Abstract
The dcuC gene of Escherichia coli encodes an alternative C4-dicarboxylate carrier (DcuC) with low transport activity. The expression of dcuC was investigated. dcuC was expressed only under anaerobic conditions; nitrate and fumarate caused slight repression and stimulation of expression, respectively. Anaerobic induction depended mainly on the transcriptional regulator FNR. Fumarate stimulation was independent of the fumarate response regulator DcuR. The expression of dcuC was not significantly inhibited by glucose, assigning a role to DcuC during glucose fermentation. The inactivation of dcuC increased fumarate-succinate exchange and fumarate uptake by DcuA and DcuB, suggesting a preferential function of DcuC in succinate efflux during glucose fermentation. Upon overexpression in a dcuC promoter mutant (dcuC*), DcuC was able to compensate for DcuA and DcuB in fumarate-succinate exchange and fumarate uptake.
Escherichia coli contains four different secondary carriers (DcuA, DcuB, DcuC, and DctA) for C4-dicarboxylates (3, 4, 22, 26). DctA is used for aerobic growth on C4-dicarboxylates (3, 12), whereas the Dcu carriers (encoded by the dcuA, dcuB, and dcuC genes) are used under anaerobic conditions and form a distinct family of carriers (4, 5, 18, 22, 25, 26). Each of the Dcu carriers is able to catalyze the uptake, antiport, and possibly also efflux of C4-dicarboxylates. DcuB is the major C4-dicarboxylate carrier for fumarate respiration with high fumarate-succinate exchange activity. It is synthesized only in the absence of oxygen and nitrate and in the presence of C4-dicarboxylates (4, 6, 7, 27). DcuA is expressed constitutively in aerobic and anaerobic growth and can substitute for DcuB (7, 22). DcuC shows the same transport modes as DcuA and DcuB (exchange, uptake, and presumably efflux of C4-dicarboxylates) (26), but the transport activities are significantly lower than for DcuA and DcuB. Thus, a mutant lacking DcuA and DcuB was severely inhibited for growth by fumarate respiration due to the limited transport activities of DcuC, whereas DcuA and DcuB were able to maintain full growth under these conditions (22, 26). These findings suggest a different physiological role for DcuC and use under different conditions. To obtain a clue as to the role of DcuC, the functions of Dcu and the conditions for Dcu synthesis were studied.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
For growth experiments and transport assays, the bacteria (Table 1) were grown under aerobic or anaerobic conditions in M9 mineral medium supplemented with acid-hydrolyzed casein (0.05%) and tryptophan (0.005%) (1, 24). Glucose (10 mM), glycerol (50 mM), sodium C4-dicarboxylates such as fumarate or succinate (50 mM), and sodium nitrate (50 mM) were included as needed.
TABLE 1.
Strains of E. coli used
| Strain | Genotype | Reference or source |
|---|---|---|
| AN387 | Parental | 1, 24 |
| MC4100 | F−araD138 Δ(argF-lac)U169 rpsL150 relA1 flbB530 deoC1 ptsF25 rbsR | 21 |
| RM101 | MC4100 but Δfnr | 19 |
| RM313 | MC4100 but arcA1 zjj::Tn10 | 19 |
| RM600 | MC4100 but narL215::Tn10 | 19 |
| VJS4322 | RK4353 but narP253::Tn10d Δ(narXL)253 | 13 |
| IMW153 | AN387 but dcuA::SpcrdcuB::KanrdcuC::mini-Tn10 (Camr) | 26 |
| IMW152 | AN387 but dcuA::SpcrdcuB::KanrdcuC* (IS5 upstream of dcuC) | This work |
| JRG2814 | AN387 but dcuA::SpcrdcuB::Kanr | 22 |
| IMW240 | MC4100 λ[Φ(dcuC′-′lacZ)] | This work |
| IMW256 | MC4100 λ[Φ(dcuC′-′lacZ)] but ΔarcA | P1 (RM313) × IMW240 |
| IMW257 | MC4100 λ[Φ(dcuC′-′lacZ)] but narL::Tetr | P1 (RM600) × IMW240 |
| IMW258 | MC4100 λ[Φ(dcuC′-′lacZ)] but narP::Camr | P1 (VJS4322) × IMW240 |
| IMW255 | MC4100 λ[Φ(dcuC′-′lacZ)] but Δfnr | RM101 × λdcuC′-′lacZ |
| IMW241 | MC4100 λ[Φ(dcuC′-′lacZ)] but dcuR::Kanr | P1 (IMW205) × IMW240 |
| IMW201 | MC4100 λ[Φ(dcuC*′-′lacZ)] | This work |
| IMW202 | MC4100 λ[Φ(dcuC*′-′lacZ)] but Δfnr | RM101 × λdcuC*′-′lacZ |
| IMW271 | MC4100 λ[Φ(dcuC*′-′lacZ)] but ΔarcA | RM313 × λdcuC*′-′lacZ |
| IMW272 | MC4100 λ[Φ(dcuC*′-′lacZ)] but ΔnarL | RM600 × λdcuC*′-′lacZ |
| IMW205 | MC4100 but dcuR::Kanr | 27 |
| IMW242 | MC4100 λ[Φ(dcuC′-′lacZ)] but citB::Spcr | P1 (IMW220) × IMW240 |
| IMW272 | MC4100 but citB::Spcr | 27 |
Genetic procedures and DNA manipulation.
The dcuC′-′lacZ fusion strain (strain IMW240) was constructed by PCR amplification of the promoter region of dcuC from genomic DNA of strain AN387 (24) with primers dcuCBam (5′-CCC CAA TAA GGA TCC CAA TG), introducing a BamHI site, and dcuCEco (5′-CCA GCG GTG AAT TCC AGA CC), introducing an EcoRI site. The resulting 1.1-kb fragment was cloned into the BamHI and EcoRI sites of the protein fusion vector pJL29 (15), yielding pMW98. The corresponding dcuC*′-′lacZ fusion (strain IMW201) was made in the same way from genomic DNA of strain IMW152 with primers dcuCEcoV (5′-GCT ATC CAG GGA TAT CCG GG), introducing an EcoRV site, and primer dcuCBam. The resulting 0.5-kb DNA fragment was cloned into the SmaI and BamHI sites of pJL29 to create plasmid pMW122. The gene fusions were transferred to the genome of E. coli MC4100 with phage λRZ5 (1, 17, 21). P1 transduction was performed as described previously (1) and checked by PCR and Southern blot analysis (22, 27).
Transport assays.
Transport of C4-dicarboxylates in cell suspensions of bacteria was measured by silicone oil centrifugation. For measurement of exchange, the bacteria were loaded with succinate and the uptake of [14C]fumarate was measured by silicone oil centrifugation (5, 22, 26). Uptake was measured by adding [14C]fumarate to energized bacteria and monitoring the increase in internal [14C]fumarate levels by silicone oil centrifugation (22, 26).
RNA isolation and primer extension.
Total RNA was isolated with the RNeasy mini kit (Qiagen). The 5′ end of the mRNA encoded by the dcuC or dcuC* gene was mapped by primer extension with primer cpe2 (5′-GAG CTC AAT GAA TGT CAG CAT AAT TTT TCC-3′), which is complementary to positions 107 to 136 of dcuC in the transcript. The primer extension was performed at 37°C for 1 h with 20 U of Moloney murine leukemia virus reverse transcriptase and [γ-32P]dATP. The extension products were purified by ethanol precipitation and subjected to denaturing polyacrylamide gel electrophoresis.
RESULTS AND DISCUSSION
Expression of dcuC′-′lacZ in response to electron acceptors and the C source.
The conditions for DcuC expression were studied with a dcuC′-′lacZ reporter gene fusion (Fig. 1). The dcuC gene was fused in frame to ′lacZ to obtain a translational protein fusion. The fusion contained the complete promoter region up to position −971 and seven codons of dcuC. The dcuC′-′lacZ fusion was inserted with phage λRZ5 into the genome of E. coli MC4100, and monolysogenic strains were used (Table 2). During anaerobic growth, dcuC was expressed with relatively high activities, but the presence of O2 caused complete repression irrespective of the growth substrate. During anaerobic growth, the addition of fumarate increased expression about twofold, whereas electron acceptors such as nitrate, dimethyl sulfoxide, and trimethylamine N-oxide (TMAO) caused slight repression (Table 2). Malate, aspartate, asparagine, and tartrate stimulated expression in a manner similar to that of fumarate (data not shown), whereas other carboxylic acids, including malonate, did not cause induction. When glucose was replaced during anaerobic growth by glycerol or other C sources, the expression of dcuC increased only negligibly, indicating that dcuC is not subject to glucose repression (Table 2).
FIG. 1.
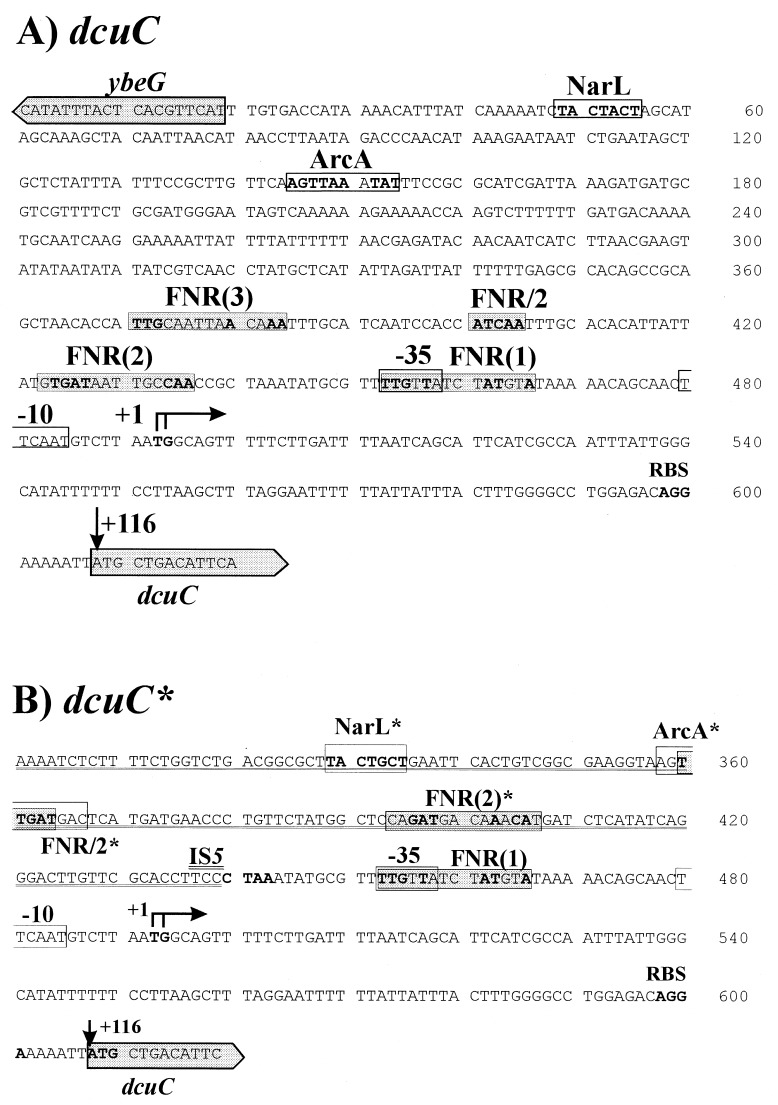
Promoter regions of dcuC (E. coli AN387) (A) and dcuC* (E. coli IMW152) (B). The putative promoter region (positions −10 and −35) is boxed, and the transcriptional start sites (+1) and putative ribosome binding sites (RBS) are shown in bold. FNR consensus sites [FNR(1) to FNR(3)] and half-sites (FNR/2) with ≥6 conserved residues (bold letters) of the FNR consensus sequence (TTGAT----ATCAA) (8, 11) are shaded. ArcA (WGT TAA TTA W, with W = A or T) (16) and NarL (TACYYMT, with Y = C or T and M = A or C) (13) consensus sequences are boxed, and the conserved residues are shown in bold. In panel B, the site of IS5 insertion is indicated (CTAA, shown in bold), and the sequence corresponding to IS5 is underlined. Sites derived from IS5 are indicated by asterisks. For the ArcA* site, the conserved residues are not indicated.
TABLE 2.
Expression of dcuC′-′lacZ as a function of electron acceptors and C4-dicarboxylates
| Growth conditiona |
dcuC′-′lacZ
expression (β-galactosidase activity, in Miller units) of strain:
|
|||
|---|---|---|---|---|
| IMW240 (wild type) | IMW255 (Δfnr)b | IMW256 (ΔarcA)b | IMW257 (narL::Tetr)b | |
| Glucose | 52 | 3 | 26 | 63 |
| Glucosec + O2 | 4 | 3 | 6 | NDd |
| Glucose + fumarate | 99 | 3 | 42 | 87 |
| Glucose + nitrate | 38 | ND | ND | 51 |
| Glucose + dimethyl sulfoxide | 37 | 3 | ND | ND |
| Glycerol + fumarate | 109 | NGe | ND | ND |
Transcriptional regulators controlling dcuC expression.
The expression of dcuC′-′lacZ in mutants deficient in regulators responding to electron acceptors was studied (Table 2). The fnr mutant, lacking the O2-responsive regulator FNR, was completely devoid of dcuC′-′lacZ expression during aerobic and anaerobic growth. The arcA mutant, which is deficient in the O2-responsive regulator ArcA (9), showed only a twofold decrease in dcuC′-′lacZ expression under anaerobic conditions. Therefore, anaerobic induction of dcuC is affected by both regulators, but FNR plays the major role. Inactivation of the narL and narP genes, encoding nitrate response regulators NarL and NarP, respectively (23), had only a weak effect on dcuC expression, in agreement with the marginal effects of nitrate. Fumarate regulation of anaerobic metabolism is mediated by the DcuSR two-component regulatory system of E. coli (6, 27). Mutants lacking the fumarate response regulator DcuR (dcuR gene) or the related CitB response regulator (citB gene) (27) showed the same fumarate stimulation of dcuC expression as the wild type. Thus fumarate stimulation of dcuC must be affected by a different regulatory system.
DcuC as the succinate efflux carrier for glucose fermentation?
According to the results obtained here, the pattern of expression of dcuC differs clearly from that of dcuA, dcuB, and dctA (3, 7, 27). DcuC is synthesized only under anaerobic conditions, and synthesis is not or is only slightly repressed by glucose or nitrate, respectively, and is slightly stimulated by fumarate. The lack of glucose repression suggests that DcuC plays a role in glucose fermentation, e.g., succinate efflux, when only the (constitutive) DcuA carrier is produced as well. Accordingly, the low rates of transport of DcuC are sufficient for succinate export during fermentation (up to 0.2 mol of succinate/mol of glucose) but not for fumarate-succinate exchange in fumarate respiration. According to the presumed functioning of DcuC as an efflux carrier, the inactivation of dcuC significantly increased the uptake and exchange of C4-dicarboxylates (Table 3). Table 3 compares the fumarate-succinate exchange and uptake activities in strains of E. coli containing different sets of Dcu carriers. When dcuB was inactivated in the strains, exchange and uptake activities for C4-dicarboxylates decreased about two- to sixfold compared to those in the parental strains. This result is in agreement with the important role of DcuB in these transport reactions of anaerobically growing E. coli. However, when dcuC was inactivated, exchange and uptake activities increased compared to those in the parental strains. The surprising finding that inactivation of a carrier increased exchange and uptake activities for the same substrates can be explained by assuming that DcuC counteracts the exchange and uptake activities effected by DcuA and DcuB and that DcuC preferentially acts as an efflux carrier in E. coli cells. Direct measurement of efflux activities was obstructed by the high rates of diffusion of C4-dicarboxylates through the membranes under the respective conditions (10).
TABLE 3.
Effects of DcuC and DcuB inactivation on exchange and uptake activities in strains with various dcu gene compositions
| Activity measured | Strain (genotype) | Carriers present | Transport activity (μmol/min/mg dry wt) | Change in activity (fold) with inactive
|
|
|---|---|---|---|---|---|
| DcuCa | DcuBb | ||||
| Exchange [14C]fumarate-succinate | AN387 (parental) | DcuA DcuB DcuC | 21.8 | 1.8 | 0.49 |
| JRG2821 (dcuA) | DcuB DcuC | 29.5 | 1.4 | 0.18 | |
| JRG2813 (dcuB) | DcuA DcuC | 10.6 | 1.5 | NAc | |
| IMW157 (dcuC) | DcuA DcuB | 39.7 | NA | 0.47 | |
| Uptake of [14C]fumarate | AN387 (parental) | DcuA DcuB DcuC | 13.9 | 1.2 | 0.39 |
| JRG2821 (dcuA) | DcuB DcuC | 11.6 | 1.4 | 0.16 | |
| JRG2813 (dcuB) | DcuA DcuC | 5.4 | 1.5 | NA | |
| IMW157 (dcuC) | DcuA DcuB | 16.7 | NA | 0.47 | |
Same as the reference strain but dcuC::mini-Tn10.
Same as the reference strain but dcuB::Kanr.
NA, not applicable.
Isolation of an IS5 promoter mutation of dcuC (dcuC*).
The dcuA dcuB double mutant grows only slowly on glycerol plus fumarate (22, 26). From the double mutant, a spontaneous mutant which had regained full anaerobic growth on glycerol plus fumarate was obtained. The nucleotide sequence of dcuC was the same in the mutant as in the wild type (26), except that an IS5 element was inserted upstream of the coding region (Fig. 1). Southern blotting and PCR analysis confirmed that dcuA and dcuB were still inactivated by the inserted resistance cassettes. The expression of dcuC in the mutant (dcuC*) was determined with a dcuC*′-′lacZ fusion. The expression of dcuC*′-′lacZ was increased by a factor of about 2.2 compared to that in the wild type, but the responses to oxygen, nitrate, and the regulators FNR, NarL, and NarP were comparable to those in the wild type (data not shown).
Functional replacement of dcuB by overexpression of dcuC in the dcuC* mutant.
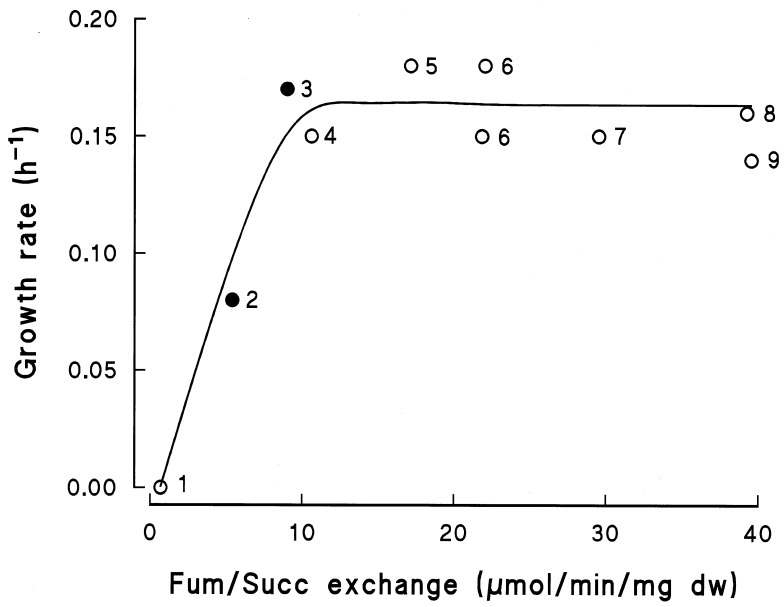
In wild-type E. coli, DcuC supports only slow growth by fumarate respiration (26). By using the dcuC* mutant, we tested whether this finding was due to restricted functioning of DcuC in the antiport mode or to limiting transport rates. In Fig. 2, the fumarate-succinate antiport activities in strains containing only dcuC or dcuC* are related to the rates of growth on glycerol plus fumarate. The increase in fumarate-succinate antiport in the dcuC* strain (about twofold) compared to that in the strain containing only dcuC was similar to the increase in dcuC or dcuC* expression and was paralleled by a similar increase in the rate of growth on glycerol plus fumarate. A further increase in fumarate-succinate antiport in strains also containing DcuA and/or DcuB caused no further increase in the growth rate. This result indicates that antiport rates of ≥10 U/mg of dry weight are sufficient to support full growth by fumarate respiration and that DcuC, in addition to its preferred function as an efflux carrier, is able to operate as a fumarate-succinate antiporter and to replace DcuB, if it is required and produced in sufficient amounts.
FIG. 2.
Rates of anaerobic growth on glycerol plus fumarate and fumarate-succinate (Fum/Succ) exchange activities for E. coli strains containing dcuC or dcuC* as the only dcu gene (●) or strains with different combinations of dcu genes (○). The strains are isogenic except for the presence of the dcu genes. 1, IMW153 (dcuA dcuB dcuC); 2, JRG2814 (dcuA dcuB); 3, IMW152 (dcuA dcuB with dcuC*); 4, JRG2813 (dcuB); 5, IMW158 (dcuB dcuC); 6, AN387 (wild type, parental strain); 7, JRG2821 (dcuA); 8, IMW159 (dcuA dcuC); and 9, IMW157 (dcuC). Growth was determined with supplemented M9 mineral medium; fumarate-succinate exchange was determined with cell suspensions of the bacteria. dw, dry weight.
Transcriptional start sites at the dcuC and dcuC* promoters.
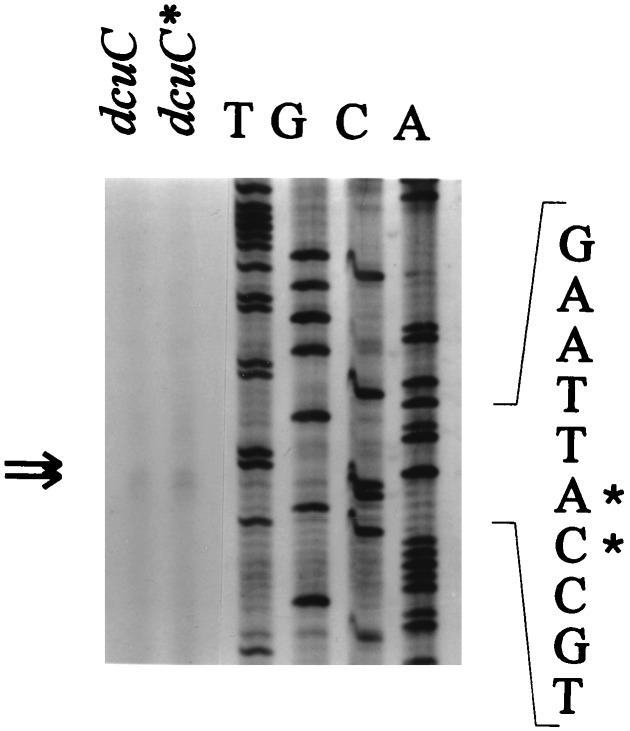
The transcriptional start sites of dcuC and dcuC* were determined with mRNA isolated from strains carrying either dcuC or dcuC*. In primer extension experiments, transcripts of the same length were produced from the mRNAs of both strains (Fig. 3) and started 116 bp upstream of the supposed translational start sites (26). In dcuC, the transcriptional start site is preceded by two potential FNR consensus sites and one half-site between positions −33.5 and −115.5. Therefore, dcuC lacks a typical FNR binding site at position −41.5 (class II site) (8, 11). In dcuC*, the promoter region at positions −35 and −10 of dcuC is retained, since the insertion site for IS5 is located at position −53 (Fig. 2). However, regulatory sites upstream of position −53, including two of the FNR consensus sites, are replaced by IS5 sequences. The IS5 element supplies two FNR consensus sites at positions −92.5 and −131 which could serve as the FNR regulatory sites of dcuC*.
FIG. 3.
Determination of the transcriptional start sites of the dcuC and dcuC* genes in strains AN387 and IMW152 by primer extension. mRNA was isolated from strains AN387 (dcuC+) and IMW152 (dcuC*) grown anaerobically on glucose plus fumarate in supplemented M9 mineral medium to an A578 of 0.5. The primer extension products (arrows) were obtained with primer cpe2. The sequencing reactions (T, G, C, and A) were performed with the same primer and pMW98 DNA. The nucleotides corresponding to the transcriptional start sites are labelled with asterisks.
Due to this situation, the FNR (1) consensus site (Fig. 1) could be used for FNR-dependent regulation of dcuC and dcuC* as well. In this case, the IS5 element would cause increased dcuC* expression by indirect effects, e.g., topological changes at the dcuC* promoter. Alternatively, other (upstream) FNR regulatory sites could be used in dcuC and dcuC*. This would mean that the IS5 element is able to provide FNR regulatory sites if inserted at appropriate positions. In any case, insertion of an IS5 element is able to increase FNR-dependent expression or to place genes under FNR control. IS5 elements frequently have been identified in or at promoters with altered expression of anaerobic pathways genes in E. coli (2, 14, 20). IS5 therefore could be important for the evolution of anaerobic pathways, either by inserting new regulatory sites or by changing the quality of adjacent promoters by affecting DNA topology.
ACKNOWLEDGMENTS
The work was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Bongaerts J, Zoske S, Weidner U, Unden G. Transcriptional regulation of the proton translocating NADH dehydrogenase genes (nuoA-N) of Escherichia coliby electron acceptors, electron donors and gene regulators. Mol Microbiol. 1995;16:521–534. doi: 10.1111/j.1365-2958.1995.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 2.Bowen S W, Hassan H M. Characterization of cis-acting regulatory mutations causing anaerobic expression of the sodA gene in Escherichia coli. Arch Biochem Biophys. 1993;302:372–379. doi: 10.1006/abbi.1993.1226. [DOI] [PubMed] [Google Scholar]
- 3.Davies, S. J., P. Golby, D. Omrani, J. R. Guest, D. J. Kelly, and S. C. Andrews. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 4.Engel P, Krämer R, Unden G. Anaerobic fumarate transport in Escherichia coli by an fnr-dependent dicarboxylate uptake system which is different from aerobic dicarboxylate uptake. J Bacteriol. 1992;174:5533–5539. doi: 10.1128/jb.174.17.5533-5539.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel P, Krämer R, Unden G. Transport of C4-dicarboxylates by anaerobically grown Escherichia coli: energetics and mechanism of exchange, uptake and efflux. Eur J Biochem. 1994;222:605–614. doi: 10.1111/j.1432-1033.1994.tb18903.x. [DOI] [PubMed] [Google Scholar]
- 6.Golby P, Davies S, Kelly D J, Guest J R, Andrews S C. Identification and characterization of a two-component sensor kinase and response regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli. J Bacteriol. 1999;181:1238–1248. doi: 10.1128/jb.181.4.1238-1248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golby P, Kelly D J, Guest J R, Andrews S C. Transcriptional regulation and organization of the dcuA and dcuB genes encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli. J Bacteriol. 1998;180:6586–6596. doi: 10.1128/jb.180.24.6586-6596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guest J R, Green J, Irvine A S, Spiro S. The FNR modulon and FNR-regulated gene expression. In: Lin E C C, Lynch A S, editors. Regulation of gene expression. New York, N.Y: Chapman & Hall; 1996. pp. 317–342. [Google Scholar]
- 9.Iuchi S, Lin E C C. Adaptation of Escherichia colito redox environments by gene expression. Mol Microbiol. 1993;9:9–15. doi: 10.1111/j.1365-2958.1993.tb01664.x. [DOI] [PubMed] [Google Scholar]
- 10.Janausch, I. G., and G. Unden. 1999. Unpublished data.
- 11.Jayaraman P S, Cole J, Busby S. Mutational analysis of the nucleotide sequence at the FNR-dependent nirB promoter of Escherichia coli. Nucleic Acids Res. 1989;17:135–145. doi: 10.1093/nar/17.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay W W, Kornberg H L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971;18:274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Kustu S, Stewart V. In vitro interaction of nitrate-responsive regulatory protein NarL with DNA target sequences in the fdnG, narG, narK and frdA operon control regions of Escherichia coliK-12. J Mol Biol. 1994;241:150–165. doi: 10.1006/jmbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Cabisco E, Obradors N, Tamarit J, Ros J, Aguilar J, Lin E C C. Evolution of an Escherichia coliprotein with increased resistance to oxidative stress. J Biol Chem. 1998;273:8308–8316. doi: 10.1074/jbc.273.14.8308. [DOI] [PubMed] [Google Scholar]
- 15.Lucht, J., and E. Bremer. Unpublished data.
- 16.Lynch A S, Lin E C C. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding and target promoters. J Bacteriol. 1996;178:6238–6249. doi: 10.1128/jb.178.21.6238-6249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrow K S, Silhavy T J, Garrett S. cis-Acting sites required for osmoregulation of ompF expression in Escherichia coliK-12. J Bacteriol. 1986;168:1165–1171. doi: 10.1128/jb.168.3.1165-1171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saier M H., Jr Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archaea and eukarya. Adv Microb Physiol. 1998;40:81–136. doi: 10.1016/s0065-2911(08)60130-7. [DOI] [PubMed] [Google Scholar]
- 19.Sawers G, Suppmann B. Anaerobic induction of pyruvate-formate lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol. 1992;174:3474–3478. doi: 10.1128/jb.174.11.3474-3478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnetz K, Rak B. IS5: a mobile enhancer of transposition in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:1244–1248. doi: 10.1073/pnas.89.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silhavy T J, Berman M, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 22.Six S, Andrews S C, Unden G, Guest J R. Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct) J Bacteriol. 1994;176:6470–6478. doi: 10.1128/jb.176.21.6470-6478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart V. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol Microbiol. 1993;9:425–434. doi: 10.1111/j.1365-2958.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- 24.Tran Q H, Bongaerts J, Vlad D, Unden G. Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coliin NADH → fumarate respiration and bioenergetic implications. Eur J Biochem. 1997;244:155–160. doi: 10.1111/j.1432-1033.1997.00155.x. [DOI] [PubMed] [Google Scholar]
- 25.Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 26.Zientz E, Six S, Unden G. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: role of the three Dcu carriers in uptake and exchange. J Bacteriol. 1996;178:7241–7247. doi: 10.1128/jb.178.24.7241-7247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zientz E, Bongaerts J, Unden G. Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR) two-component regulatory system. J Bacteriol. 1998;180:5421–5425. doi: 10.1128/jb.180.20.5421-5425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]