveness, NVAC is the attack rate among the unvaccinated and VAC is the attack rate among vaccinated individuals.
Results
The cohort
The study cohort included 1639 adolescents with IRD, aged 12–18 years. We collected data on the three most prevalent diagnoses of adolescents with IRD in Israel: FMF, JIA and SLE. Patients with FMF (n = 1168) comprised 71.3% of the study cohort, followed by JIA at 23.2% (n = 380) and SLE at 5.5% (n = 91).
Table 1 presents the data regarding vaccination status and COVID-19 infection among the entire cohort. A total of 1188 (72.5%) adolescents with IRD were vaccinated: 179 (10.9%) had received one dose, 745 (45.5%) of the patient cohort had received two doses of vaccine and 264 (16.1%) had received three doses. The remaining 451 (27.5%) adolescents with IRD were not vaccinated at all.
Table 1.
Vaccination status and COVID-19 infection among adolescents with IRD
| Vaccine doses | Total | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Adolescents with IRD | 1639 | 451 (27.5%) | 179 (10.9%) | 745 (45.5%) | 264 (16.1%) |
| COVID-19 infection | 277 | 234 | 22 | 20 | 1 |
| Infections/vaccinateda | 16.9% | 51.9% | 12.3% | 2.7% | 0.4% |
| Vaccine effectivenessb | 76.3% | 94.8% | 99.2% |
Percentage of COVID-19 infection per vaccine dose.
Vaccine effectiveness calculated according to the formula: VE = (NVAC – VAC)/NVAC. IRD: inflammatory rheumatic disease; VE: vaccine effectiveness; NVAC: attack rate among the unvaccinated, VAC: attack rate among the vaccinated.
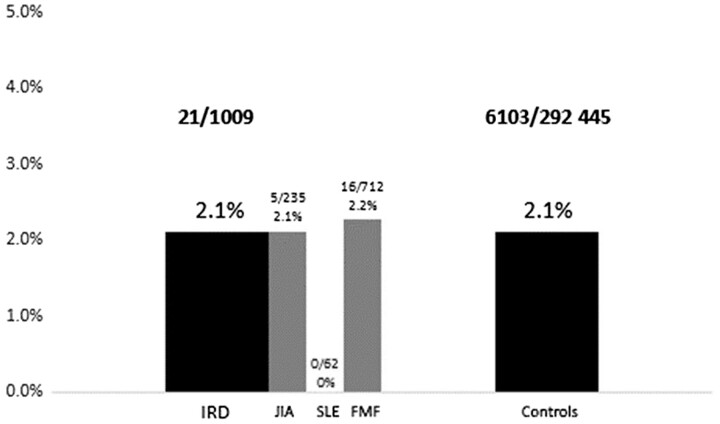
COVID-19 infection was diagnosed among 16.9% (n = 277) of the adolescents with IRD and 51.9% (n = 234) of the non-vaccinated adolescents with IRD. Among the adolescents with IRD who received at least two vaccine doses, 21/1009 (2.1%) were infected with COVID-19 after receiving their second or third dose of vaccine.
The median follow-up times after receiving vaccination for the study cohort were 21.6 weeks [interquartile range (IQR) 14.7–39.1], 19.0 weeks (IQR 13.6–36.9) and 8.9 weeks (IQR 7.3–11.6) after one, two and three doses of vaccine, respectively.
The estimated vaccine effectiveness for documented COVID-19 infection for adolescents with IRD was 76.3% after the first dose, 94.8% after the second dose and 99.2% after the third dose.
The controls
The control group included 524 471 adolescents without IRD in the same age range as the study cohort. Table 2 presents the data regarding vaccination status and COVID-19 infection among the controls, of which 342 701 (65.3%) were vaccinated. Within the control group, 226 156 (43.1%) adolescents received two doses of vaccine and 66 289 (12.6%) received three doses. COVID-19 infection was diagnosed among 15.3% (n = 80 167) of the adolescents in the control group, and in 37.2% of those who were not vaccinated. The median follow-up times after receiving vaccination for the controls were 21.3 weeks (IQR 14.3–38.7), 18.6 weeks (IQR 12.3–36.6) and 8.9 weeks (IQR 7.3–11.7) following one, two and three doses of vaccine, respectively.
Table 2.
Vaccination status and COVID-19 infection among controls without IRD
| Vaccine doses | Total | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Controls without IRD | 524 471 | 181 770 (34.7%) | 50 256 (9.6%) | 226 156 (43.1%) | 66 289 (12.6%) |
| COVID-19 infection | 80 167 | 67 719 | 6,345 | 5,943 | 160 |
| Infections/Vaccinateda | 15.3% | 37.2% | 12.6% | 2.6% | 0.2% |
| Vaccine effectivenessb | 66.1% | 93% | 99.5% |
Percentage of COVID-19 infection per vaccine dose.
Vaccine effectiveness calculated according to the formula: VE = (NVAC – VAC)/NVAC. IRD: inflammatory rheumatic disease; VE: vaccine effectiveness; NVAC: attack rate among the unvaccinated; VAC: attack rate among the vaccinated.
Infection occurred in 6103/292 445 (2.1%) adolescents after a second or third dose of vaccine. Thus, the estimated vaccine effectiveness for documented COVID-19 infection for adolescents without IRD was 66.1% after the first dose, 93% after the second dose and 99.5% after the third dose.
COVID-19 infection was diagnosed among 51.9% (n = 234) of the non-vaccinated adolescents with IRD as compared with 37.2% (n = 67 719) of the unvaccinated controls (P < 0.001).
Fig. 1 presents the comparison between adolescents with IRD and controls without IRD who were infected with COVID-19 after being vaccinated with at least two doses (21/1009 compared with 6103/292 445, respectively). The infection rates were not significantly different between these two groups (χ2 = 0.01, P = 0.99).
Fig. 1.
Comparison of COVID-19 infection rates after the second dose, between adolescents with IRD and controls
IRD: autoimmune inflammatory rheumatic disease.
Effectiveness of COVID-19 vaccination among patients with IRD vs controls
Table 3 and Fig. 1 present the comparison between patients with IRD (FMF, JIA and SLE) and controls. Among the 380 adolescents with JIA who were evaluated in the study, 235 (61.8%) were vaccinated at least twice. Five adolescents (5/235, 2.1%) were infected with COVID-19 after receiving a second vaccine dose. The time span of infection from the second dose was 22–28 weeks. None of these 5 received immunomodulating drugs on a regular basis. None of the adolescents who received three doses of the vaccine was infected with COVID-19.
Table 3.
COVID-19 infection following vaccination among paediatric patients with IRD compared with controls without IRD
| Vaccine doses | Total | 0 |
1 |
2 |
3 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | P-value | N (%) | P-value | N (%) | P-value | N (%) | P-value | |||
| Subjects | ||||||||||
| Controls without IRD | Total subjects | 524 471 | 181 770 | 50 256 | 226 156 | 66 289 | ||||
| COVID-19 positive rate per dose | 80 167 (15.3%) | 67 719 (37.2%) | 6345 (12.6%) | 5943 (2.6%) | 160 (0.2%) | |||||
| JIA | Total | 380 | 111 | 34 | 164 | 71 | ||||
| COVID-19 positive rate per dose | 51 (13.4%) | 46 (41.4%) | 0.36 | 0 (0%) | <0.01 | 5 (3%) | 0.14 | 0 (0%) | 0.53 | |
| SLE | Total | 91 | 20 | 9 | 34 | 28 | ||||
| COVID-19 positive rate per dose | 15 (16.5%) | 13 (65%) | 0.79 | 2 (22.2%) | 0.38 | 0 (0%) | 0.33 | 0 (0%) | 0.25 | |
| FMF | Total | 1168 | 320 | 136 | 547 | 165 | ||||
| COVID-19 positive rate per dose | 211 (18%) | 175 (54.7%) | 0.001 | 20 (14.7%) | 0.46 | 15 (2.7%) | 0.18 | 1 (0.6%) | 0.34 | |
IRD: inflammatory rheumatic disease.
The results showed a significant difference in the rates of COVID-19 infection between JIA patients and controls following one dose of vaccine (0% vs 12.6%, respectively, P < 0.01). There was no significant difference in the rates of COVID-19 between unvaccinated adolescents with JIA and controls (P = 0.36), and for those who received two (P = 0.14) or three doses (P = 0.53) of the vaccine.
Overall, 47 (12.4%) of patients with JIA were treated with MTX, 84 (22.1%) with anti-TNF, and 7 (1.8%) with tocilizumab (Table 4). None of the adolescents with JIA receiving immunomodulation were infected with COVID-19 after the second dose of vaccine.
Table 4.
COVID-19 infection following vaccination in relation to immunomodulatory treatment
| Medication | COVID-19 infection |
||||
|---|---|---|---|---|---|
| Vaccine dose N | 0 | 1 | 2 | 3 | |
| Adalimumab | 39 | 4 | 0 | 0 | 0 |
| Etanercept | 39 | 8 | 0 | 0 | 0 |
| Infliximab | 5 | 0 | 0 | 0 | 0 |
| Golimumab | 1 | 0 | 0 | 0 | 0 |
| MTX | 47 | 7 | 0 | 0 | 0 |
| HCQ | 37 | 2 | 1 | 0 | 0 |
| MMF | 23 | 0 | 0 | 0 | 0 |
| AZA | 8 | 0 | 0 | 0 | 0 |
| SSZ | 5 | 1 | 0 | 0 | 0 |
| Colchicine | 793 | 108 | 0 | 13 | 1 |
| Canakinumab | 31 | 7 | 0 | 0 | 0 |
| Anakinra | 9 | 1 | 0 | 0 | 0 |
| Tocilizumab | 7 | 0 | 0 | 0 | 0 |
Among 91 adolescents with SLE, 62 (68.1%) received at least two doses of vaccine and 2 were infected after one dose. None of the patients with SLE who received two or three doses of the vaccine were infected with COVID-19 after completing the series of vaccinations.
There were no significant differences in the post-vaccination COVID-19 rates among adolescents with SLE compared with controls, for any of the time points—one dose (P = 0.38), two doses (P = 0.33) or three doses (P = 0.25)—or between non-vaccinated adolescents with SLE and controls (P = 0.79).
Among the 23 (25.3%) of adolescents with SLE who were treated with MMF, none were infected with COVID-19. Similarly, no infections with COVID-19 were documented for adolescents with SLE treated with AZA (n = 3) or MTX (n = 1) (Table 4).
A total of 1168 adolescents with FMF were evaluated in this study. Among them, 712 (61%) were vaccinated at least twice, of whom 16/712 (2.2%) became infected with COVID-19.
Significantly more non-vaccinated adolescents with FMF contracted COVID-19 compared with non-vaccinated controls (54.7% vs 37.2%, respectively, P = 0.001). There was no significant difference in infection rates with COVID-19 between adolescents with FMF and controls after one dose (P = 0.46), two doses (P = 0.18) or three doses (P = 0.34) of vaccine.
Among the adolescents with FMF, 793 (67.9%) were treated with colchicine, 31 (2.6%) with canakinumab and 9 (0.8%) with anakinra. None of the adolescents treated with canakinumab or anakinra were infected with COVID-19 after the second dose of vaccine.
Hospitalizations
Four patients with IRD (0.2%) were hospitalized due to COVID-19 infection, 3 with SLE and 1 with JIA. All were unvaccinated and had mild disease. There were no hospitalizations due to MIS-C. No deaths due to COVID-19 infection or MIS-C were reported among adolescents with IRD. Among the control group, 420 (0.08%) were hospitalized due to COVID-19 infection or MIS-C: 74% with mild disease and 14% with moderate/severe disease. Disease severity during hospitalization was not documented in 12%. The only death was that of an obese, but otherwise healthy, 16-year-old boy who died due to MIS-C.
Discussion
This study compared the effectiveness of the BNT162b2 mRNA COVID-19 vaccine for adolescents with an IRD vs controls without IRD. The estimated vaccine effectiveness was very high for both groups. Among adolescents with IRD, it was 76.3% after the first dose, 94.8% after the second dose and 99.2% after the third dose.
Data from adults with IRD receiving the BNT162b2 vaccine demonstrate safety and immunogenicity in most patients with IRD, with 86% seropositivity. However, antibody levels to the S1/S2 spike protein were significantly lower among patients with IRD compared with controls [20]. Information on the safety and immunogenicity of COVID vaccines for adolescents with IRD is limited. Heshin-Bekenstein et al. [21] recently reported a study on 91 adolescents with IRD and 42 healthy controls from four centres in Israel and Slovenia. The results demonstrated that the BNT162b2 COVID-19 vaccine has a good safety profile. IRD patients had an adequate immunogenic response, but significantly lower antibody titres, when compared with healthy controls.
In the past decade, knowledge about the safety and immunogenicity of vaccines in paediatric patients with IRD has increased significantly. Evidence has accumulated that vaccines, including live-attenuated vaccines, are safe for paediatric patients with IRD on immunosuppression [22–24]. Regarding immunogenicity, numerous studies have found that vaccinations were immunogenic in paediatric patients with IRD, including those receiving CSs, MTX or TNF inhibitors [25–26].
Data on effectiveness remains scarce. No studies were powered to assess the effectiveness of vaccinations in paediatric patients with IRD. These studies are difficult to perform because the risk of infection is low, due to high herd immunity. Most of the vaccines included in national immunization programs have resulted in the elimination of these diseases, making current studies regarding their effectiveness impossible [27]. The ongoing COVID-19 pandemic is a rare opportunity to examine the immunogenicity and effectiveness of a vaccine among different populations, including adolescents with IRD, and to compare the data with that for adolescents without IRD.
Randomized clinical trials of mRNA-based vaccines reported that the efficacy for preventing COVID-19 is 94% to 95% in persons 16 years of age or older [11]. Follow-up research evaluating adolescents ages 12–15 years found that the BNT162b2 vaccine was safe, immunogenic and resulted in an observed efficacy of 100% against COVID-19 infection [12]. Our study found that the estimated vaccine effectiveness for documented COVID-19 infection for adolescents with IRD was almost 95% after the second dose of vaccine and above 99% after the third dose.
The current study demonstrates that immunomodulatory drug treatment did not affect the effectiveness of the vaccine. No COVID-19 infections were documented for adolescents treated with c-DMARDS or b-DMARDS who received two or three doses of vaccine. Recent studies evaluating the immunogenicity of the COVID-19 vaccine in adults and adolescents with IRD found reduced humoral response in patients treated with MMF [21, 28]. We found that the effectiveness of the vaccine for adolescents treated with MMF was 100% after the second dose.
In addition, it was found that unvaccinated adolescents with IRD were at increased risk of contracting COVID-19, compared with adolescents without IRD. In subgroup analysis, it was found that this difference was evident only in adolescents with FMF (54.7% in unvaccinated adolescents with FMF as compared with 37.2% in unvaccinated controls). In a recent study, Stella et al. proposed that pyrin may have evolved to combat pathogens, including viral infections [29]. On the other hand, COVID-19 infection may induce an intense inflammatory response known as a ‘cytokine storm’ [30], raising the question of the susceptibility and severity of SARS CoV-2 infection among patients with innate immunity disorders, such as those with FMF. Our study was the first to find that patients with FMF were at increased risk of contracting COVID-19 infection as compared with controls.
Another finding of our study was that only about 60% of the adolescents with IRD received two or three doses of vaccine. Although vaccination coverage was slightly better among patients with IRD compared with controls, we should aim for higher vaccination rates for all adolescents and especially those with IRD, due to their increased risk for disease flare if infected and increased risk for a more severe clinical course of COVID-19 infection.
The findings presented here can reassure families and patients with IRD who are being treated with immunomodulation regarding the effectiveness of the COVID-19 vaccine. This might improve the sub-optimal vaccination coverage for this population.
Our study has some limitations to take into consideration. This is a retrospective nationwide study that relies on medical data gathered from the electronic medical records of patients with IRD. Due to security and privacy reasons, we could not validate the ICD-9 codes for this cohort. Another limitation was the absence of data regarding glucocorticoid use in our study. We decided not to include glucocorticoid use in our study due to our inability to validate the accurate dosage prescribed. Moreover, we could not confirm whether it had been prescribed for a rheumatologic condition (rather than other concomitant conditions, e.g. asthma). Another limitation was that infection rates of adolescents with IRD, especially individuals taking immunomodulatory drugs, might be influenced by better health-promoting behaviour in this group, e.g. a higher tendency to wear masks and stay at home. Our analysis could not be fully controlled for aspects related to human behaviour. Nonetheless, the data size, consistency of the results, and valid outcomes such as PCR positivity and hospitalization rates are reassuring about the effect of the vaccination.
This is the first study to evaluate the effectiveness of the COVID-19 vaccine in adolescents with IRD. Access to the database from the largest health-care organization in Israel enabled us to analyse a large cohort of adolescents with IRD and compare it with a large control group.
In conclusion, this nationwide observational study conducted during the COVID-19 pandemic demonstrated that the effectiveness of the BNT162b2 mRNA vaccine was excellent among patients with IRD treated with immunomodulatory drugs, and similar to the effectiveness among controls without IRD. Our results can encourage adolescents with IRD to get vaccinated against COVID-19 and, thus, improve the suboptimal vaccination coverage for this population.
Acknowledgements
We thank Faye Schreiber, MS, for editing the manuscript.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Amit Ziv, Pediatric Rheumatology Unit, Meir Medical Center, Kfar Saba; Sackler School of Medicine, Tel Aviv University.
Merav Heshin-Bekenstein, Sackler School of Medicine, Tel Aviv University; Pediatric Rheumatology Service, Dana Children’s Hospital, Tel Aviv Medical Center, Tel Aviv.
Ruby Haviv, Pediatric Rheumatology Unit, Meir Medical Center, Kfar Saba; Sackler School of Medicine, Tel Aviv University.
Shaye Kivity, Sackler School of Medicine, Tel Aviv University; Rheumatology Unit, Meir Medical Center, Kfar Saba.
Doron Netzer, Community Medical Services Division, Clalit Health Services, Tel Aviv.
Shlomit Yaron, Community Medical Services Division, Clalit Health Services, Tel Aviv.
Yoav Schur, Community Medical Services Division, Clalit Health Services, Tel Aviv.
Tsipi Egert, Inbar, NPO, Ramat Gan.
Yona Egert, Inbar, NPO, Ramat Gan.
Yaron Sela, The Research Center for Internet Psychology, School of Communications, Reichman University, Herzliya.
Philip J Hashkes, Pediatric Rheumatology Unit, Shaare Zedek Medical Center; Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel.
Yosef Uziel, Pediatric Rheumatology Unit, Meir Medical Center, Kfar Saba; Sackler School of Medicine, Tel Aviv University.
Data availability statement
All the data relevant to this study are included in the manuscript, tables, and figures. Additional data will be provided upon request.
References
- 1. Leung K, Wu JT, Liu D. et al. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet 2020;395:1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castagnoli R, Votto M, Licari A. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020;174:882–9. [DOI] [PubMed] [Google Scholar]
- 3. Chao JY, Derespina KR, Herold BC. et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr 2020;223:14–19.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeBiasi RL, Song X, Delaney M. et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr 2020;223:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liguoro I, Pilotto C, Bonanni M. et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020;179:1029–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ben-Shimol S, Livni G, Megged O. et al. COVID-19 in a subset of hospitalized children in Israel. J Pediatric Infect Dis Soc 2021;10:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buonsenso D, Munblit D, De Rose C. et al. Preliminary evidence on long COVID in children. Acta paediatr 2021;110:2208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Health. Results of the Long-COVID Survey Among Children in Israel. https://www.gov.il/en/departments/news/13092021-01 (3 November 2021, date last accessed).
- 9. Kelvin AA, Halperin S.. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis 2020;20:633–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polack FP, Thomas SJ, Kitchin N. et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frenck RW, Klein NP, Kitchin N. et al. ; C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med 2021;385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodworth KR, Moulia D, Collins JP. et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years — United States, November 2021. MMWR Morb Mortal Wkly Rep 2021;70:1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammerman A, Sergienko R, Friger M. et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med 2022;386:1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glatman-Freedman A, Bromberg M, Dichtiar R. et al. The BNT162b2 vaccine effectiveness against new COVID-19 cases and complications of breakthrough cases: a nation-wide retrospective longitudinal multiple cohort analysis using individualised data. EBioMedicine 2021;72:103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glatman-Freedman A, Hershkovitz Y, Kaufman Z. et al. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 delta variant infection, Israel, 2021. Emerg Infec Dis 2021;27:2919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beukelman T, Xie F, Chen L. et al. ; SABER Collaboration. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012;64:2773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becker I, Horneff G.. Risk of serious infection in juvenile idiopathic arthritis patients associated with TNF-inhibitors and disease activity in the German BIKER registry. Arthritis Care Res 2017;69:552–60. [DOI] [PubMed] [Google Scholar]
- 18. Heijstek MW, De Bruin LMO, Bijl M. et al. ; EULAR. EULAR recommendations for vaccination in pediatric patients with rheumatic diseases. Ann Rheum Dis 2011;70:1704–12. [DOI] [PubMed] [Google Scholar]
- 19. Toplak N, Uziel Y.. Vaccination for children on biologics. Curr Rheumatol Rep 2020;22:26. [DOI] [PubMed] [Google Scholar]
- 20. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 21. Heshin Bekenstein M, Ziv A, Toplak N. et al. Safety and immunogenicity of BNT162b2 mRNA COVID-19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatology (Oxford) 2022. doi: 10.1093/rheumatology/keac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uziel Y, Moshe V, Onozo B. et al. ; PReS working party of Vaccination Study Group. Live attenuated MMR/V booster vaccines in children with rheumatic diseases on immunosuppressive therapy are safe: multicenter, retrospective data collection. Vaccine 2020;38:2198–201. [DOI] [PubMed] [Google Scholar]
- 23. Jeyaratnam J, Ter Haar NM, Lachmann HJ. et al. The safety of live-attenuated vaccines in patients using IL-1 or IL-6 blockade: an international survey. Pediatr Rheumatol 2018;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Speth F, Hinze CH, Andel S. et al. Varicella-zoster-virus vaccination in immunosuppressed children with rheumatic diseases using a pre-vaccination check list. Pediatr Rheumatol Online J 2018;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorelik M, Elizalde A, Wong Williams KW. et al. Immunogenicity of sequential 13-valent conjugated and 23-valent unconjugated pneumococcal vaccines in a population of children with lupus. Lupus 2018;27:2228–35. [DOI] [PubMed] [Google Scholar]
- 26. Heijstek MW, Van Gageldonk PGM, Berbers GAM et al Differences in persistence of measles, mumps, rubella, diphtheria and tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis 2012;71:948–54. [DOI] [PubMed] [Google Scholar]
- 27. Banaszkiewicz A, Radzikowski A.. Efficacy, effectiveness, immunogenicity – are not the same in vaccinology. World J Gastroenterol 2013;19:7217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun-Moscovici Y, Kaplan M, Braun M. et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 29. Stella A, Lamkanfi M, Portincasa P.. Familial Mediterranean fever and COVID-19: friends or foes? Front Immunol 2020;11:574593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soy M, Keser G, Atagunduz P. et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 2020;39:2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this study are included in the manuscript, tables, and figures. Additional data will be provided upon request.