Abstract
Post-COVID cognitive deficits (often referred to as ‘brain fog’) are common and have large impacts on patients’ level of functioning. No specific intervention exists to mitigate this burden.
This study tested the hypothesis, inspired by recent experimental research, that post-COVID cognitive deficits can be prevented by inhibiting receptor-interacting protein kinase. Using electronic health record data, we compared the cognitive outcomes of propensity score-matched cohorts of patients with epilepsy taking phenytoin (a commonly used receptor-interacting protein kinase inhibitor) versus valproate or levetiracetam at the time of COVID-19 diagnosis. Patients taking phenytoin at the time of COVID-19 were at a significantly lower risk of cognitive deficits in the 6 months after COVID-19 infection than a matched cohort of patients receiving levetiracetam (hazard ratio 0.78, 95% confidence interval 0.63–0.97, P = 0.024) or valproate (hazard ratio 0.73, 95% confidence interval 0.58–0.93, P = 0.011). In secondary analyses, results were robust when controlling for subtype of epilepsy, and showed specificity to cognitive deficits in that similar associations were not seen with other ‘long-COVID’ outcomes such as persistent breathlessness or pain. These findings provide pharmacoepidemiological support for the hypothesis that receptor-interacting protein kinase signaling is involved in post-COVID cognitive deficits. These results should prompt empirical investigations of receptor-interacting protein kinase inhibitors in the prevention of post-COVID cognitive deficits.
Keywords: RIPK signaling, endotheliopathy, Mpro, brain fog, long COVID
Little is known about the causes and treatments of post-COVID ‘brain fog’. Taquet et al. report that people taking phenytoin at the time of COVID-19 infection were less likely to later develop brain fog, suggesting that the latter might be due to damage to the blood vessels in the brain, which might be preventable with medications.
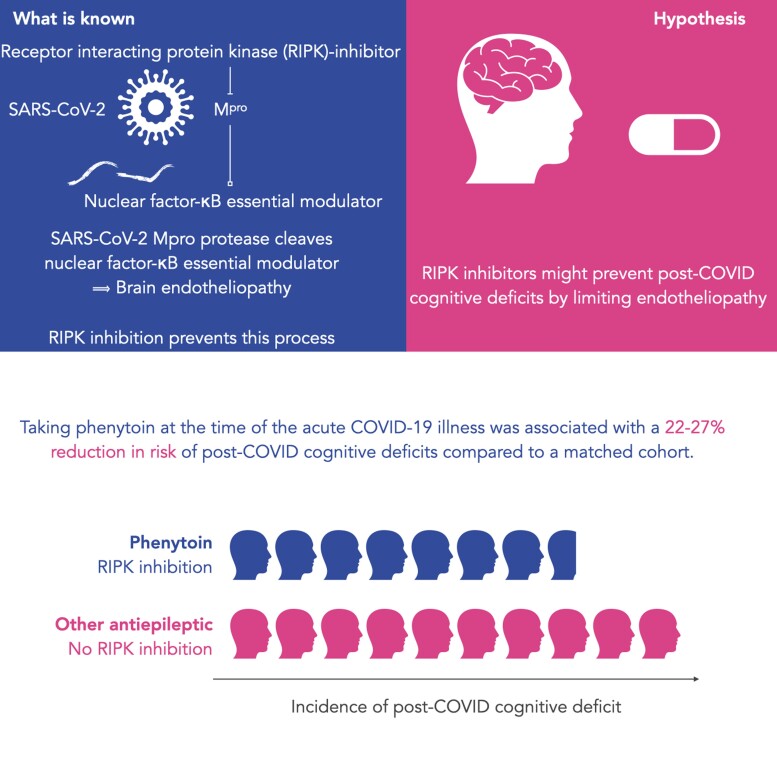
Graphical Abstract
Graphical Abstract.
Introduction
A proportion of patients experience long-lasting symptoms in the weeks and months after a diagnosis of COVID-19.1–3 Of those symptoms, cognitive impairment (also referred to as ‘brain fog’) is particularly worrisome: it is one of the most common,4,5 can affect those with even relatively mild acute COVID-19 illness1,5 and results in the inability to work for many affected patients.3 While emerging research is starting to characterize the clinical presentation of post-COVID cognitive deficits,6 its pathogenesis remains elusive. Identifying therapeutic targets is critical to reducing the burden of this COVID-19 complication.
Endotheliopathy has been hypothesized as one potential mechanism underlying post-COVID cognitive deficits.7 According to recent research, microvascular brain pathology following COVID-19 can be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease Mpro cleaving nuclear factor-κB essential modulator thus inducing the death of brain endothelial cells.8 The same study showed that pharmacologically inhibiting receptor-interacting protein kinase (RIPK) signaling prevents the Mpro-induced microvascular pathology.8
This research leads to the following hypothesis: exposure to a pharmacological inhibitor of RIPK signaling at the time of COVID-19 infection reduces the risk of post-COVID cognitive deficits. In this study, we tested this hypothesis using a retrospective cohort study based on electronic health records (EHRs) data. While many pharmacological agents inhibit RIPK signaling,9 most are only used in very rare clinical scenarios (e.g. sunitinib for the treatment of advanced renal cell carcinoma or pancreatic neuroendocrine tumors). The exception is phenytoin which is used as an anti-epileptic drug and which, among its other effects, is a RIPK1 inhibitor protecting against necroptosis.10,11 In this study, we compared the incidence of post-COVID cognitive deficits between patients exposed to phenytoin and matched cohorts of patients exposed to other anti-epileptic drugs at the time of their COVID-19 diagnosis.
Materials and methods
Data
The study used TriNetX Analytics, a federated network of linked EHRs recording anonymized data from 59 healthcare organizations (HCOs), primarily in the USA, totaling 81 million patients. Available data include demographics, diagnoses (using ICD-10 codes), procedures (using CPT codes), medications (encoded as RxNorm codes and VA classes) and measurements (e.g. body mass index). The HCOs in the network are a mixture of hospitals, primary care and specialist providers, and they contribute data from uninsured and insured patients. Data de-identification is formally attested as per Section §164.514(b)(1) of the HIPAA Privacy Rule, superseding TriNetX’s waiver from the Western Institutional Review Board; no further ethical approval was thus needed. As the study uses fully anonymized routinely collected data, no consent from participants was required. Within TriNetX, cohorts can be defined based on inclusion and exclusion criteria, propensity score-matched on a set of covariates and the outcomes can be compared between matched cohorts. For further details about TriNetX, its data provenance and functionalities, see Supplementary material.
Cohorts
The primary cohort was defined as all individuals who met the following three criteria:
The individual has a diagnosis of epilepsy or recurrent seizures (ICD-10 code G40).
The individual has a confirmed diagnosis of COVID-19 or a positive polymerase chain reaction (PCR) test for SARS-CoV-2 between 20 January 2020 (date of the first recorded COVID-19 case in the USA) and 29 November 2021.
The individual has phenytoin recorded in their medications on the day of their COVID-19 diagnosis or positive PCR test for SARS-CoV-2 or within 3 months before it.
Two control cohorts were defined. They both also met inclusion Criteria A and B, but instead of C, they had to meet C′ (first control cohort) or C″ (second control cohort):
C′. The individual has levetiracetam recorded in their medications on the day of their COVID-19 diagnosis or positive PCR test for SARS-CoV-2 or within 3 months before it.
C″. The individual has valproate recorded in their medications on the day of their COVID-19 diagnosis or positive PCR test for SARS-CoV-2 or within 3 months before it.
In addition, individuals who had any record of receiving phenytoin during the study period or up to 6 months before the start of the study period (20 January 2020) were excluded from both control cohorts.
Details of the cohort definition including EHR codes can be found in Supplementary material.
Covariates
As in our previous studies, a set of risk factors for COVID-19 and for more severe COVID-19 illness was used1: age, sex, race, ethnicity, obesity, hypertension, diabetes, chronic kidney disease, asthma, chronic lower respiratory diseases, nicotine dependence, substance misuse, previous psychiatric illness, ischemic heart disease and other forms of heart disease, socioeconomic deprivation, cancer (and hematological cancer in particular), chronic liver disease, stroke, dementia, organ transplant, rheumatoid arthritis, lupus, psoriasis and disorders involving an immune mechanism. In addition, because of the comorbidity between epilepsy and mood disorders, cohorts were also matched for specific mood disorder diagnoses. Cohorts were also matched for previous or concurrent use of specific medications with known association with COVID-19 including any anti-depressant, fluvoxamine specifically,12 any anti-psychotic and clozapine specifically.13 Given the theoretical possibility that angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers might affect the pathogenesis of COVID-19 sequelae (despite the absence of association with incidence of and mortality from COVID-19),14 these two classes of drugs were also included as covariates.
In total, 74 variables were used as covariates. More details including ICD-10 codes are provided in Supplementary material. Cohorts were matched for all these variables, as described below.
Outcomes
The outcome period was 6 months after COVID-19 diagnosis. The primary outcome was a composite of ICD-10 codes capturing the range of diagnostic codes that patients presenting with ‘brain fog’ might receive, as defined in our previous study.1 Specifically the following codes were used: F01 (‘Vascular dementia’), F02 (‘Dementia in other disease classified elsewhere’), F03 (‘Unspecified dementia’), F05 (‘Delirium due to known physiological condition’), F06.8 (‘Other specified mental disorders due to known physiological condition’), G30 (‘Alzheimer’s disease’), G31.0 (‘Frontotemporal dementia’), G31.83 (‘Dementia with Lewy bodies’), G31.84 [‘Mild cognitive impairment’ (MCI)], G93.40 (‘Encephalopathy, unspecified’), R40 (‘Somnolence, stupor and coma’), R41 (‘Other symptoms and signs involving cognitive functions and awareness’) or R48 (‘Dyslexia and other symbolic dysfunction’).
As secondary outcomes, the above ICD-10 codes were classified into five categories: dementia (F01-F03, G30, G31.0 or G31.83), delirium (F05), MCI (G31.84), encephalopathy (G93.40) and other cognitive deficits (any of the other codes: F06.8, R40, R41 or R48).
Statistical analyses
Propensity score matching was used to create cohorts with matched baseline characteristics,15 and carried out within the TriNetX network. Propensity score 1:1 matching used a greedy nearest neighbour matching approach with a calliper distance of 0.1 pooled standard deviations of the logit of the propensity score. Any characteristic with a standardized mean difference (SMD) between cohorts <0.1 is considered well matched.16 The incidence of each outcome was estimated using the Kaplan–Meier estimator. Comparisons between cohorts were made using a log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using the Cox model. The proportional hazard assumption was tested using the generalized Schoenfeld approach. The E-value was used to quantify sensitivity of the findings to unmeasured confounders.17 Statistical analyses were conducted in R version 3.6.3 except for the matching of cohorts which was performed within TriNetX. Statistical significance was set at two-sided P-values <0.05.
Secondary analyses
To assess whether the association between phenytoin exposure and post-COVID cognitive impairment also affects the incidence towards the later phase of the follow up, the statistical analysis was repeated with the weighted log-rank test with a Fleming–Harrington function with parameters p = 0, q = 1, which has optimal power to detect late effects of exposure on outcomes.18
Since levetiracetam and valproate are approved for the treatment of primary generalized epilepsy and focal epilepsies, whereas phenytoin is mostly used for focal epilepsies, and since patients with intellectual disability are more likely to have focal than generalized epilepsy,19 we ran a robustness analysis in which the epilepsy subtype (generalized epilepsy versus other) and intellectual disabilities (F70–F79) were added to the list of covariates.
We tested the specificity of the association of phenytoin exposure with post-COVID cognitive deficit by including, as putative negative control outcomes, another eight common long-COVID clinical features as defined in our previous study,1 namely chest/throat pain, headache, myalgia, other pain, abnormal breathing, abdominal symptoms, fatigue and anxiety/depression. Each of them was captured with the same ICD-10 codes as used in our previous study (see Supplementary material for details).
As another secondary analysis, we compared patients with high phenytoin level (≥10 μg/mL, i.e. above minimum therapeutic level) versus low (<10 μg/mL, i.e. below therapeutic level). This exploratory analysis was unmatched as the sample size was too low to achieve appropriate matching and because both cohorts were receiving phenytoin thus reducing the potential for large confounding effects.
Data availability
The TriNetX system returned the results of these analyses as csv files which were downloaded and archived. Data presented in this study and Supplementary material can be freely accessed at https://osf.io/wxtgu. Additionally, TriNetX will grant access to researchers if they have a specific concern (via the third-party agreement option).
Results
A total of 668 individuals with SARS-CoV-2 infections had phenytoin recorded as a medication in their EHR within the 3 months preceding a diagnosis of COVID-19 or a confirmed PCR test for SARS-CoV-2. In the control cohorts, 4192 and 1344 individuals with SARS-CoV-2 infections had respectively levetiracetam and valproate recorded as a medication in their EHR within the 3 months preceding a diagnosis of COVID-19 or a confirmed PCR test for SARS-CoV-2. After 1:1 matching, 663 patients on phenytoin were compared with 663 patients on levetiracetam, and 505 patients on phenytoin were compared with 505 patients on valproate. Baseline characteristics of all cohorts are presented in Table 1 and Supplementary Tables 1 and 2. Adequate matching was achieved for all covariates in all comparisons.
Table 1.
Baseline characteristics
| Comparison with levetiracetam | Comparison with valproate | |||||
|---|---|---|---|---|---|---|
| Phenytoin | Levetiracetam | SMD | Phenytoin | Valproate | SMD | |
| Number | 663 | 663 | – | 505 | 505 | – |
| Demographics | ||||||
| Age, mean (SD), years | 60.9 (15.7) | 61.3 (19.3) | 0.02 | 58.7 (15.6) | 57.9 (19.7) | 0.04 |
| Sex, n (%) | ||||||
| Female | 277 (41.8) | 268 (40.4) | 0.03 | 213 (42.2) | 209 (41.4) | 0.02 |
| Male | 386 (58.2) | 395 (59.6) | 0.03 | 292 (57.8) | 296 (58.6) | 0.02 |
| Race, n (%) | ||||||
| White | 369 (55.7) | 376 (56.7) | 0.02 | 300 (59.4) | 291 (57.6) | 0.04 |
| Black or African-American | 212 (32.0) | 216 (32.6) | 0.01 | 145 (28.7) | 152 (30.1) | 0.03 |
| Unknown | 65 (9.8) | 57 (8.6) | 0.04 | 54 (10.7) | 51 (10.1) | 0.02 |
| Comorbidities, n (%) | ||||||
| Overweight and obesity | 212 (32.0) | 212 (32.0) | 0 | 163 (32.3) | 159 (31.5) | 0.02 |
| Hypertensive disease | 463 (69.8) | 482 (72.7) | 0.06 | 340 (67.3) | 342 (67.7) | 0.008 |
| Type 2 diabetes mellitus | 213 (32.1) | 220 (33.2) | 0.02 | 172 (34.1) | 171 (33.9) | 0.004 |
| Asthma | 89 (13.4) | 86 (13.0) | 0.01 | 72 (14.3) | 73 (14.5) | 0.006 |
| Other chronic obstructive pulmonary disease | 116 (17.5) | 117 (17.6) | 0.004 | 89 (17.6) | 81 (16.0) | 0.04 |
| Nicotine dependence | 141 (21.3) | 149 (22.5) | 0.03 | 100 (19.8) | 92 (18.2) | 0.04 |
| Psychiatric comorbidities | ||||||
| Substance misuse | 197 (29.7) | 205 (30.9) | 0.03 | 142 (28.1) | 135 (26.7) | 0.03 |
| Psychotic disorders | 82 (12.4) | 86 (13.0) | 0.02 | 76 (15.1) | 70 (13.9) | 0.03 |
| Mood disorders | 247 (37.3) | 244 (36.8) | 0.009 | 207 (41.0) | 211 (41.8) | 0.02 |
| Anxiety disorders | 237 (35.7) | 246 (37.1) | 0.03 | 191 (37.8) | 185 (36.6) | 0.02 |
| Ischemic heart diseases | 210 (31.7) | 217 (32.7) | 0.02 | 152 (30.1) | 147 (29.1) | 0.02 |
| Other forms of heart disease | 317 (47.8) | 314 (47.4) | 0.009 | 241 (47.7) | 236 (46.7) | 0.02 |
| Chronic kidney disease (CKD) | 116 (17.5) | 114 (17.2) | 0.008 | 90 (17.8) | 90 (17.8) | 0 |
| Cerebral infarction | 121 (18.2) | 135 (20.4) | 0.05 | 91 (18.0) | 100 (19.8) | 0.05 |
| Unspecified dementia | 107 (16.1) | 105 (15.8) | 0.008 | 83 (16.4) | 83 (16.4) | 0 |
| Medications, n (%) | ||||||
| Anti-depressants | 311 (46.9) | 323 (48.7) | 0.04 | 247 (48.9) | 246 (48.7) | 0.004 |
| Anti-psychotics | 208 (31.4) | 209 (31.5) | 0.003 | 182 (36.0) | 184 (36.4) | 0.008 |
| ACE inhibitors | 209 (31.5) | 227 (34.2) | 0.06 | 165 (32.7) | 151 (29.9) | 0.06 |
| Angiotensin II inhibitors | 97 (14.6) | 100 (15.1) | 0.01 | 67 (13.3) | 65 (12.9) | 0.01 |
Only a subset of the most common and representative baseline characteristics is presented. All other baseline characteristics can be found in Supplementary Tables 1 and 2.
SMD, Standardized mean difference.
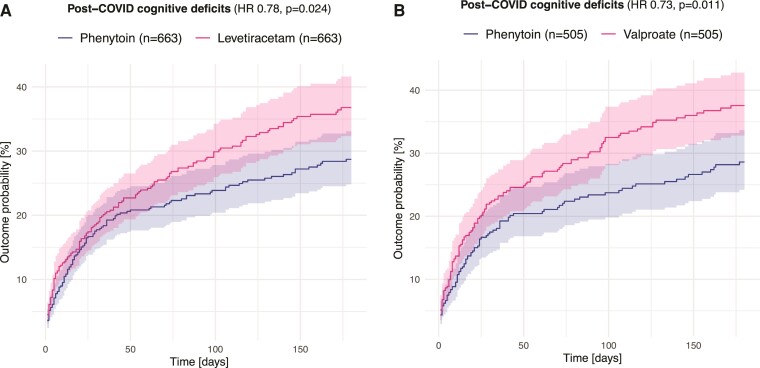
Patients taking phenytoin at the time of COVID-19 were at a significantly lower risk of post-COVID cognitive deficits than a matched cohort of patients receiving levetiracetam (HR 0.78, 95% CI 0.63–0.97, P = 0.024; E-value 1.66; no evidence of violation of proportionality assumption: P = 0.42; Fig. 1A) or valproate (HR 0.73, 95% CI 0.58–0.93, P = 0.011; E-value 1.79; no evidence of violation of proportionality assumption: P = 0.77; Fig. 1B) in the following 6 months. This relationship was sustained when primarily focusing on the later phase of follow up (weighted log-rank P = 0.021 when comparing with levetiracetam, and P = 0.026 for valproate). It was also robust to adjustment for type of epilepsy and intellectual disabilities (HR 0.80, 95% CI 0.70–0.91, P = 0.0005 for the comparison with levetiracetam and HR 0.86, 95% CI 0.75–0.99, P = 0.039 for the comparison with valproate).
Figure 1.
Kaplan–Meier curves for the incidence of cognitive deficits within the first 6 months after a diagnosis of COVID-19. Comparison is made between patients on phenytoin and those on (A) levetiracetam and (B) valproate. Shaded areas represent 95% confidence intervals.
The association was found to be specific to post-COVID cognitive deficits as no significant associations were observed for any of the other long-COVID features (all P > 0.05) except for a significantly lower risk of headache in those exposed to phenytoin compared with valproate (HR 0.59, 95% CI 0.38–0.93, P = 0.02) but not compared with levetiracetam (see Supplementary Table 3).
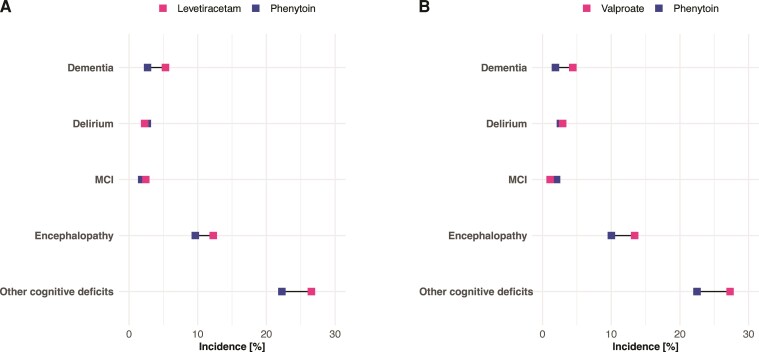
The incidence of most components of the composite endpoint representing cognitive deficits was lower in the phenytoin cohort, with notable contributions from ‘other cognitive deficits’ (Fig. 2).
Figure 2.
Cumulative 6 months incidence of different components of the primary composite endpoint. Comparison is made between phenytoin and (A) levetiracetam and (B) valproate. Note that because an individual may have more than one outcome, the sum of the incidence might exceed the incidence of the composite endpoint. Horizontal bars represent 95% confidence intervals. MCI = mild cognitive impairment.
Patients with a therapeutic phenytoin level (n = 240) were at a lower risk of post-COVID cognitive impairment than those with a subtherapeutic phenytoin level (n = 219; the total number is less than the number of individuals on phenytoin because several patients on phenytoin did not have a level recorded during the pandemic), but this did not reach statistical significance (HR 0.76, 95% CI 0.55–1.05, P = 0.091), except when focusing on the later phase of follow up (weighted log-rank P = 0.021).
Discussion
The findings in this study based on EHRs show that receiving phenytoin (versus levetiracetam or valproate) around the time of infection with SARS-CoV-2 is associated with a 22–27% lower risk of post-COVID cognitive deficits over the following 6 months compared with other anti-epileptic drugs.
These findings provide the first empirical support for the hypothesis that exposure to a pharmacological inhibitor of RIPK signaling around the time of COVID-19 infection reduces the risk of post-COVID cognitive deficits. This in turn suggests that the pathogenesis of post-COVID cognitive deficits involves (at least in some cases) cerebral endotheliopathy. The downstream mechanism from endotheliopathy to cognitive impairment might follow the general pathophysiological pathway of small vessel diseases which is a leading cause of cognitive decline.20 Experimental research on the role of RIPK signaling in COVID-related neuropathology has been limited to small vessels.8 It is unclear if the same pathological process is responsible for large vessel ischemic stroke after COVID-19 and the rate of ischemic strokes was too low in our population for meaningful comparisons between matched cohorts (for completeness, the results in terms of ischemic strokes are presented in Supplementary Fig. 1 showing non-statistically significant decreased rates in individuals on phenytoin compared with those on levetiracetam or valproate).
‘Other cognitive symptoms’ was the most common category of cognitive sequelae of COVID-19, which likely reflects the ‘brain fog’, word finding difficulties or poor concentration reported in surveys of long-COVID.1 The fact that (i) this is also the outcome with the largest difference in incidence (in absolute terms) in those receiving phenytoin, (ii) the risk of delirium (which is typically restricted to the acute phase) appears unaffected by phenytoin and (iii) differences between cohorts are as notable or even more notable in later stages of the follow up suggest that endotheliopathy via RIPK signaling pathway might play a particularly important role in post-acute cognitive symptoms as part of long COVID.
Our study is observational; hence, no causal effect can be demonstrated and residual confounding (despite extensive matching) may persist. However, any unmeasured confounders would need to be associated with both the difference in exposure and post-COVID cognitive deficits with a relative risk of 1.66-fold each (i.e. the E-value) to explain away the observed association, which seems unlikely. In addition, it is worth noting that in the absence of COVID-19, levetiracetam is known to better preserve or even improve cognitive function,21,22 whereas phenytoin has recognized cognitive side effects.23 The choice of control cohort might therefore have led to a conservative estimate of the association between phenytoin and post-COVID cognitive deficits, potentially offsetting any positive bias from unmeasured confounders.
There are other limitations to this study besides those general to studies based on EHR data (summarized in Supplementary material). First, in the absence of a diagnostic code specific to the ‘brain fog’ reported by patients following COVID-19 infection, we used a pragmatic list of ICD-10 codes that are likely to capture this phenomenon and which were already used in a previous study.1 Second, while we have hypothesized a specific mechanism, no direct information can be gleaned from EHR data to demonstrate the mechanism at play; prospective studies with measurements of biomarkers for endotheliopathy are needed to address this issue. In particular, further studies should assess whether phenytoin inhibits RIPK signaling at anti-epileptic concentrations since in vivo studies were conducted by injecting phenytoin intraperitoneally in mice.10,11 Third, it is possible that the results do not apply to COVID-19 patients who do not have epilepsy. While this possibility cannot be ruled out from our data, endotheliopathy has been recognized as a core pathophysiological feature of COVID-19.24
These findings give support for a potential pathophysiological mechanism of post-COVID cognitive impairment, which is amenable to pharmacological intervention. The side-effect profile of phenytoin makes it relatively unattractive for repurposing as a preventative treatment for post-COVID cognitive deficits. However, more selective inhibitors of the RIPK signaling pathway could merit testing to prevent the cognitive sequelae of COVID-19, or trialled as secondary prevention for patients with positive biomarkers for endotheliopathy.25
Supplementary Material
Acknowledgement
The authors thank Arjune Sen and Masud Husain for helpful discussion.
Abbreviations
- EHR =
electronic health records
- HR =
hazard ratio
- MCI =
mild cognitive impairment
- RIPK =
receptor-interacting protein kinase
- SMD =
standardized mean difference
Contributor Information
Maxime Taquet, Department of Psychiatry, University of Oxford, Oxford, UK; Oxford Health NHS Foundation Trust, Oxford, UK.
Paul J Harrison, Department of Psychiatry, University of Oxford, Oxford, UK; Oxford Health NHS Foundation Trust, Oxford, UK.
Funding
Work supported by the National Institute for Health and Care Research (NIHR) Oxford Health Biomedical Research Centre (grant BRC-1215-20005), the Wolfson Foundation and MQ Mental Health Research. M.T. is an NIHR Academic Clinical Fellow and NIHR Oxford Health BRC Senior Research Fellow. The views expressed are those of the authors and not necessarily those of the UK National Health Service, NIHR, or the UK Department of Health.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-Month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8(5):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis HE, Assaf GS, McCorkell L, et al. . Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans RA, McAuley H, Harrison EM, et al. . Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham EL, Clark JR, Orban ZS, et al. . Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hampshire A, Trender W, Chamberlain SR, et al. . Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021;39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 2021;144(12):3576–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wenzel J, Lampe J, Müller-Fielitz H, et al. . The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24(11):1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martens S, Hofmans S, Declercq W, Augustyns K, Vandenabeele P. Inhibitors targeting RIPK1/RIPK3: Old and new drugs. Trends Pharmacol Sci. 2020;41(3):209–224. [DOI] [PubMed] [Google Scholar]
- 10. Von Mässenhausen A, Tonnus W, Himmerkus N, et al. . Phenytoin inhibits necroptosis. Cell Death Dis. 2018;9(3):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moerke C, Jaco I, Dewitz C, et al. . The anticonvulsive Phenhydan® suppresses extrinsic cell death. Cell Death Differ. 2019;26(9):1631–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lenze EJ, Mattar C, Zorumski CF, et al. . Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial. JAMA 2020;324(22):2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vai B, Mazza MG, Colli CD, et al. . Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: A systematic review and meta-analysis. Lancet Psychiatry 2021;8(9):797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fosbøl EL, Butt JH, Østergaard L, et al. . Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020;324(2):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39(1):33–38. [Google Scholar]
- 16. Haukoos JS, Lewis RJ. The propensity score. JAMA 2015;314(15):1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 18. Royston P, Parmar MKB. A simulation study comparing the power of nine tests of the treatment effect in randomized controlled trials with a time-to-event outcome. Trials 2020;21(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Airaksinen EM, Matilainen R, Mononen T, et al. . A population-based study on epilepsy in mentally retarded children. Epilepsia 2000;41(9):1214–1220. [DOI] [PubMed] [Google Scholar]
- 20. Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. [DOI] [PubMed] [Google Scholar]
- 21. Gomer B, Wagner K, Frings L, et al. . The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10(3):486–494. [DOI] [PubMed] [Google Scholar]
- 22. Magalhães JC, Gongora M, Vicente R, et al. . The influence of levetiracetam in cognitive performance in healthy individuals: Neuropsychological, behavioral and electrophysiological approach. Clin Psychopharmacol Neurosci. 2015;13(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jokeit H, Krämer G, Ebner A. Do antiepileptic drugs accelerate forgetting? Epilepsy Behav 2005;6(3):430–432. [DOI] [PubMed] [Google Scholar]
- 24. Gu SX, Tyagi T, Jain K, et al. . Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18(3):194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuadrado-Godia E, Dwivedi P, Sharma S, et al. . Cerebral small vessel disease: A review focusing on pathophysiology, biomarkers, and machine learning strategies. J Stroke Cerebrovasc Dis. 2018;20(3):302–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The TriNetX system returned the results of these analyses as csv files which were downloaded and archived. Data presented in this study and Supplementary material can be freely accessed at https://osf.io/wxtgu. Additionally, TriNetX will grant access to researchers if they have a specific concern (via the third-party agreement option).