PURPOSE
Tisagenlecleucel is a CD19-specific chimeric antigen receptor T-cell therapy, US Food and Drug Administration–approved for children, adolescents, and young adults (CAYA) with relapsed and/or refractory (RR) B-cell acute lymphoblastic leukemia (B-ALL). The US Food and Drug Administration registration for tisagenlecleucel was based on a complete response (CR) rate of 81%, 12-month overall survival (OS) of 76%, and event-free survival (EFS) of 50%. We report clinical outcomes and analyze covariates of outcomes after commercial tisagenlecleucel.
METHODS
We conducted a retrospective, multi-institutional study of CAYA with RR B-ALL across 15 US institutions, who underwent leukapheresis shipment to Novartis for commercial tisagenlecleucel. A total of 200 patients were included in an intent-to-treat response analysis, and 185 infused patients were analyzed for survival and toxicity.
RESULTS
Intent-to-treat analysis demonstrates a 79% morphologic CR rate (95% CI, 72 to 84). The infused cohort had an 85% CR (95% CI, 79 to 89) and 12-month OS of 72% and EFS of 50%, with 335 days of median follow-up. Notably, 48% of patients had low-disease burden (< 5% bone marrow lymphoblasts, no CNS3, or other extramedullary disease), or undetectable disease, pretisagenlecleucel. Univariate and multivariate analyses associate high-disease burden (HB, ≥ 5% bone marrow lymphoblasts, CNS3, or non-CNS extramedullary) with inferior outcomes, with a 12-month OS of 58% and EFS of 31% compared with low-disease burden (OS; 85%, EFS; 70%) and undetectable disease (OS; 95%, EFS; 72%; P < .0001 for OS and EFS). Grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 21% and 7% overall and 35% and 9% in patients with HB, respectively.
CONCLUSION
Commercial tisagenlecleucel in CAYA RR B-ALL demonstrates efficacy and tolerability. This first analysis of commercial tisagenlecleucel stratified by disease burden identifies HB preinfusion to associate with inferior OS and EFS and increased toxicity.
BACKGROUND
Survival in pediatric B-cell acute lymphoblastic leukemia (B-ALL) has improved dramatically over the past 50 years 1-4 ; however, relapsed and/or refractory (RR) B-ALL remains a dominant cause of pediatric cancer–related mortality. 1,5-7 Chimeric antigen receptor (CAR) T cells targeting CD19 have proven to be effective in achieving early responses in pediatric B-ALL with complete response (CR) rates of 65%-90% across varied CAR constructs and institutions. 8-12 The landmark ELIANA trial studying tisagenlecleucel, autologous CD19-specific CAR T cells, demonstrated an 81% CR rate in 75 infused children, adolescents, and young adults (CAYA; eligibility included ≥ 3 years at screening, ≤ 21 years at diagnosis, and ≥ 5% bone marrow [BM] lymphoblasts at screening). Twelve-month overall survival (OS) and event-free survival (EFS) rates were 76% and 50%, respectively. Significant toxicity was reported with 49% ≥ grade 3 cytokine release syndrome (CRS), as graded by UPENN criteria and 13% ≥ grade 3 neurotoxicity, 9 as graded by common terminology criteria for adverse events. This study led to US Food and Drug Administration approval of tisagenlecleucel (Kymriah) in August 2017 for the treatment of CAYA < age 26 years, with ≥ 2nd RR B-ALL.
CONTEXT
Key Objective
After tisagenlecleucel commercialization, cross-institutional practices relating to tisagenlecleucel administration are heterogeneous and data-capturing is limited. Our key objective was to interrogate data from the commercial pediatric tisagenlecleucel experience to establish outcomes in the real-world setting and understand variables affecting post–chimeric antigen receptor outcomes. To approach this, we developed a national consortium, permitting cross-institutional reporting and analysis. Beyond overall outcome reporting, we distinctly report response, toxicity, and survival outcomes, as stratified by disease burden.
Knowledge Generated
Univariate and multivariate analyses studying covariates of response, toxicity, and survival demonstrate that patients with high-disease burden, defined by ≥ 5% bone marrow lymphoblasts, any circulating lymphoblasts and/or CNS3, or non-CNS extramedullary disease, have a decreased response rate, increased toxicity, and decreased overall and event-free survival.
Relevance
We identify patients entering tisagenlecleucel with high-disease burden to be a high-risk patient population who may benefit from further interventional strategies to consolidate chimeric antigen receptor–mediated outcomes.
After tisagenlecleucel commercialization, cross-institutional clinical practice and data-capturing have been heterogeneous and limited. To understand the functionality of commercial tisagenlecleucel, we conducted a retrospective, multi-institutional study measuring the relationship between baseline characteristics and clinical outcomes.
METHODS
Study Design
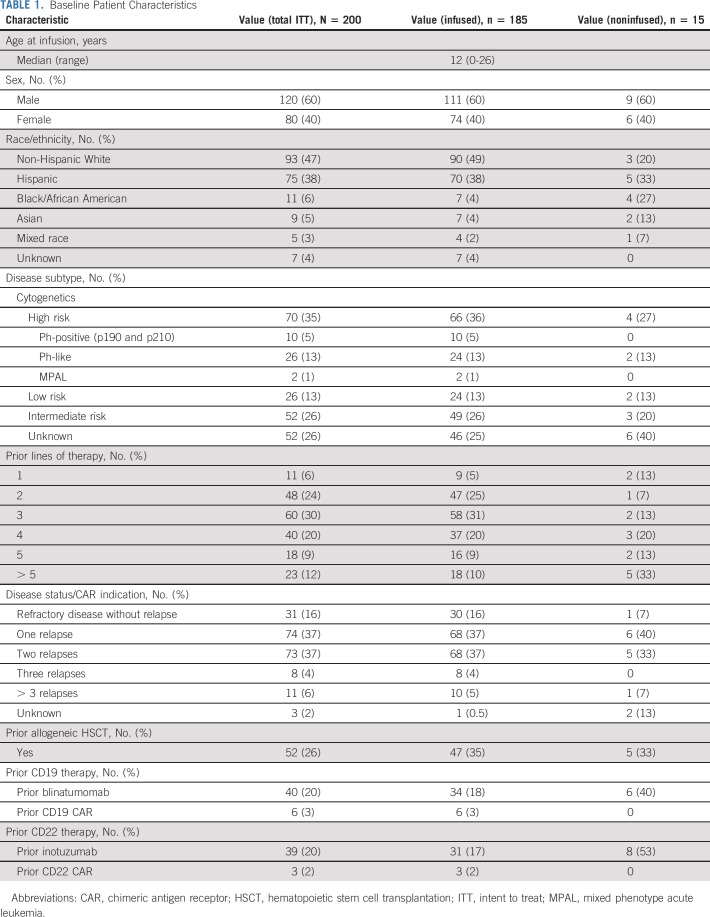
We formed a national consortium comprising 15 pediatric centers delivering standard-of-care tisagenlecleucel (Data Supplement, online only). Centers obtained independent institutional review board approval, collecting deidentified data in a Health Insurance Portability and Accountability Act–compliant, REDCap database. Patients with RR B-ALL who underwent leukapheresis shipment to Novartis (Hanover, NJ) for commercial tisagenlecleucel manufacturing, from August 30, 2017, through March 6, 2020, were included in an intent-to-treat response analysis (Table 1). All infused patients, including those on an expanded managed access protocol (MAP, NCT03601442) or with individual investigational new drug (s-IND) approval, with 28 days of minimum follow-up as of data cutoff, were included for response, toxicity, and survival analyses. MAP/s-IND was included because of intent for commercial product and previous work establishing comparable outcomes with products meeting manufacturing specifications. 13
TABLE 1.
Baseline Patient Characteristics
Study Aims and Clinical Outcome Assessment
Our primary aim was to establish overall day 28 (d28) response rate after standard-of-care tisagenlecleucel, as measured by BM and/or peripheral blood morphology and minimal residual disease (MRD) by flow cytometry, with negativity defined by the performing laboratory's threshold. Extramedullary (EM) disease was assessed by CSF, imaging, and/or physical examination. CR is defined as < 5% BM lymphoblasts and absence of circulating lymphoblasts and EM disease. Secondary aims included analysis of OS and EFS at 6 and 12 months after tisagenlecleucel. OS and EFS were measured from time of CAR infusion to occurrence of event or censored at the last follow-up. OS considered all-cause death as event, and EFS events included lack of d28 CR, relapse, secondary malignancy, and death. We establish duration of remission (DOR) and duration of B-cell aplasia (DBA), as measured from CAR infusion in only patients achieving CR. Although DOR and DBA are reported from CAR infusion, establishment of CR and B-cell aplasia (BCA) was only confirmed at d28 (Data Supplement). BCA is defined by CD19 recovery, as per institutional threshold. Because CRS grading algorithms changes over time, CRS was retrospectively graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) 14 for all patients. Neurotoxicity was graded by ASTCT 14 (69%), CAR-related encephalopathy syndrome (CRES) 15 (16%), and others (16%), per institutional standard. We additionally analyzed the secondary end point, time to relapse and/or second malignancy, treating nonrelapse death, and hematopoietic stem cell transplantation (HSCT) as competing risks.
Our exploratory aim was to evaluate risk factors for response and survival, including baseline age at diagnosis, sex, cytogenetics, prior therapy lines, prior HSCT, prior CD19-directed therapy, disease burden, time from diagnosis to CAR infusion, and RR status. A distinct therapy line was defined as initiation of a new treatment plan because of disease recurrence, refractory disease, or toxicity. Time from diagnosis to CAR infusion, number of prior therapy lines, and number of relapses are considered as continuous predictors in our multivariate Cox model while age at diagnosis was categorized into groups using the cut points that reflect known clinical risk groups: 0-2, 3-9, 10-12, 13-20, and ≥ 21 years.
Univariate and multivariate findings drove detailed outcome analysis in patients with variable disease burden, as reported at the time of last pre-CAR disease evaluation. High-disease burden (HB) is defined by ≥ 5% BM lymphoblasts, any peripheral blood lymphoblasts, CNS3 status, or non-CNS EM site of disease; low-disease burden (LB) is defined by morphologic or flow cytometry detectable BM or CNS disease not meeting HB criteria, and patients without morphologic or flow cytometry detectable disease or EM disease were categorized as undetectable (UD).
Statistical Analysis
We report frequency and percent for d28 CR, CRS, and neurotoxicity with Fisher's exact test to test differences between subgroups. Kaplan-Meier (KM) curves were plotted by baseline characteristics and compared using log-rank tests for OS, EFS, DOR, and DBA. Continuous factors (prior therapy lines and number of prior relapses) were categorized in KM curves for descriptive visualization but were kept as continuous in subsequent regression models. A multivariate Cox model for OS was constructed, including all baseline factors except cytogenic risk and EM disease because of data missingness. The Schoenfeld residual test was used for assessing proportional hazards assumption.
In the exploratory analysis of time to relapse and/or second malignancy treating nonrelapse death and HSCT as competing risks, we generated cumulative incidence curves for each competing event. We used a cause-specific hazard regression model to estimate biological associations between various risk factors and the cause-specific hazard of relapse. 16-18 We additionally used KM curves to report OS, EFS, and DOR from the time of HSCT in patients receiving HSCT while in CAR-mediated remission.
RESULTS
Patients
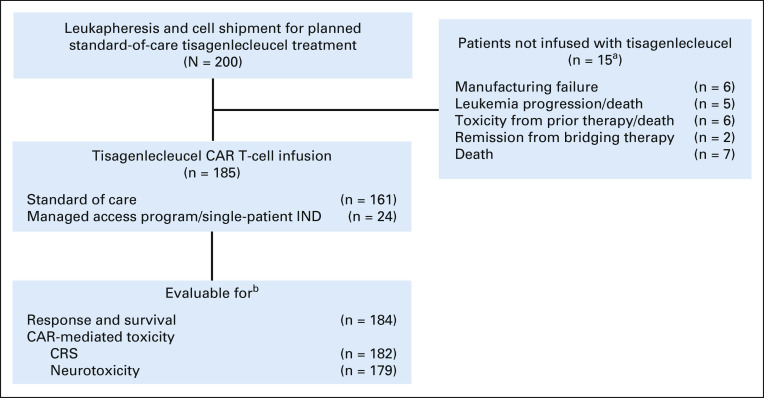
A total of 200 patients who underwent cell shipment to Novartis for planned standard-of-care tisagenlecleucel therapy were included in this analysis. Of 200 patients, 92.5% (185) of patients were infused and 7.5% (15) were not infused. Eighty-seven percent (161) of infused products met manufacturing release criteria, and 13% (24) were delivered on the MAP or with s-IND approval (Fig 1). Seventy-one percent (17) of MAP/sIND products were nonconforming because of subthreshold viability, all with viability ranging from 70% to 80%.
FIG 1.
Patient flow diagram. aPatients had concurrent disease progression, toxicities from prior therapy and/or death, therefore cumulative sum > 15. bPatients excluded because of incomplete reporting. CAR, chimeric antigen receptor; CRS, cytokine release syndrome; IND, investigational new drug.
The median follow-up from tisagenlecleucel was 335 days (range, 6-863; follow-up < d28 reflect early deaths). The median age at infusion was 12 years (range, 0-26). Thirteen patients were < 3 years at the time of infusion, and six were > 21 years at the time of diagnosis. Table 1 includes expanded patient and disease characteristics. Sixteen percent (30) of patients were treated for up-front refractory disease (seven with ≥ 5% and 23 with < 5% end-of-induction BM lymphoblasts) and 83% (154) for relapsed disease (67 in first relapse and 87 in ≥ second relapse). One patient was treated upfront because of high-risk treatment-related B-ALL and HSCT contraindication. Reasons for treatment in the first relapse include chemorefractoriness (54), prior HSCT (9), HSCT otherwise contraindicated (15; 12 of whom were additionally chemorefractory), and salvage chemotherapy contraindicated (1; Data Supplement).
Patients underwent a median of three prior therapy lines (range, 1-10) with a median duration of 33 months (range, 3-171) between diagnosis and infusion. Twenty-one percent (38) of patients received pretisagenlecleucel CD19-targeted therapies, and 18% (34) received prior CD22-targeted therapies. Twenty-five percent (47) received HSCT pretisagenlecleucel, five of whom received > 1 HSCT. Among infused patients, 51% (94) had HB, 22% (41) had LB, and 25% (46) had UD disease; four had indeterminate disease burden. Seventeen percent (13) had CNS disease (CNS2 [n = 7]; CNS3 [n = 6]), and 8% (15) had non-CNS EM disease (craniofacial [6], bone [3], testes [3], soft tissue [3], renal [2], skin [1], ocular [1], and lung [1]). Fifty-nine percent (105) had no bridging therapy, and 41% (73) had bridging therapy between disease burden assessment and lymphodepletion, with a median duration between assessment and tisagenlecleucel in these cohorts of 2 weeks (range, 0-12) and 8 weeks (range, 1-22), respectively. Details of lymphodepletion, apheresis, CAR product, and infusion are included in the Data Supplement.
Efficacy
Intent-to-treat response analysis, excluding patients noninfused because of CR from prior therapy, demonstrated a 79% (156 of 197; 95% CI, 72 to 84) d28 CR rate. Of 184 infused evaluable patients, d28 CR rate was 85% (156 of 184; 95% CI, 79 to 89). A similar CR rate (83%) was observed in the subgroup of patients receiving MAP/s-IND products. Among 134 patients infused with detectable disease at pretisagenlecleucel evaluation, the CR rate was 81% (108 of 134; 95% CI, 73 to 86). Of infused patients achieving morphologic CR, MRD testing by flow cytometry was available for 98% (153) with 97% (148) achieving MRD-negative CR. MRD testing using next-generation sequencing was available for 17% (32) of infused patients, of whom five with CR by morphology and flow cytometry had detectable next-generation sequencing MRD.
Median OS has not yet been reached. Among 184 infused, evaluable patients, 6-month OS was 85% and 12-month OS was 72%. Six-month EFS was 62%, and 12-month EFS was 50%. Of 156 patients with d28 CR, DOR at 6 and 12 months was 75% and 62%, respectively (Fig 2). Thirty-seven percent (57 of 156) of responders experienced relapse. The median time from infusion to relapse was 101 days (range, 30-645). At the time of relapse, 41% (22 of 52) of evaluable patients had CD19-negative disease and 59% (30 of 52) had continued CD19 expression. In three patients, CD19 negativity was associated with myeloid transformation, two of whom had KMT2A (mixed-lineage leukemia) rearrangement. Of 129 infused patients with evaluable BCA, the probability of maintaining BCA at 6 and 12 months after infusion was 66% and 55%, respectively (Fig 2).
FIG 2.
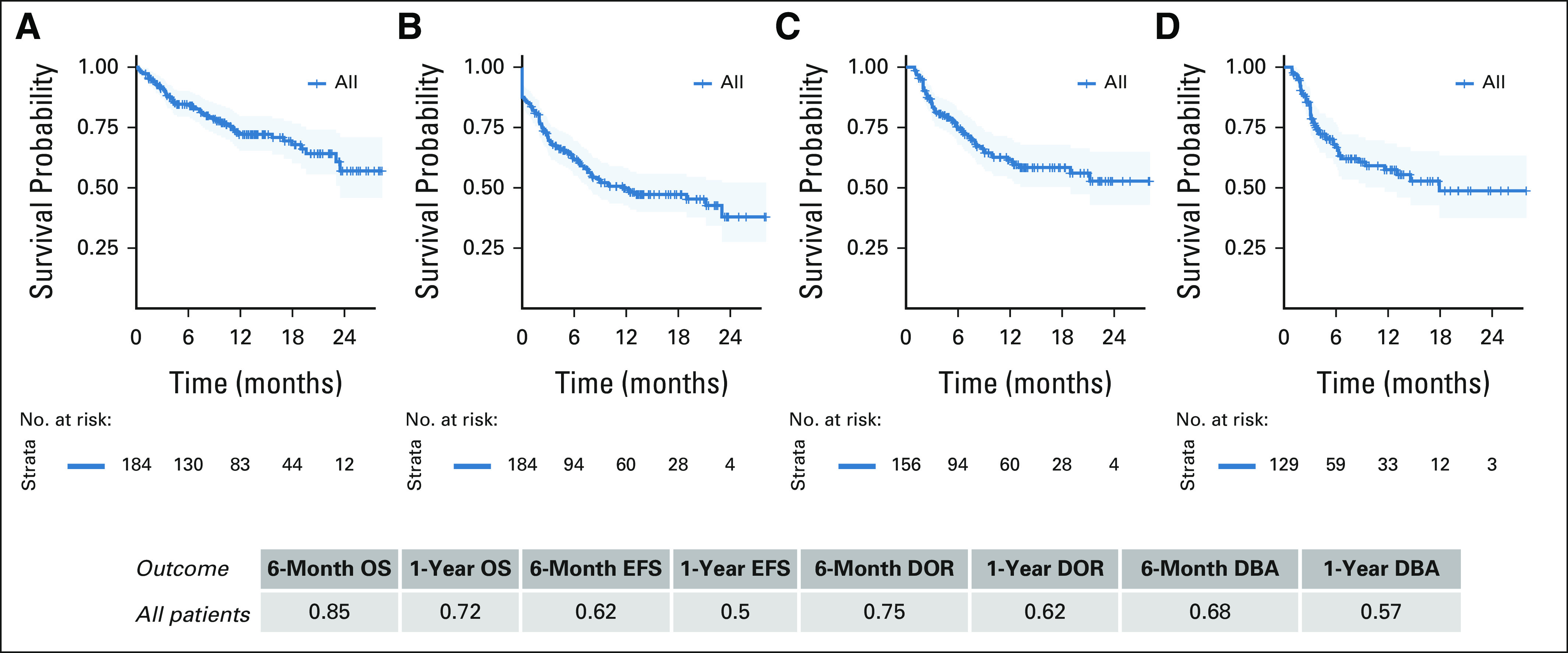
(A) OS, (B) EFS, (C) DOR, and (D) DBA outcomes of infused cohort. OS and EFS estimates in all infused, evaluable patients. DOR and DBA estimates in patients who achieved morphologic remission. DBA, duration of B-cell aplasia; DOR, duration of remission; EFS, event-free survival; OS, overall survival.
Of patients achieving CR, 28% (41 of 156) underwent post-CAR HSCT (median time from tisagenlecleucel to HSCT; 199 days, range, 36-565). Twenty patients (five with ongoing BCA and 15 with BCA loss) underwent HSCT while in remission after tisagenlecleucel, without intervening relapse (median time from tisagenlecleucel to HSCT; 126.5 days, range, 36-436). Survival analysis limited to patients proceeding to HSCT while in post-tisagenlecleucel remission demonstrates OS, EFS, and DOR (Data Supplement). Nineteen patients proceeded to HSCT after relapse post-tisagenlecleucel. Two patients went to HSCT with the evidence of myelodysplastic syndrome. We demonstrate cumulative incidence of death, RR B-ALL and/or myelodysplastic syndrome, and HSCT as competing events (Data Supplement).
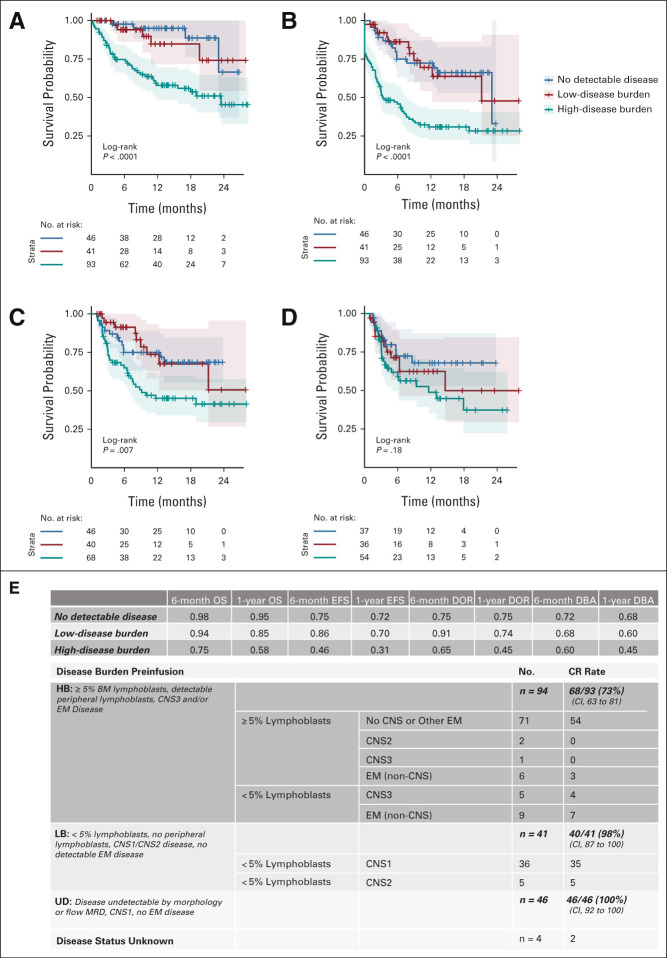
Both univariate (Data Supplement) and multivariate analyses (Fig 3, Data Supplement) implicate baseline disease burden as strongly associating with outcomes. The HB cohort had decreased morphologic CR rate of 73% (95% CI, 63 to 81), as compared with 98% (95% CI, 87 to 100) in LB and 100% (95% CI, 92 to 100) in UD (P < .0001). OS, EFS, and DOR were lower among patients with HB at 6 and 12 months (Fig 4). Importantly, 12-month OS and EFS were 58% and 31% in HB, respectively, as compared with 85% OS and 70% EFS in LB and 95% OS and 72% EFS in UD (P < .0001 for OS and EFS). Analysis is limited to patients with baseline disease evaluation < 3 weeks pretisagenlecleucel, and no interval bridging therapy validates inferior HB outcomes (Data Supplement). Multivariate analysis additionally associates age 3-10 years at diagnosis (P = .006) and increased time from diagnosis to infusion (P < .001) with improved OS and expectedly associates increased prior therapy lines (P = .022) and relapses (P = .005) with decreased OS. Expanded univariate analysis is shown in the Data Supplement. Prior HSCT associates with improved OS on multivariate analysis (P = .019), but univariate analysis did not associate prior HSCT or CD19-directed therapy with survival outcomes, DOR, or DBA (Data Supplement). Among 35 patients who underwent leukapheresis while in remission, improved EFS is seen compared with patients collected with active disease (Data Supplement).
FIG 3.
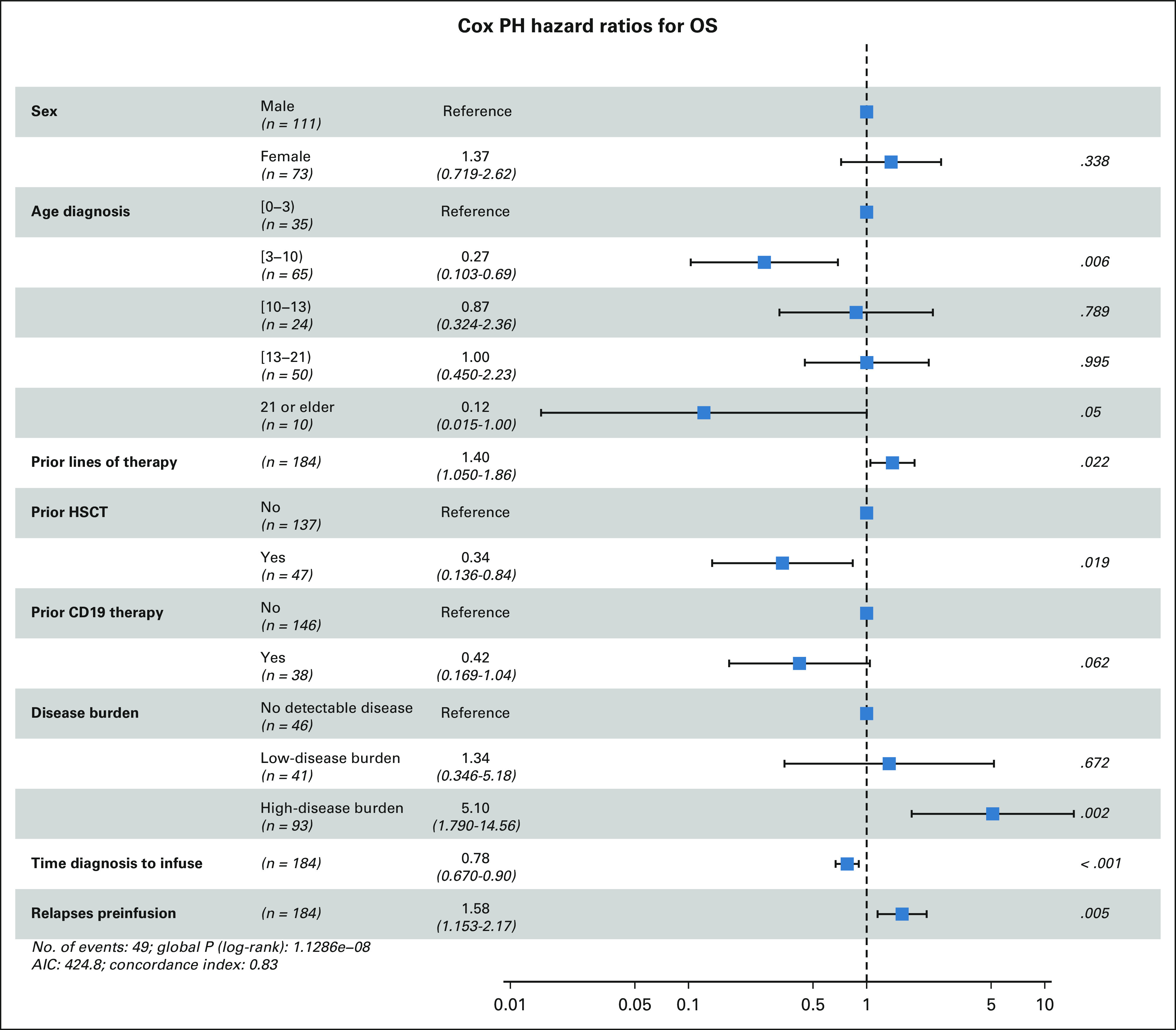
Association of baseline characteristics with OS in a multivariate analysis of tisagenlecleucel recipients. AIC, akaike information criterion; HSCT, hematopoietic stem cell transplantation; OS, overall survival.
FIG 4.
(A) OS, (B) EFS, (C) DOR, and (D) DBA outcomes stratified by disease burden. OS and EFS estimates in all infused patients with measurable disease burden. DOR and DBA estimates in patients who achieved morphologic remisson. (E) Disease burden stratification and day 28 CR rate. BM, bone marrow; CR, complete response; DBA, duration of B-cell aplasia; DOR, duration of remission; EFS, event-free survival; EM, extramedullary; HB, high-disease burden; LB, low-disease burden; MRD, minimal residual disease; OS, overall survival; UD, undetectable disease.
Safety
Among 185 patients treated with tisagenlecleucel, d28 safety was evaluable in 183 (incomplete data; n = 2). Thirty-eight percent (69 of 183) and 39% (72 of 183) received lymphodepletion and tisagenlecleucel in the outpatient setting, respectively. Four patients had infusion reactions including two with anaphylaxis (Data Supplement). CRS classification as per ASTCT guidelines demonstrates overall and ≥ grade 3 CRS rates of 63% (116 of 183) and 21% (39 of 183), respectively, with one CRS-attributed death (Table 2). The median CRS onset time was 5 days (0-14 days) with a median duration of 4 days (range, 1-42). Hemophagocytic lymphohistiocytosis was reported in one patient and was managed using anakinra, dexamethasone, and etoposide.
TABLE 2.
Summary of Safety Using Commercial Tisagenlecleucel
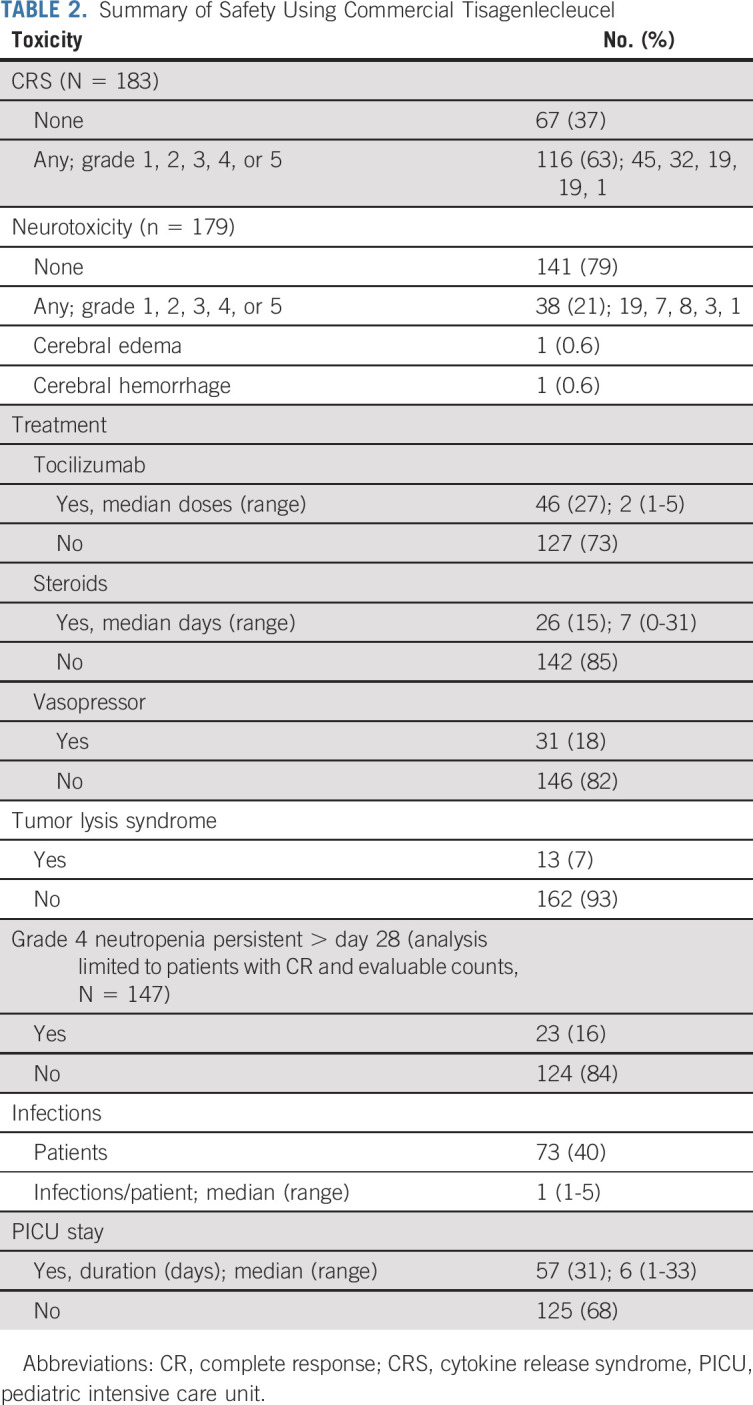
Overall and ≥ grade 3 neurotoxicity were reported in 21% (38 of 179) and 7% (12 of 179) of patients, respectively (Table 2). One patient had cerebral edema that resolved without neurologic sequelae. A singular neurotoxicity-related death was reported with fatal cerebral hemorrhage in context of coagulopathy, CRS, and pretisagenlecleucel stroke. The median neurotoxicity onset was 6 days (range, 3-25) post-tisagenlecleucel, with a median duration of 5 days (range, 1-203 days; outlier because of prolonged facial palsy). Prior CNS3 disease did not associate with increased neurotoxicity frequency or severity (Data Supplement).
Eighty-two percent (151 of 184) of overall cohort had CAR-related hospital admissions before d28 with a median inpatient days of 14.5 (range, 1-75), and 31% (57 of 184) were admitted to the pediatric intensive care unit with a median duration of 6 days (range, 1-33). Details of tocilizumab, steroids, and vasopressor administration are reported in Table 2. Alternative therapies included anakinra (n = 3) and siltuximab (n = 1).
Grade 4 neutropenia occurred in 118 of 175 evaluable patients, with a median duration among 94 evaluable neutropenic patients of 14 days (range, 1-76). Seven percent (13 of 175) experienced tumor lysis syndrome, and 40% (73 of 181) experienced at least one infectious complication postinfusion (median onset 68 days, range, 1-559). Fifty-one deaths occurred after tisagenlecleucel, five occurring < d28 (leukemia [n = 1], infection [n = 2], CRS [n = 1], and neurotoxicity [n = 1]). Of remaining 46 deaths, nine died without active leukemia (infection [n = 3], HSCT complications [n = 5], and cardiac [n = 1]). Nonrelapse mortality rate post-tisagenlecleucel is 7% (13 of 184).
Univariate analysis of toxicity identified disease burden and relapse versus refractory status to associate with increased CRS severity (Data Supplement). Patients with HB had increased overall CRS (79.3% [HB] v 51.2 [LB] and 41.3 [UD], P < .0001) and ≥ grade 3 CRS rates (34.7% [HB] v 9.8 [LB] and 0.4 [UD], P < .0001). Forty-three percent of patients with HB required pediatric intensive care unit–level care compared with 22% LB and 13% UD (P < .0001). Neurotoxicity did not significantly differ between subgroups (Data Supplement). Univariate outcome analysis associated ≥ grade 3 CRS with decreased OS and EFS (P < .0001; Data Supplement).
DISCUSSION
Multi-institutional, retrospective data on 185 CAYA patients infused with commercial tisagenlecleucel demonstrate overall comparable response, OS, and EFS rates with previous reported series. 9,10 However, increased disease burden associates with inferior tisagenlecleucel outcomes. It remains unclear if disease burden is a surrogate of distinct biology driving inferior outcomes or this is a modifiable factor that is responsive to debulking before CAR infusion.
Although ELIANA required > 5% BM lymphoblasts with a median of 75% at enrollment, 9 the US Food and Drug Administration–approved indication for tisagenlecleucel does not require a threshold disease burden. In our series, 48% of infused patients had LB (< 5% BM lymphoblasts, no CNS3, or other EM) or UD disease at the last evaluation before CAR. Disease burden on ELIANA was assessed at enrollment and may shift during manufacturing or with bridging chemotherapy. Direct comparisons across studies are, therefore, inherently challenged; however, our study likely represents a cohort with an overall lower-disease burden than ELIANA.
Impact of disease burden was previously described in adults with ALL receiving CD19-targeting (19-28z) CAR T cells where patients with LB or UD disease had longer EFS and OS, as compared with patients with ≥ 5% BM lymphoblasts or EM disease. 19 Pediatric data using the same construct support that minimal disease burden positively affect response. 12 In the commercial setting, the Center for International Blood and Marrow Transplant Research described comparable tisagenlecleucel efficacy with previous pivotal trials, across pediatric and adult leukemia and lymphoma. 20 Similar to our data set, high response rates were seen in patients entering CAR without detectable disease. However, survival outcomes were not stratified across disease burden. Real-world study of axicabtagene ciloleucel (Yescarta) in adult B-cell lymphoma identifies increased lactate dehydrogenase, a biomarker correlating with disease bulk, as an independent variable of poor response. 21
Conversely, previous work in pediatrics supports that low absolute numbers of CD19-expressing target cells (both healthy and malignant B cells) at the time of CAR infusion associate with shortened CAR T-cell engraftment. 10 Although we found 64% of patients with LB or UD disease maintained BCA for at least 1 year, we did not assess normal B-cell numbers preinfusion.
Decreased toxicity in our cohort, as compared with ELIANA, may relate to lower-disease burden or an evolving threshold for intervention. Similar to previous work associating tumor burden with post-CAR toxicity in pediatric ALL, 8 we report increased CRS incidence and severity in patients infused with HB. Thirty-nine percent of our cohort were infused as outpatients. The low rate of infusion-related toxicity, lag between tisagenlecleucel-infusion and CRS-onset (median; 5 days), and experience that CRS starts with low-grade symptoms support outpatient tisagenlecleucel infusion as a safe practice.
Interestingly, compared with ELIANA where 15 of 16 patients with evaluable antigen status relapsed with CD19-negative disease, we report that majority of relapses have preserved CD19 surface expression in this cohort (59% CD19-positive v 41% CD19-negative). Whether this finding relates to differences in tisagenlecleucel expansion and persistence because of lower disease/CD19 antigen burden, or alternative factors, including varying thresholds for defining antigen downregulation, warrants further study. Intriguingly, this analysis did not find patients with lower-disease burden to have decreased DBA.
Limitations of this study derive from the retrospective nature and heterogeneity of reporting across centers and evolving definitions of disease response and relapse. Additionally, evolving toxicity grading systems were adopted by participating institutions at different times. Although CRS was regraded according to ASTCT, data were not available to regrade neurotoxicity. Timing of the last disease burden assessment before tisagenlecleucel varied across centers, with possible changes in burden during the bridging/manufacturing window. If disease burden changed before infusion, it would tend to blunt the impact of disease burden on outcomes observed in our analysis. That disease burden remained highly prognostic suggests a true effect. Nonetheless, further study to quantify the impact of disease burden assessed immediately pretisagenlecleucel will be important. Finally, we are unable to assess the impact of planned consolidative HSCT after tisagenlecleucel given the rarity with which this was undertaken in this study cohort.
In conclusion, we report the feasibility of commercial tisagenlecleucel delivery, with comparable overall response and survival outcomes with the landmark ELIANA trial and decreased toxicity and antigen downregulation in context of lower overall disease burden. Importantly, disease burden associates with response, survival, and toxicity, and patients with HB emerge as a distinct high-risk population who may benefit from further interventional strategies to optimize CAR-mediated outcomes.
ACKNOWLEDGMENT
We acknowledge the following individuals for their major roles in supporting successful execution of this multi-institutional study. Regulatory support: S.M. and E.E. Administrative support: Anika Dove and Daisy Torres. Legal Council and Contracting: Neil Morimoto. Data Management: Anne Marcy, Michelle Fujimoto, Jennifer Sheppard, Jean Sosna, Victoria Koch, Katie Doherty, Emily Bakinowski, Elizabeth Klein, Daritzya Baraja, Courtney Newbold, Glenn McWillians, Maggie Dyer, Kasey Abrahamnson, Angie Peltz, Ahmed Tahoun, Mary Suarez, Megan Hanby, Stacy Cooper, and Brad Muller.
Christine L. Phillips
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Heather E. Stefanski
Honoraria: Novartis
Steven P. Margossian
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Michael R. Verneris
Stock and Other Ownership Interests: Fate Therapeutics
Honoraria: Novartis, Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: patent to make innate lymphiod cells. It is not licensed.
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead company
Muna Qayed
Consulting or Advisory Role: Novartis, Mesoblast
Travel, Accommodations, Expenses: Novartis
Michelle Hermiston
Consulting or Advisory Role: Novartis, Sobi
Patents, Royalties, Other Intellectual Property: Spouse has patents pending for platform technology with application to oncology, diagnostics, anti-infections and for anti-bleeding technology (I).
Prakash Satwani
Consulting or Advisory Role: Mesoblast, Takeda
Speakers' Bureau: Sobi
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1047592
Rachel Wilcox
Research Funding: Novartis, AlloVir
Travel, Accommodations, Expenses: Novartis
Rayne H. Rouce
Honoraria: Novartis Pharmaceuticals UK Ltd, Kite/Gilead
Research Funding: Tessa Therapeutics (Inst)
Valentin V. Barsan
Leadership: Tessera Therapeutics, Navio
Stock and Other Ownership Interests: Illumina, Natera, Pacific Biosciences, InVitae
Consulting or Advisory Role: Guardant Health
Kevin J. Curran
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Mesoblast
Research Funding: Juno Therapeutics
Crystal L. Mackall
Leadership: Syncopation Life Sciences
Stock and Other Ownership Interests: Lyell Immunopharma, Alimera Sciences, Vor Pharmaceuticals, Apricity Health, Syncopation Life Sciences, Ensoma, Mammoth
Consulting or Advisory Role: Bryology, Vor Biopharma, Apricity Health, TPG, Alimera Sciences, PACT Pharma, Nektar, Lyell Immunopharma, NeoImmuneTech, Syncopation Life Sciences, Bristol Myers Squibb, Immatics, GlaxoSmithKline, Ensoma, Mammoth
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: I am an inventor on numerous patents related to chimeric antigen receptor therapeutics and received royalties from NIH for the CD22-CAR patent licensed to Juno therapeutics.
Travel, Accommodations, Expenses: NeoImmuneTech, Roche, Nektar
Other Relationship: Lyell Immunopharma, Syncopation Life Sciences
Theodore W. Laetsch
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 62nd American Society of Hematology (ASH) Virtual Annual Meeting & Exposition, December 6, 2020.
SUPPORT
Supported by a St Baldrick's/Stand Up 2 Cancer Pediatric Dream Team Translational Cancer Research Grant (C.L.M.). Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M. is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Crystal L. Mackall
Administrative support: Liora M. Schultz, Sharon Mavroukakis, Emily Egeler
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disease Burden Affects Outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia After Commercial Tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christine L. Phillips
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Heather E. Stefanski
Honoraria: Novartis
Steven P. Margossian
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Michael R. Verneris
Stock and Other Ownership Interests: Fate Therapeutics
Honoraria: Novartis, Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: patent to make innate lymphiod cells. It is not licensed.
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead company
Muna Qayed
Consulting or Advisory Role: Novartis, Mesoblast
Travel, Accommodations, Expenses: Novartis
Michelle Hermiston
Consulting or Advisory Role: Novartis, Sobi
Patents, Royalties, Other Intellectual Property: Spouse has patents pending for platform technology with application to oncology, diagnostics, anti-infections and for anti-bleeding technology (I).
Prakash Satwani
Consulting or Advisory Role: Mesoblast, Takeda
Speakers' Bureau: Sobi
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1047592
Rachel Wilcox
Research Funding: Novartis, AlloVir
Travel, Accommodations, Expenses: Novartis
Rayne H. Rouce
Honoraria: Novartis Pharmaceuticals UK Ltd, Kite/Gilead
Research Funding: Tessa Therapeutics (Inst)
Valentin V. Barsan
Leadership: Tessera Therapeutics, Navio
Stock and Other Ownership Interests: Illumina, Natera, Pacific Biosciences, InVitae
Consulting or Advisory Role: Guardant Health
Kevin J. Curran
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Mesoblast
Research Funding: Juno Therapeutics
Crystal L. Mackall
Leadership: Syncopation Life Sciences
Stock and Other Ownership Interests: Lyell Immunopharma, Alimera Sciences, Vor Pharmaceuticals, Apricity Health, Syncopation Life Sciences, Ensoma, Mammoth
Consulting or Advisory Role: Bryology, Vor Biopharma, Apricity Health, TPG, Alimera Sciences, PACT Pharma, Nektar, Lyell Immunopharma, NeoImmuneTech, Syncopation Life Sciences, Bristol Myers Squibb, Immatics, GlaxoSmithKline, Ensoma, Mammoth
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: I am an inventor on numerous patents related to chimeric antigen receptor therapeutics and received royalties from NIH for the CD22-CAR patent licensed to Juno therapeutics.
Travel, Accommodations, Expenses: NeoImmuneTech, Roche, Nektar
Other Relationship: Lyell Immunopharma, Syncopation Life Sciences
Theodore W. Laetsch
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicans' Education Resource, Y-mAbs Therapeutics
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2. Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: Results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 7. Oskarsson T, Söderhäll S, Arvidson J, et al. Relapsed childhood acute lymphoblastic leukemia in the nordic countries: Prognostic factors, treatment and outcome. Haematologica. 2016;101:68–76. doi: 10.3324/haematol.2015.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–2368. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossoff J, Baggott C, Prabhu S, et al. Real-world treatment of pediatric patients with relapsed/refractory B-cell acute lymphoblasstic leukemia using tisagenlecleucel that is out of specification for commercial release. Blood. 2020;136(suppl 1):42–44. [Google Scholar]
- 14. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 15. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36:4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oyama MA, Shaw PA, Ellenberg SS. Considerations for analysis of time-to-event outcomes subject to competing risks in veterinary clinical studies. J Vet Cardiol. 2018;20:143–153. doi: 10.1016/j.jvc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 19. Park JH, Rivière I, Gonen M, et al. Long-Term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–5424. doi: 10.1182/bloodadvances.2020003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: Results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]