Abstract
Background
The COVID-19 pandemic necessitated a rapid shift to telemedicine to minimize patient and provider exposure risks. While telemedicine has been used in a variety of primary and specialty care settings for many years, it has been slow to be adopted in oncology care. Health care provider and administrator perspectives on factors affecting telemedicine use in oncology settings are not well understood, and the conditions associated with the COVID-19 pandemic offered the opportunity to study the adoption of telemedicine and the resulting provider and staff perspectives on its use.
Objective
The aim of this paper is to study the factors that influenced telemedicine uptake and sustained use in outpatient oncology clinics at a US cancer center to inform future telemedicine practices.
Methods
We used purposive sampling to recruit a mix of oncology specialty providers, practice managers, as well as nursing and administrative staff representing 5 outpatient oncology clinics affiliated with the Dartmouth Cancer Center, a large regional cancer center in the northeast of United States, to participate in semistructured interviews conducted over 6 weeks in spring 2021. The interview guide was informed by the 5 domains of the Consolidated Framework for Implementation Research, which include inner and outer setting factors, characteristics of the intervention (ie, telemedicine modality), individual-level factors (eg, provider and patient characteristics), and implementation processes. In total, 11 providers, 3 leaders, and 6 staff participated following verbal consent, and thematic saturation was reached across the full sample. We used a mixed deductive and inductive qualitative analysis approach to study the main influences on telemedicine uptake, implementation, and sustainability during the first year of the COVID-19 pandemic across the 5 settings.
Results
The predominant influencers of telemedicine adoption in this study were individual provider experiences and assumptions about patient preference and accessibility. Providers’ early telemedicine experiences, especially if negative, influenced preferences for telephone over video and affected sustained use. Telemedicine was most favorably viewed for lower-acuity cancer care, visits less dependent on physical exam, and for patient and caregiver education. A lack of clinical champions, leadership guidance, and vision hindered the implementation of standardized practices and were cited as essential for telemedicine sustainability. Respondents expressed anxiety about sustaining telemedicine use if reimbursements for telephonic visits diminished or ceased. Opportunities to enhance future efforts include a need to provide additional guidance supporting telemedicine use cases and evidence of effectiveness in oncology care and to address provider concerns with communication quality.
Conclusions
In a setting of decentralized care processes, early challenges in telemedicine implementation had an outsized impact on the nature and amount of sustained use. Proactively designed telemedicine care processes with attention to patient needs will be essential to support a sustained role for telemedicine in cancer care.
Keywords: telemedicine, telehealth, remote consultation, clinical oncology, implementation science, qualitative research, telemedicine methods, telemedicine organization and administration, telemedicine trends, clinical oncology methods, clinical oncology organization and administration, oncology, digital health, virtual care, COVID-19
Introduction
The COVID-19 pandemic led to an unprecedented need to deliver care for cancer and other conditions remotely [1-4]. Telemedicine has long been touted as a promising but underused mode of delivering cancer care, especially in rural areas where access is often constrained by the need to travel significant distances [5-12]. While technologies to support telemedicine have been around for decades [13-15], it was only when the public health emergency occurred locally—necessitating the curtailing of all nonessential in-person contact in March 2020 [16-21]—that our region in the rural New England region of the United States experienced a rapid uptake. At the start of the pandemic, telemedicine support at the Dartmouth Cancer Center (DCC) was provided by a small department used to handling a fraction of the visits experienced during the pandemic (outpatient televisit rates increased some 10,000%). The basic visit process entailed a multistep setup requiring the patient to download, install, and configure a computer software or smartphone app, or to be available for a phone call for a telephone visit once payment policy shifted to permit telephone visits [16-20,22]. After the first 3 months of the pandemic, the video visit process simplified to one where video visits could occur via a much simpler application accessible via the patient portal either on a computer or smartphone. Resources supporting the transition to televisits were largely limited to web-based training materials for learning to use the telemedicine platform, without the capacity for providing technical support or individualized workflow adaptations at the department or clinic level.
Quantitative analysis of telemedicine use (including the use of either telephone or video to provide real-time care similar to an in-person office visit) within the DCC over a 1-year time frame from pandemic onset revealed relatively low use compared to other specialties [23], and further analysis showed significant variability in use by clinic site corresponding to a larger magnitude of difference in telemedicine use rates compared with patient, geographic, or medical factors [24].
In a broader context, published studies of use trends of telehealth for cancer care suggest disparities in telehealth use, with patients in urban settings favoring telehealth more than rural [25], as well as other groups including older adults and patients of color [26]. Recent qualitative studies of telehealth for cancer care during the COVID-19 pandemic suggest that there is a subset of care situations within survivorship that is acceptable to providers and patients alike [27], and that telehealth has broadly been acceptable to many patients and providers even as concerns about a lack of physical exam are raised [28].
To better understand the underlying factors to the observed local variation in use amid the rapid transition in care delivery, we conducted a rapid-cycle qualitative study of semistructured interviews with a diverse mix of oncology providers and clinic staff in the spring of 2020.
Methods
Study Setting
The DCC, an affiliate of Dartmouth Health, serves the bistate region of New Hampshire and Vermont as well as parts of New York, Massachusetts, and Maine with headquarters at Dartmouth-Hitchcock Medical Center (DHMC) in Lebanon, New Hampshire. DCC operates 5 oncology clinics serving 18,000 to 20,000 patients per year across the catchment area. The proportion of patients who are dual eligible for both Medicaid (state-sponsored insurance for eligible low-income patients) and Medicare (nationally sponsored insurance for eligible older adults aged 65 or older or with specific disabilities) ranges from 19.1% to 25% across the oncology clinic sites (Table 1).
Table 1.
Patient demographic characteristics as a percentage of total population served by DCCa across oncology clinics in 2019.
| Characteristics | Clinics | |||||
|
|
A (n=2341), n (%) | B (n=9923), n (%) | C (n=2631), n (%) | D (n=1882), n (%) | E (n=1502), n (%) | |
| Race | ||||||
|
|
White | 2271 (97.0) | 97.3 (9655) | 2394 (91.0) | 1673 (88.9) | 1458 (97.1) |
|
|
Black | 12 (0.5) | 60 (0.6) | 84 (3.2) | 47 (2.5) | 6 (0.4) |
|
|
Hispanic | 14 (0.6) | 99 (1.0) | 84 (3.2) | 85 (4.5) | 11 (0.7) |
| Sex | ||||||
|
|
Female | 1367 (58.4) | 5487 (55.3) | 1584 (60.2) | 1150 (61.1) | 765 (50.9) |
|
|
Male | 974 (41.6) | 4436 (44.7) | 1047 (39.8) | 732 (38.9) | 737 (49.1) |
| Medicaidb | 447 (19.1) | 1935 (19.5) | 658 (25.0) | 356 (18.9) | 360 (24.0) | |
| Age | ||||||
|
|
>65 | 1470 (62.8) | 5517 (55.6) | 1181 (44.9) | 804 (42.7) | 993 (66.1) |
|
|
>85 | 206 (8.8) | 506 (5.1) | 134 (5.1) | 83 (4.4) | 93 (6.2) |
aDCC: Dartmouth Cancer Center.
bIncludes those dual eligible for Medicaid and Medicare.
Approximately 71% of patients seen in 2020 resided in rural settings [29]. The COVID-19 pandemic impacted care starting in mid-March 2020, with Dartmouth-Hitchcock Medical Center responding to a state-mandated lockdown by postponing or transitioning all nonessential care to telemedicine on March 18, 2020. Restrictions continued until April 30, 2020, at which point efforts sought to normalize care volumes through screening processes and visitor restrictions while continuing to incorporate telemedicine where appropriate.
Sampling and Recruitment
Across the 5 clinic locations, we recruited a purposive sample of oncology clinical providers, leaders, regional practice managers overseeing telemedicine implementation, and nonphysician staff (eg, schedulers and nurse managers) to participate in semistructured interviews. Of the 67 medical doctors and nurse practitioners employed at DCC who used some amount of telemedicine between January 2020 and October 2020, we sampled 30 clinical providers representing low-to-high telemedicine use, a mix of oncology specialties, the 5 clinic locations, and varied proportion of rural patients served. Leaders, practice managers, and clinical providers were recruited through direct email invitations from study leaders. Following interviews with regional managers, we used snowball sampling to identify a mix of other nonphysician staff members representing all 5 clinics with direct experience supporting telemedicine during the pandemic.
Data Collection and Analysis
We used the Consolidated Framework for Implementation Research (CFIR) to inform data collection and analysis. The CFIR includes over 30 evidence-based constructs grouped within the 5 domains of intervention characteristics, outer setting, inner setting, characteristics of individuals targeted by the intervention, and the process of implementation. The CFIR was developed to examine complex interventions across different settings, including hospitals as well as primary care and telehealth settings [30-34]. We created 2 semistructured interview guides for providers and staff, which explored constructs from all 5 CFIR domains with particular emphasis on identifying barriers and facilitators to telemedicine uptake and sustainability associated with inner or outer contextual factors; telemedicine technology and functionality; provider experiences, knowledge, and attitudes toward telemedicine technology given typical clinical workflows; perceptions of patient and caregiver attitudes and capabilities in using the technology; and overall implementation processes and adaptability. Following the creation of the guides and use in a few initial interviews, we made final modifications to the question wording and probes to improve interview clarity, flow, and consistency among the interviewers.
Two researchers (DV and RB) conducted semistructured interviews with cancer care providers, and 1 researcher (JAT) conducted all staff interviews. None of the interviewers had explicit clinical experience or roles within the institution, and all of them were unknown to interview participants. All 3 researchers listened to a sample of the early interviews and then met to debrief, adjust the interview guide as noted above, and reach consensus on interview methods before completing future interviews. The interviews lasted approximately 45 minutes and were recorded with participant permission for later transcription.
We used the web-based transcription service, “Rev.com,” to create word-for-word transcripts of the interviews that were then uploaded into the qualitative analysis platform Dedoose (Socio Cultural Research Consultants, LLC). Two researchers (DV and RB) coded and analyzed the transcripts using mixed inductive and deductive methods [35,36]. The 2 researchers coded a sample of transcripts separately and then met to debrief and reach consensus. The researchers used a mix of in-person debriefing sessions, emails, and internal Dedoose memos to reach consensus and discuss any personal or professional biases that arose throughout the analysis process. If the coding team was unable to reach consensus, additional members of the study team were consulted (JAT, MM, and AT). Thematic saturation was reached across the full sample as evidenced by no new themes and subthemes coming forth in exploring the main CFIR constructs of interest [37].
Ethical Considerations
The study received Dartmouth Health institutional review board approval (STUDY 02000578). The participants were provided with an information sheet and opportunity to ask questions prior to participation. The participants were verbally consented, and permission was obtained for the audio recording of interviews. Names, titles, and practice settings were deidentified and will not be included in any published data.
Results
Overview
We conducted 11/20 (55%) provider, 3/20 (15%) leader, and 6/20 (30%) staff interviews (Table 2). All providers reported adopting some form of telemedicine for a significant proportion of visits in the early months of the pandemic (March-June 2020), predominantly via phone (vs video), consistent with our prior quantitative analysis [24]. Telemedicine use (video and phone combined) by the providers in this sample also mirrored that of the entire DCC oncology service, which peaked at an average weekly telehealth visit rate of 26% of visits in the initial lockdown phase, settling out to an average of 10%-12% after lockdown [23].
Table 2.
Interview participant demographics.
| Category | Value, n (%) | |
| Position level |
|
|
|
|
Provider (medical doctors and nurse practitioners) | 11 (55) |
|
|
Leaders or practice managers | 3 (15) |
|
|
Staff (schedulers and registered nurse managers) | 6 (30) |
| Provider telemedicine use | ||
|
|
Low (0%-9%) | 3 (28) |
|
|
Medium (10%-17%) | 4 (36) |
|
|
High (18%-30%) | 4 (36) |
| Years in role |
|
|
|
|
Less than 1 | 5 (25) |
|
|
1-4 | 5 (25) |
|
|
5-10 | 3 (15) |
|
|
11-15 | 2 (10) |
|
|
>15 | 4 (20) |
|
|
Not specified | 1 (5) |
The qualitative findings shed light on these use patterns. Figure 1 presents the factors having the greatest influence on uptake and sustained use of telemedicine that emerged in the staff and provider narratives by the CFIR domain. Figure 2 presents a summary of the factors across all CFIR domains, which emerged as either facilitating telemedicine use or acting as barriers dissuading or constraining telemedicine use. The results are summarized below; select quotations can be found in Table 3.
Figure 1.
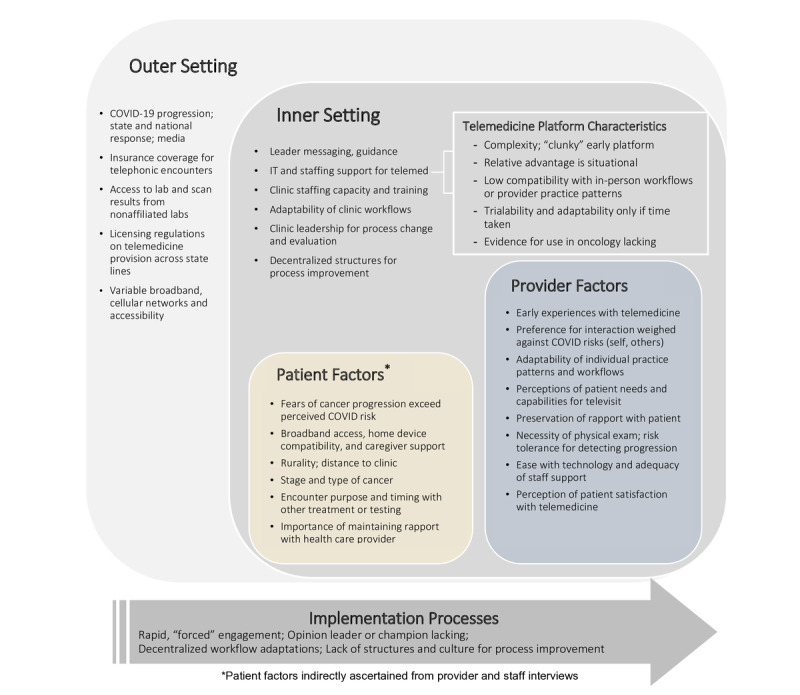
Main themes organized by the Consolidated Framework for Implementation Research construct; telemed: telemedicine.
Figure 2.
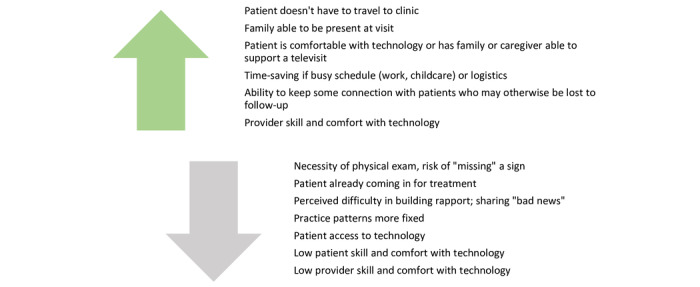
Facilitators and barriers to telemedicine uptake.
Table 3.
Selected quotations organized by the Consolidated Framework for Implementation Research (CFIR) domain.
| CFIR domain | Exemplar quotes |
| Outer setting |
|
| Inner setting |
|
| Intervention characteristics |
|
| Characteristics of individuals |
|
| Implementation processes |
|
Outer Setting Factors
The pandemic and the associated public health response by national, state, and institutional leaders were both a trigger for implementing telemedicine and a source for widespread disruption in usual clinical practice workflows. All interview participants described a considerable amount of initial confusion in the transition to telemedicine due to questions about reimbursement allowances (eg, whether Medicaid, Medicare, or commercial insurances covered telephone as well as video visits, and whether payment rates would be the same as in-person care) and provider licensing regulations, compounded by mixed media messages and unknowns related to the spread and exposure of the virus. Another challenge to the early transition reported mainly by staff participants was associated with the shift by many patients to using local, nonaffiliated clinics for lab testing, which were not linked to the electronic health record. Providers and staff alike reported this necessitated additional staff time to obtain and integrate results into the record for providers to have during a telemedicine visit; if it was not obtained, it caused scheduling disruptions. Another external setting factor that emerged was the major policy change allowing reimbursement of telephonic visits at rates on par with in-person or video-based visits. This policy change was a key factor to overcoming technology frustrations experienced in early video visits and was cited by most respondents, providers, and staff alike, as it is important for the sustained use of telemedicine in oncology.
Inner Setting Factors
According to the participants, practice and provider workflows for using telemedicine were nonexistent at all oncology clinics at the start of the pandemic. Existing clinic workflows for in-person visits were reported to be largely incompatible with the new flows needed for the telehealth transition, and staff and providers alike wished for greater guidance from DCC leaders to help with initial implementation. As noted above, there was considerable confusion and questions about which visits could and could not be carried out via telemedicine in the early days, and the respondents overall reported a lack of clear direction or support from an internal champion to address questions. Moreover, the providers reported that the overall pace of care did not allow for dedicated time to effectively engage with training materials on their own, and there was no institution-wide push to ensure all providers complete telemedicine training.
Clinic leaders, schedulers, and providers reported taking matters into their own hands to develop ad hoc strategies to make the shift to telemedicine early on. Workflows and staff responsibilities were modified to support telemedicine visits. On some teams, staff were tasked with calling patients in advance to prepare them for the telemedicine visit, practice with the technology, and gather medication and medical history information; however, clinics rarely had the staffing resources to carry this out consistently. As the pandemic evolved, clinic teams continued to refine internal workflows, patient messaging, and coordination with new lab vendors to support telemedicine use, all with a high degree of variation across clinics and largely based on local preferences of providers and perceived patient needs.
Intervention (Telemedicine) Characteristics
Provider dissatisfaction with the telemedicine user interface, particularly with video visits in the early days of the pandemic, emerged as a critical variable in determining ultimate use of the technology for patient care and preferences for phone over video. The providers described the telemedicine platform in use at the onset of the pandemic as “clunky” and requiring multiple steps to log in. Many reported quickly transitioning to phone visits because of the technical challenges both they and their patients encountered with the video interface, citing frustration and wasted time trying to establish and maintain a successful video connection. Even after a year, a few providers in our sample had still not conducted a video visit after hearing about colleagues’ experiences. Following an institutional switch to a different telemedicine platform in the summer of 2020, the participants reported improved connectivity and visit satisfaction, although not enough to convince those more hesitant with the technology to reattempt video visits.
Telemedicine was perceived as holding relative advantage over in-person visits for some clinical situations described below. Moreover, the technology offered providers time savings and greater flexibility in scheduling visits around research, meetings, and serving multiple clinic sites, while it was also reported as reducing travel demands for rural patients and those with busy work, home, and school schedules. Despite these advantages, respondents wanted more evidence of the efficacy of telemedicine, particularly in the context of cancer care where many feared missing disease progression when conducting clinical exams virtually.
Trialability and adaptability with the telemedicine technology happened to varying degrees among clinic teams as reported in the interviews. Novel uses for telemedicine in oncology care emerged during the implementation, most notably in the form of what was locally referred to as “chemo teaches” (meetings to prepare patients and caregivers on what to expect while undergoing chemotherapy) and other patient or family education. Telemedicine allowed family members who were geographically remote or working to participate in education sessions and visits. The easing of state licensure restrictions (also an external setting factor) enabled several providers to provide telemedicine consultation to patients outside of their usual geographic area, supporting continuity for patients who needed to travel as well as enabling new consultations and second opinions.
Characteristics of Individuals
Provider-Level Characteristics and Preferences
Of all the factors influencing telemedicine uptake and implementation, provider preference had the greatest effect on both the ratio of telemedicine to in-person visits and the modality of those telemedicine visits (phone vs video). A combination of early negative experiences with video, comfort with technology (or lack thereof), convenience, and perceptions of patient preferences contributed to a majority of providers in our sample, almost exclusively opting for telephonic visits if in-person visits were not possible.
Preferences were also influenced by attitudes around risk of COVID-19 exposure (self, staff, and patients) balanced against the degree to which providers valued direct patient interactions to connect with patients and assess clinical conditions. Provider willingness to experiment with the technology and adapt individual practice workflows was more of a predictor of telemedicine use than clinical specialty or years in practice (ie, provider age).
Most providers in our sample felt it was harder to achieve their preferred level of rapport with their patients in televisits (phone or video), though some found video visits afforded new ways to connect with patients by observing them in home settings and family encounters. For difficult conversations or when health literacy was in question, in-person and video visits were universally preferred. In the narratives, providers often couched their own preferences around supporting their patients’ preferences (real or perceived). The providers reported using patient preferences to determine visit type yet acknowledged that patient willingness to use telemedicine (either telephonic or video) could be modified by messaging about the different options during appointment scheduling.
Perceptions of Patient-Level Characteristics and Preferences
A patient’s geographic distance to the medical center had a mixed effect on telemedicine use. According to staff and providers, for some rural patients, telemedicine offered a solution against frequent, lengthy trips into the clinic for more routine visits (especially in poor weather conditions or when transportation assistance was needed). For other patients, the providers cited reports of poor internet connectivity or cell service, which hindered telemedicine use. The participants gave examples that suggested they would assess a patient’s skill or comfort with technology in determining whether to offer a telemedicine visit. Older, rural patients were reported to be more likely to choose phone or in-person visits rather than using telemedicine technology because of a lack of familiarity with technology. Family or caregiver support (eg, in assisted living settings) was observed by staff and providers to buffer against technology challenges. Younger patients were cited as being more willing to engage with technology but were constrained by other factors, including busy work and family schedules that led providers to offer telephonic visits more often than video visits.
Staff and providers agreed regarding the clinical situations better suited for telemedicine. These included patients with less aggressive or more stable cancers such as hematological cancers; cancers for which a physical exam was less important because scans or lab results largely dictated treatment decisions; patients in remission; or clinical situations where visits could reasonably alternate between in-person and telemedicine (eg, if the patient needed monthly monitoring). These considerations are summarized in Table 4.
Table 4.
Situations in cancer care better and worse suited to telemedicine use, as reported from staff and provider interviews.
| Category | Better for telemedicine | Worse for telemedicine |
| Cancer type |
|
|
| Visit type |
|
|
aGI: gastrointestinal.
bGU: genitourinary.
For more rapidly progressing cancers such as breast, gastrointestinal or genitourinary, as well as head and neck cancers, the providers had a strong preference for seeing their patients in person, as they were concerned they may miss important disease progression that could influence treatment decisions. In these cases, they reported a heavy reliance on the physical exam and other aspects of an in-person visit to assess a patient’s response to and tolerance of treatment, especially around important decision points in care.
Overall, providers and staff reported that while telemedicine can be incorporated into oncology care, the nature of oncology and the fact that patients with oncology-related needs are already coming in for treatment do not lend themselves to a high level of telemedicine adoption. Patient and provider perceptions of confidentiality and privacy concerns in using technology did not emerge as a main theme in this study.
Implementation Processes
Interview participants voiced a desire for a clear vision for telemedicine use in oncology, substantiated by evidence, supported by recognized champions, and standardized through official policies such as continued reimbursement for telephonic visits in specific clinical situations.
Logistical and workflow improvement recommendations included staff support to virtual “room” patients at the start of a telemedicine visit, establishing dedicated space for televisits, where equipment was already set up, establishing preappointment protocols and scheduling processes to ensure patients were adequately prepared, and clarifying roles and training to ensure clinics had the capacity to support both in-person and televisits in a smooth fashion. Challenges and burdens of staff time in obtaining lab results from outside vendors indicate a need for formal partnerships, data sharing agreements, and integrated electronic systems to share results more efficiently.
The participants identified a need to continue to improve accessibility and ease in using the telemedicine technology for patients and providers alike. Translation services were a challenge for some to incorporate within telemedicine visits. The providers voiced a need for more training and peer-to-peer learning opportunities to gain greater ease in adjusting their visit flow, maximizing the information obtained from patients in a digital setting, and ensuring understanding on the part of patients and caregivers.
Discussion
Principal Findings
Telemedicine use in oncology, as characterized by the participants in this sample, reflected a complex interaction of multiple factors beyond pandemic-specific circumstances. A relative void of institutional steering and support allowed provider opinions about the relative benefit (eg, patient convenience or improved access) and risks (eg, concerns about compromising clinical care quality, impaired rapport building, and reduced communication quality) to drive variable use of telemedicine. A larger context of no clear oncologic standard of care pertaining to the efficacy and safety of telemedicine to fall back on further enabled a wide range of opinions and practices. These dynamics were skewed by technology challenges early in adoption, which led to preferential engagement with telephone over video for visit modality.
While there were clear positive impressions of telemedicine among staff and providers to support its ongoing use, at the time of this study, there were no significant continuing efforts or conversations among care teams or at a center- or department-wide level around long-term adaptation for sustained use. The presence of a local champion (an individual on work units who formally or informally promotes a process or intervention to their colleagues) is generally regarded as important to successful and sustained adoption of telemedicine [38,39] and is a core part of the “diffusion of innovation” model as put forth by Rogers and expanded upon by Greenhalgh et al [40]; such an individual was not apparent within the oncology department in our interviews. Study team members involved in telehealth deployment across this period noted that telemedicine champions seemed to already exist and emerge organically in other services at the organization; it is unclear precisely why this did not occur for oncology at our center, and a proactive effort to identify or designate a champion would be useful for future innovation efforts. Organizational learning and process improvement specific to telemedicine was slow to emerge, and expanded messaging and infrastructure in these areas could facilitate sustained, ongoing process improvement. Such approaches could provide an opportunity to revisit and shift some of the patterns set early in pandemic-forced adoption toward patient-oriented and shared goals (eg, minimizing frequency of patient transport when clinically feasible) and away from anecdotal impressions of care team members (eg, assumptions that certain patients or demographics are best served via face-to-face or telemedicine without directly eliciting preferences, or telemedicine use depending on provider comfort with technology rather than clinical context).
Comparison With Prior Work
Organizational barriers may explain in part the differences in telemedicine use in our study versus the work by Patt et al [15], who reported less significant barriers in uptake and a >95% reported rate of video telemedicine use in a survey-based study; our study furthers theirs in that it used in-depth semistructured interviews rather than a survey tool to gather data for analysis.
Our findings align with larger theoretical frameworks around the implementation of novel processes and innovations, including the CFIR model as well as diffusion of innovation. These models all support the complex interplay of a myriad of factors influencing the success of any innovation and underscore the advantages of being able to plan and prepare for systemwide transitions such as this; such a preparation was not possible with the sudden shift in patient care necessitated by the COVID-19 pandemic. We noted the most influential factors pertaining to our rural, multisite cancer center above, including elements specific to the innovation itself (technologic challenges and the impacts of using telemedicine for the patient encounter), communication channels (a relative lack of leadership or operational support both in implementation and ongoing improvement work), and adopters (individual attitudes and motivations to adopt change).
Limitations
While DCC serves a broad population base, most of the patients are located within Northern New England, and it is quite likely that other institutions with their own distinct populations and institutional cultures will have different challenges and opportunities. It is also possible that implementation in other circumstances without the rapid adoption due to a pandemic may have different dynamics and key factors for implementation. Our sample was sufficient to reach thematic saturation on major themes, but it leaves room for a more detailed exploration of some of the subthemes that emerged, including variation in provider messaging to patients around the visit options (in-person, telephonic, and video), provider and staff comfort with technology, and specific operational practices to minimize schedule disruption associated with telemedicine visits.
While staff and providers offered important insights to the attitudes, challenges, needs, and feedback of their patients, we did not directly interview patients for this study. It is notable that studies such as that carried out by Smith et al [28] included patients and caregivers in their interviews and found similar themes to our work regarding the acceptability and efficiency of telehealth generally for cancer care, alongside concerns regarding the lack of physical exam. Further investigation and analysis of patient perceptions of telemedicine use in cancer care—especially as we transition to a postpandemic environment where more patients are familiar with telemedicine and novelty—should further extend understanding of the risks and benefits of telemedicine use in oncology settings to equitably serve the needs of diverse populations.
Conclusion
In a setting of decentralized care processes, early challenges in telemedicine implementation had an outsized impact on the nature and amount of sustained use. Proactively designed telemedicine care processes with attention to patient needs will be essential to supporting a sustained role for telemedicine in cancer care.
Acknowledgments
The team wishes to thank Jennifer Snide for her support in the quantitative analysis that helped inform this study. We would also like to thank Stephen D Leach for championing the study’s recruitment efforts.
This work was supported by the National Cancer Institute at the National Institutes of Health (P30CA023108-S5). The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The research team had full autonomy in all aspects of the study.
Abbreviations
- CFIR
Consolidated Framework for Implementation Research
- DCC
Dartmouth Cancer Center
Footnotes
Authors' Contributions: MM, RB, JAAT, KMC, ML, TDT, WZ, and ANAT conceptualized the study; RB, DV, JAAT, TDT, and WZ participated in data curation and formal analysis; ANAT was responsible for funding acquisition; RB, DV, JAAT, and KMC carried out the study investigation. MM, RB, DV, JAAT, KMC, ML, and ANAT contributed to study methodology; RB, DV, JAAT, KMC, and ML carried out project administration; RB, DV, JAAT, KMC, ML, TDT, WZ, and ANAT where responsible for providing resources; RB and DV provided software support; ANAT carried out study supervision; MM, RB, DV, JAAT, KMC, and ML validated the study; MM and RB carried out study visualization; MM, RB, DV, and JAAT where responsible for writing—original draft; and MM, RB, DV, JAAT, KMC, ML, TDT, WZ, and ANAT carried out the writing—review and editing.
Conflicts of Interest: None declared.
References
- 1.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020 Apr 30;382(18):1679–1681. doi: 10.1056/nejmp2003539. [DOI] [PubMed] [Google Scholar]
- 2.Gelburd R. The Coronavirus Pandemic and the Transformation of Telehealth. U.S. News. 2020. Jun 02, [2022-07-29]. https://www.usnews.com/news/healthiest-communities/articles/2020-06-02/covid-19-and-the-transformation-of-telehealth .
- 3.Elkaddoum R, Haddad FG, Eid R, Kourie HR. Telemedicine for cancer patients during COVID-19 pandemic: between threats and opportunities. Future Oncol. 2020 Jun;16(18):1225–1227. doi: 10.2217/fon-2020-0324. https://www.futuremedicine.com/doi/abs/10.2217/fon-2020-0324?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latifi R, Doarn CR. Perspective on COVID-19: finally, telemedicine at center stage. Telemed J E Health. 2020 Sep 01;26(9):1106–1109. doi: 10.1089/tmj.2020.0132. [DOI] [PubMed] [Google Scholar]
- 5.Doolittle GC, Allen A. Practising oncology via telemedicine. J Telemed Telecare. 1997 Jun 23;3(2):63–70. doi: 10.1258/1357633971930869. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez J, Kuperman E, Kaboli PJ. Using telehealth as a tool for rural hospitals in the COVID-19 pandemic response. J Rural Health. 2021 Jan;37(1):161–164. doi: 10.1111/jrh.12443. http://europepmc.org/abstract/MED/32277777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabesan S. Medical models of teleoncology: current status and future directions. Asia Pac J Clin Oncol. 2014 Sep 17;10(3):200–4. doi: 10.1111/ajco.12225. [DOI] [PubMed] [Google Scholar]
- 8.Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. American Society of Clinical Oncology Educational Book. 2018 May;(38):540–545. doi: 10.1200/edbk_200141. [DOI] [PubMed] [Google Scholar]
- 9.Worster B, Swartz K. Telemedicine and palliative care: an increasing role in supportive oncology. Curr Oncol Rep. 2017 Jun 18;19(6):37. doi: 10.1007/s11912-017-0600-y.10.1007/s11912-017-0600-y [DOI] [PubMed] [Google Scholar]
- 10.Cox A, Lucas G, Marcu A, Piano M, Grosvenor W, Mold F, Maguire R, Ream E. Cancer survivors' experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017 Jan 09;19(1):e11. doi: 10.2196/jmir.6575. https://www.jmir.org/2017/1/e11/ v19i1e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beswick DM, Vashi A, Song Y, Pham R, Holsinger FC, Rayl JD, Walker B, Chardos J, Yuan A, Benadam-Lenrow E, Davis D, Sung CK, Divi V, Sirjani DB. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016 Jun 21;38(6):925–9. doi: 10.1002/hed.24386. [DOI] [PubMed] [Google Scholar]
- 12.Levit LA, Byatt L, Lyss AP, Paskett ED, Levit K, Kirkwood K, Schenkel C, Schilsky RL. Closing the rural cancer care gap: three institutional approaches. JCO Oncology Practice. 2020 Jul;16(7):422–430. doi: 10.1200/op.20.00174. [DOI] [PubMed] [Google Scholar]
- 13.Stacey D, Carley M, Ballantyne B, Skrutkowski M, Whynot A, Pan-Canadian Oncology Symptom TriageRemote Support COSTaRS Team Perceived factors influencing nurses' use of evidence-informed protocols for remote cancer treatment-related symptom management: A mixed methods study. Eur J Oncol Nurs. 2015 Jun;19(3):268–77. doi: 10.1016/j.ejon.2014.11.002. https://linkinghub.elsevier.com/retrieve/pii/S1462-3889(14)00191-4 .S1462-3889(14)00191-4 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez JA, Betancourt JR, Sequist TD, Ganguli I. Differences in the use of telephone and video telemedicine visits during the COVID-19 pandemic. Am J Manag Care. 2021 Jan 14;27(1):21–26. doi: 10.37765/ajmc.2021.88573. https://www.ajmc.com/pubMed.php?pii=88573 .88573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patt D, Wilfong L, Kanipe K, Paulson RS. Telemedicine for cancer care: implementation across a multicenter community oncology practice. Am J Manag Care. 2020 Dec 18;26(10 Spec No):SP330–SP332. doi: 10.37765/ajmc.2020.88560. https://www.ajmc.com/pubMed.php?pii=88560 .88560 [DOI] [PubMed] [Google Scholar]
- 16.New Hampshire Medicaid Telehealth Fact Sheet during COVID-19. State of New Hampshire. [2022-07-29]. https://www.nhadaca.org/Announcements/8842951 .
- 17.NH Medicaid Telehealth Informational Bulletin COVID-19 Preparedness and Response. New Hampshire Department of Health and Human Services. [2022-07-29]. https://www.dhhs.nh.gov/sites/g/files/ehbemt476/files/documents/2021-11/telehealth040220.pdf .
- 18.In Depth: Telehealth and COVID19 in N.H. New Hampshire Public Radio. 2020. [2022-07-29]. https://www.nhpr.org/post/depth-covid-19-and-telemedicine-nh#stream/0 .
- 19.Telehealth. Department of Vermont Health Access. [2022-07-29]. https://dvha.vermont.gov/providers/telehealth .
- 20.New Hampshire telemedicine policy, simplified. eVisit. [2022-07-29]. https://evisit.com/state-telemedicine-policy/new-hampshire/
- 21.2020 COVID-19 Emergency Orders. State of New Hampshire. [2022-07-29]. https://www.governor.nh.gov/news-and-media/covid-19-emergency-orders-2020 .
- 22.March 2021 report to the Congress: Medicare payment policy. Medpac. 2021. [2022-07-29]. https://www.medpac.gov/document/march-2021-report-to-the-congress-medicare-payment-policy/
- 23.Curtis K, Alford-Teaster J, Lowry M, Mackwood M, Snide J, Tosteson T, Tosteson A. Pandemic use of telehealth by oncology at a rural academic medical center. Telemed J E Health. 2022 Apr;28(4):501–508. doi: 10.1089/tmj.2020.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackwood MB, Tosteson TD, Alford-Teaster JA, Curtis KM, Lowry ML, Snide JA, Zhao W, Tosteson AN. Factors influencing telemedicine use at a Northern New England cancer center during the COVID-19 pandemic. JCO Oncology Practice. 2022 Jul;18(7):e1141–e1153. doi: 10.1200/op.21.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchett J, Borah BJ, Dholakia R, Moriarty JP, Ahn H, Huang M, Khera N, Kharfan-Dabaja M, Ticku J, Leppin AL, Tilburt JC, Paludo J, Haddad TC. Patient- and provider-level factors associated with telehealth utilization across a multisite, multiregional cancer practice. JCO. 2022 Jun 01;40(16_suppl):1512–1512. doi: 10.1200/jco.2022.40.16_suppl.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jewett PI, Vogel RI, Ghebre R, Hui JYC, Parsons HM, Rao A, Sagaram S, Blaes AH. Telehealth in cancer care during COVID-19: disparities by age, race/ethnicity, and residential status. J Cancer Surviv. 2022 Feb 20;16(1):44–51. doi: 10.1007/s11764-021-01133-4. http://europepmc.org/abstract/MED/34800257 .10.1007/s11764-021-01133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arem H, Moses J, Cisneros C, Blondeau B, Nekhlyudov L, Killackey M, Pratt-Chapman ML. Cancer provider and survivor experiences with telehealth during the COVID-19 pandemic. JCO Oncology Practice. 2022 Apr;18(4):e452–e461. doi: 10.1200/op.21.00401. [DOI] [PubMed] [Google Scholar]
- 28.Smith S, Smith A, Kennett W, Vinod SK. Exploring cancer patients', caregivers', and clinicians' utilisation and experiences of telehealth services during COVID-19: A qualitative study. Patient Educ Couns. 2022 Jun 06;:A. doi: 10.1016/j.pec.2022.06.001. http://europepmc.org/abstract/MED/35688719 .S0738-3991(22)00263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidelines for using rural-urban classification systems for community health assessment. Washington State Department of Health. [2022-07-29]. https://www.doh.wa.gov/Portals/1/Documents/1500/RUCAGuide.pdf .
- 30.Birken SA, Powell BJ, Presseau J, Kirk MA, Lorencatto F, Gould NJ, Shea CM, Weiner BJ, Francis JJ, Yu Y, Haines E, Damschroder LJ. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the Theoretical Domains Framework (TDF): a systematic review. Implement Sci. 2017 Jan 05;12(1):2. doi: 10.1186/s13012-016-0534-z. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-016-0534-z .10.1186/s13012-016-0534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009 Aug 07;4(1):50. doi: 10.1186/1748-5908-4-50. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-4-50 .1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR) Implement Sci. 2013 May 10;8(1):51. doi: 10.1186/1748-5908-8-51. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-8-51 .1748-5908-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keith RE, Crosson JC, O'Malley AS, Cromp D, Taylor EF. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci. 2017 Feb 10;12(1):15. doi: 10.1186/s13012-017-0550-7. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-017-0550-7 .10.1186/s13012-017-0550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damschroder LJ, Reardon CM, Sperber N, Robinson CH, Fickel JJ, Oddone EZ. Implementation evaluation of the Telephone Lifestyle Coaching (TLC) program: organizational factors associated with successful implementation. Transl Behav Med. 2017 Jun 29;7(2):233–241. doi: 10.1007/s13142-016-0424-6. http://europepmc.org/abstract/MED/27688249 .10.1007/s13142-016-0424-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. International Journal of Qualitative Methods. 2016 Nov 29;5(1):80–92. doi: 10.1177/160940690600500107. [DOI] [Google Scholar]
- 36.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007 Aug;42(4):1758–72. doi: 10.1111/j.1475-6773.2006.00684.x. http://europepmc.org/abstract/MED/17286625 .HESR684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guest G, Bunce A, Johnson L. How many interviews are enough? Field Methods. 2016 Jul 21;18(1):59–82. doi: 10.1177/1525822x05279903. [DOI] [Google Scholar]
- 38.Brewster L, Mountain G, Wessels B, Kelly C, Hawley M. Factors affecting front line staff acceptance of telehealth technologies: a mixed-method systematic review. J Adv Nurs. 2014 Jan 20;70(1):21–33. doi: 10.1111/jan.12196. [DOI] [PubMed] [Google Scholar]
- 39.Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012 Dec 01;18(8):490–2. doi: 10.1258/jtt.2012.gth115.jtt.2012.GTH115 [DOI] [PubMed] [Google Scholar]
- 40.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly. 2004 Dec;82(4):581–629. doi: 10.1111/j.0887-378x.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


