PURPOSE
The recommended duration of adjuvant fluoropyrimidine and oxaliplatin chemotherapy for patients with stage III colon cancer is based on tumor classification into clinically low-risk (T1-3 N1) and high-risk (T4 or N2) groups. We determined whether Immunoscore can enhance prognostication within these risk groups.
MATERIALS AND METHODS
Patients with stage III colon carcinomas (N = 600) were randomly selected from the infusional fluorouracil, leucovorin, and oxaliplatin arm of adjuvant trial NCCTG N0147 (Alliance for Clinical Trials in Oncology). Tumors were evaluated for Immunoscore that quantifies CD3+ and CD8+ T-cell densities in the tumor center and invasive margin by digital image analysis. Disease-free survival (DFS) by Immunoscore was analyzed using a multivariable Cox regression model in each risk group with adjustment for covariates including KRAS, BRAFV600E, and mismatch repair status.
RESULTS
Of 559 cancers with Immunoscore data, 299 (53.5%) were classified as clinically low-risk (T1-3 N1) and 260 (46.5%) as clinically high-risk (T4 and/or N2). Among patients with low-risk tumors, those with Immunoscore-Low versus Immunoscore-High tumors had significantly worse 5-year DFS rates (77.5% v 91.8%; hazard ratio, 1.70; 95% CI, 1.03 to 2.79; P = .037). Among patients with high-risk tumors, those with Immunoscore-Low versus Immunoscore-High tumors also had significantly worse DFS (55.3% v 70.3%; hazard ratio, 1.65; 95% CI, 1.11 to 2.47; P = .013). Tumors that were low-risk/Immunoscore-Low had similar outcomes as did tumors that were high-risk/Immunoscore-High (P = .174). Prognostication was significantly improved in multivariable models where Immunoscore was added to clinical risk parameters and limited biomarkers (likelihood ratio test P = .0003).
CONCLUSION
Immunoscore can refine patient prognosis beyond clinical risk group classification, suggesting its potential utility for adjuvant decision making.
INTRODUCTION
Immunoscore quantifies cytotoxic T cells in the tumor microenvironment by combining CD3+ and CD8+ T-cell densities in the tumor core and its invasive margin. Previous studies have validated the consensus Immunoscore as an independent prognostic variable in patients with stage I-III colorectal cancer,1,2 including the IDEA France3 and NCCTG N01474 phase III adjuvant chemotherapy trials. Immunoscore has been incorporated into the European Society for Medical Oncology (ESMO) Clinical Practice Guidelines (2020) for localized colon cancer5 and the Pan-Asian adapted ESMO Clinical Practice Guidelines (2021) to refine patient prognosis. Although use of a fluoropyrimidine plus oxaliplatin is standard adjuvant chemotherapy for stage III colon cancers, the recommended duration of this treatment is currently based on T and N stage risk classification as demonstrated in the IDEA collaboration6 and endorsed by the National Comprehensive Cancer Network and ESMO. Patients with clinically low-risk (T1-3 N1) tumors are recommended to receive at least 3 months of adjuvant fluoropyrimidine plus oxaliplatin treatment, whereas those with clinically high-risk (T4 and/or N2) tumors should receive 6 months of treatment.6 Before results from the IDEA collaboration, the standard of care was to administer 6 months of adjuvant infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy. Here, we determined the potential of Immunoscore to refine patient prognosis within clinically low-risk and high-risk groups from the FOLFOX treatment arm of a completed phase III adjuvant chemotherapy trial (NCCTG N0147; Alliance for Clinical Trials in Oncology).
CONTEXT
Key Objective
Immunoscore quantifies T-cell densities in the tumor microenvironment and is an independent prognostic variable in patients with localized colon cancer. We determined whether Immunoscore can enhance prognostication within clinically low-risk (T1-3 N1) and high-risk (T4 or N2) groups, which informs the recommended duration of adjuvant chemotherapy for patients with stage III colon cancer.
Knowledge Generated
Immunoscore was shown to prognostically stratify patients within clinically low-risk and high-risk groups. Five-year disease-free survival rates ranged from 55.3% to 91.8% on the basis of the Immunoscore and risk group–combined variable. Prognostication was significantly improved in multivariable analysis where Immunoscore was added to the models.
Relevance
Immunoscore can enhance patient prognostication beyond clinical risk categorization, suggesting its potential ability to inform decision making for adjuvant therapy.
MATERIALS AND METHODS
Study Population
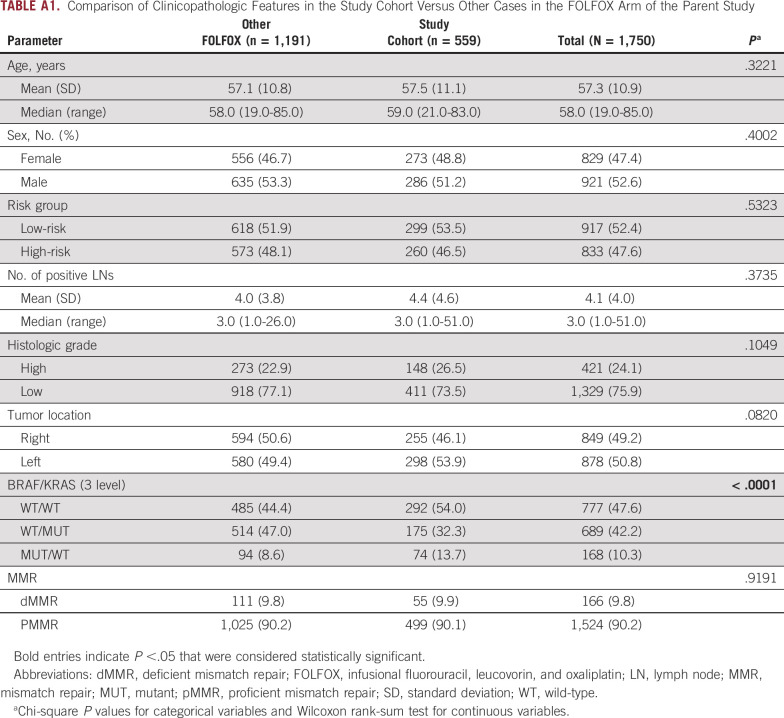
Patients with curatively resected, stage III colon adenocarcinomas (N = 600) were randomly selected from the FOLFOX alone arm of adjuvant trial NCCTG N0147 (Alliance for Clinical Trials in Oncology). This trial evaluated FOLFOX for 6 months with or without cetuximab, and the addition of cetuximab to FOLFOX was not associated with a significant difference in the primary end point of disease-free survival (DFS).7 Primary tumor sidedness was defined as relative to the splenic flexure, where left-sided tumors included those located at the splenic flexure. The median patient follow-up for survival in the N0147 study population was 83 months. Patient clinicopathologic features and limited biomarker data in the study cohort did not differ significantly from other cases in the FOLFOX arm of the N0147 study except for the BRAF/KRAS variable (Appendix Table A1).
Immunoscore
Immunohistochemical staining of CD3+ and CD8+ T cells was performed on formalin-fixed, paraffin-embedded tumor sections, as previously described.2 Briefly, using image analysis software, the density of CD3+ and CD8+ T cells was quantified by the number of cells per mm2 in the tumor center and invasive margin.2,8,9 Densities were converted into percentiles (0%-100%), and the mean of four percentiles (two markers and two regions) was calculated and converted into an Immunoscore. The two-level classification uses low (0%-1% and 0%-25%) and high (2%-4% and 25%-100%) density scores. After quality control criteria were implemented, there were 559 cases with available results that were analyzed blinded to patient clinical outcome data.
Analysis of tumors for mutations in KRAS and BRAFV600E genes and mismatch repair (MMR) status had been previously performed.10 A separate cohort of patients (N = 1,532) from the N0147 trial with tumor-infiltrating lymphocyte (TIL) densities quantified using light microscopy was used for assessment of concordance with Immunoscore in an exploratory analysis. Optimal cut points for TIL densities for association with DFS were previously determined.11
The study was approved by the Mayo Clinic Institutional Review Board. Each participant signed an Institutional Review Board—approved, protocol-specific informed consent. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. The N0147 trial had been registered at ClinicalTrials.gov identifier (NCT00079274).
Statistical Analysis
DFS was defined as time from random assignment to recurrence or death because of all causes, whichever occurred first. The distribution of DFS was evaluated by the Kaplan-Meier method. DFS by Immunoscore was analyzed by a multivariable Cox regression model in the overall cohort and in each clinical risk group with adjustment for covariates as shown. A backward elimination procedure (P value of .05 as a threshold for remaining in the model) was applied to identify the most important covariates to predict DFS. Model fitting was compared using the likelihood ratio test. The final model was tested for the proportional hazards assumption. Adjusted hazard ratios (HRs) and 95% CIs are reported. For interaction P values, the threshold for statistical significance was < .05. Two-sided P values are reported; P <.05 was considered statistically significant.
RESULTS
Characteristics of the study population are as follows: the median patient age was 59 years, 51.2% of study participants were men, and 46.1% had right-sided tumors (Appendix Table A1). Of 559 cancers with an Immunoscore result, 299 (53.5%) were categorized as clinically low-risk (T1-3 N1) and 260 (46.5%) as clinically high-risk (T4 and/or N2). There were 59 (9.9%) tumors with deficient DNA MMR. Tumor BRAF/KRAS mutation status is as follows: Mutant (MUT; V600E)/wild-type (WT; n = 74; 13.7%), WT/MUT (n = 175; 32.3%), and WT/WT (n = 292; 54.0%). Tumor recurrence occurred in 164 of 559 (29.3%) patients, and 137 died during the study follow-up.
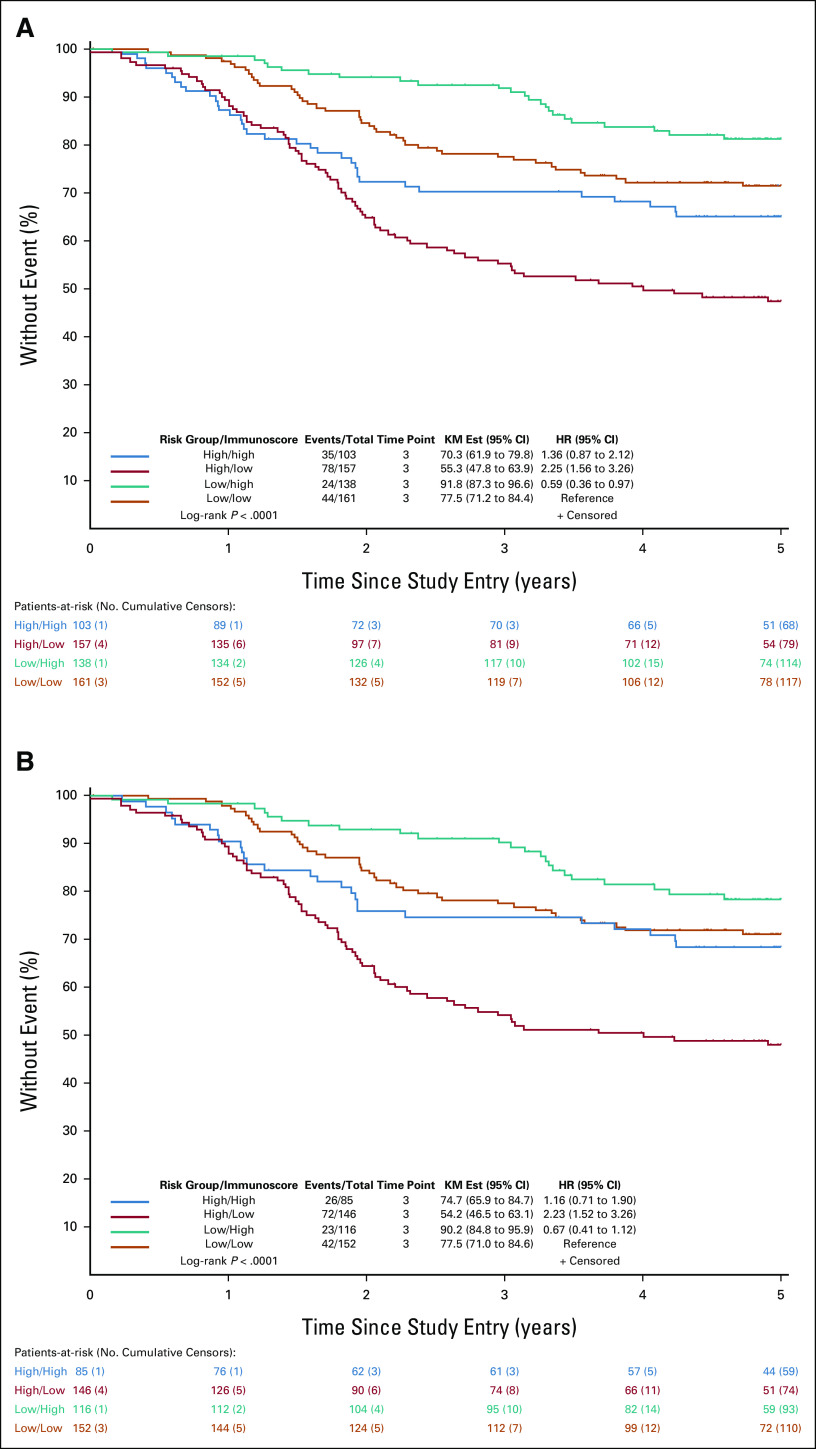
We determined whether Immunoscore could prognostically stratify patients within clinically low-risk and high-risk groups. Patient DFS rates were examined by clinical risk group and Immunoscore as a combined variable in the overall study cohort. Although the poorest DFS was observed for patients whose tumors were high-risk/Immunoscore-Low (5-year DFS, 55.3%), tumors that were low-risk/Immunoscore-High had the most favorable 5-year DFS (5-year DFS, 91.8%; P < .0001; Fig 1A). Patients with clinically low-risk/Immunoscore-Low tumors had significantly poorer DFS compared with those with low-risk/Immunoscore-High tumors (HR, 1.70 [95% CI, 1.03 to 2.79]; P = .037; 5-year DFS: 77.5% v 91.8%). Patients with clinically high-risk/Immunoscore-Low tumors had significantly poorer DFS than did those with high-risk/Immunoscore-High (HR, 1.65 [95% CI, 1.11 to 2.47]; P = .013; 5-year DFS: 55.3% v 70.3%; Fig 1). Importantly, we found that DFS of patients whose tumors were low-risk/Immunoscore-Low did not differ significantly from those whose tumors were high-risk/Immunoscore-High (HR, 1.36 [95% CI, 0.87 to 2.12]; P = .174; 5-year DFS: 77.5% v 70.3%; Fig 1).
FIG 1.
(A) Patient 5-year DFS by clinical risk group/Immunoscore in patients with stage III colon cancer treated with adjuvant infusional fluorouracil, leucovorin, and oxaliplatin. Risk group is categorized as low-risk (T1-3 N1) or high-risk (T4 and/or N2), and Immunoscore is dichotomized as Immunoscore-Low or Immunoscore-High. Multivariable HRs and 95% CIs are shown. (B) Analysis was also performed with the study cohort restricted to cancers showing pMMR (DFS by risk group/Immunoscore [pMMR]). DFS, disease-free survival; Est, estimate; HR, hazard ratio; KM, Kaplan-Meier; pMMR, proficient mismatch repair.
Immunoscore was also analyzed by risk group for DFS when restricting the cohort to patients whose tumors showed proficient MMR (pMMR). We observed relatively similar DFS rates for pMMR tumors as in the overall study cohort (Fig 1B). Again, patients with tumors that were low-risk/Immunoscore-Low had similar DFS rates compared with tumors that were high-risk/Immunoscore-High (HR, 1.16 [95% CI, 0.71 to 1.90]; P = .544; 5-year DFS: 77.5% v 74.7%; Fig 1B). Patients with pMMR cancers that were high-risk/Immunoscore-Low had a significantly poorer DFS compared with all other groups (P < .0001).
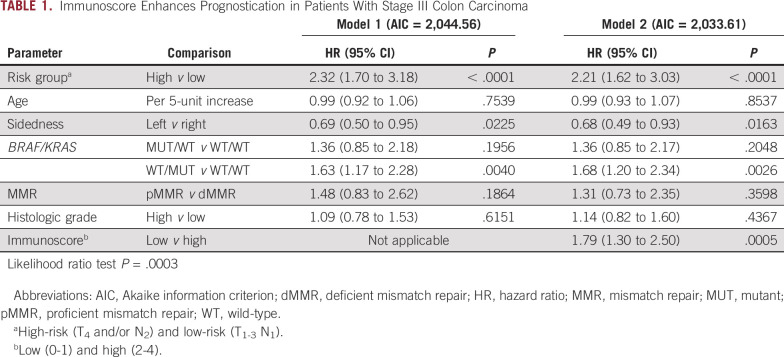
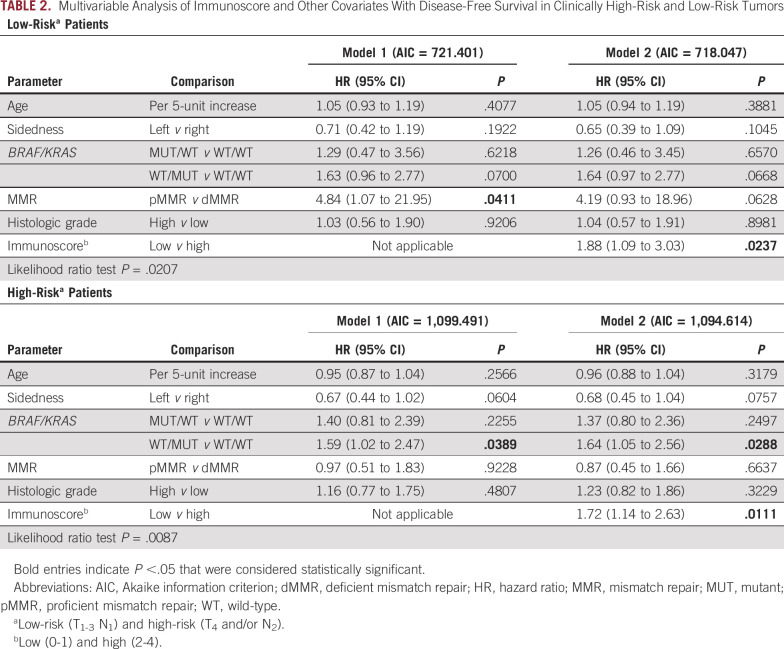
In the overall cohort, we generated and compared a multivariable model that included clinicopathologic and biomarker risk parameters with another model that included the same parameters plus Immunoscore. This comparison revealed a lower Akaike information criterion value and a statistically significant improvement in prognostication for the model containing Immunoscore (likelihood ratio P = .0003, Table 1). In these models, risk group, tumor sidedness, MUT KRAS, and Immunose were each significantly associated with DFS (Table 1). In the multivariable analysis, the proportional hazards assumption was met (chi-square test, P = .069). Of note, a statistically significant interaction was found between Immunoscore and MMR status (Padj = .043) that was limited to low-risk tumors. We then generated multivariable models that evaluated Immunoscore within each risk group. We found that Immunoscore-Low versus Immunoscore-High was significantly associated with poorer DFS in both low-risk and high-risk tumors (Table 2). Among clinically low-risk tumors, the statistically significant association of MMR status with DFS was lost when Immunoscore was added to the model (Table 2). Among high-risk tumors, the finding that MUT KRAS was associated with significantly worse DFS was maintained when Immunoscore was added to the model. We also generated multivariable models in tumors where Immunoscore was Low or High and found that the addition of clinical risk group was significantly associated with patient DFS (Appendix Table A2).
TABLE 1.
Immunoscore Enhances Prognostication in Patients With Stage III Colon Carcinoma
TABLE 2.
Multivariable Analysis of Immunoscore and Other Covariates With Disease-Free Survival in Clinically High-Risk and Low-Risk Tumors
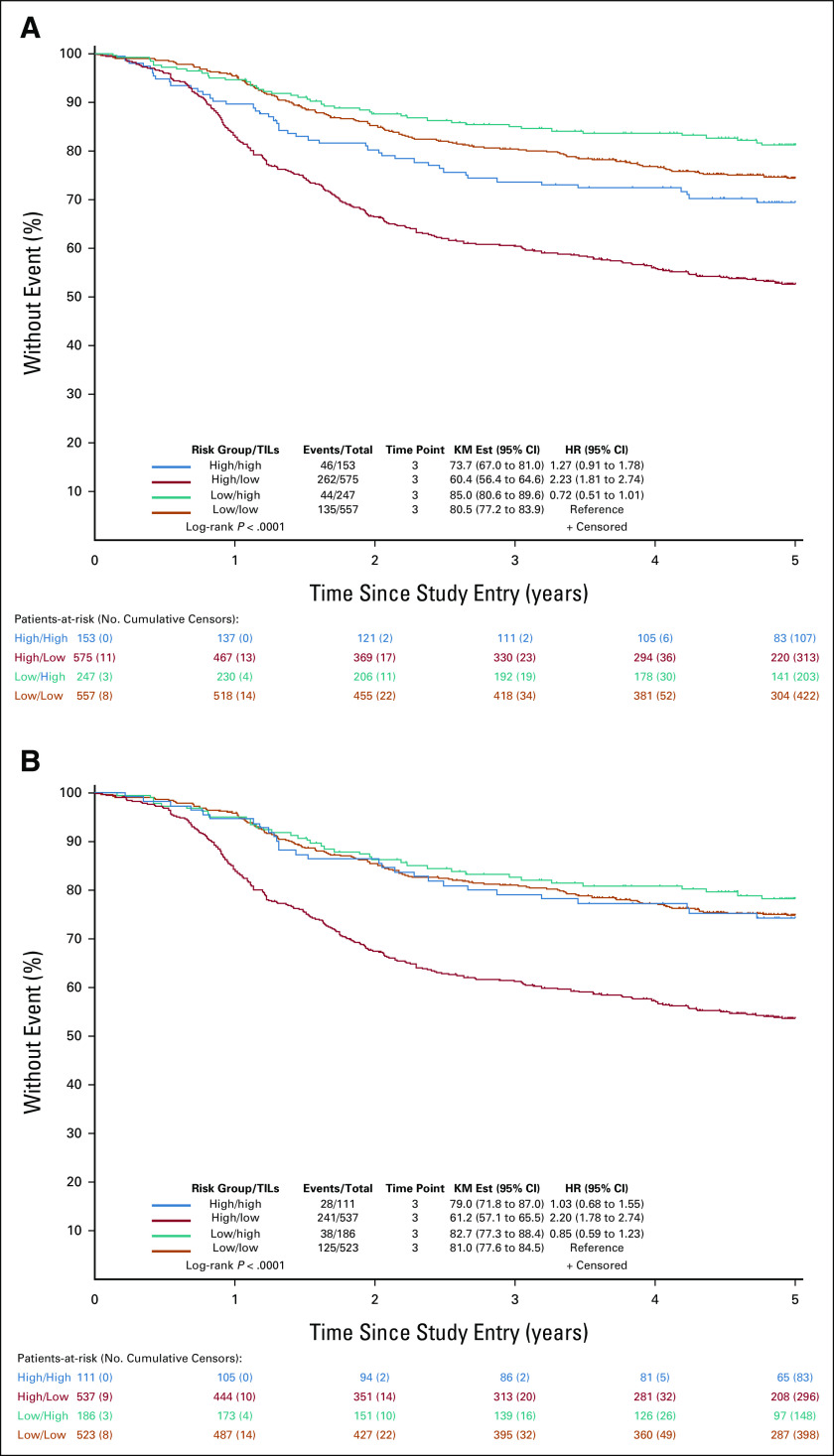
Finally, we compared our results for Immunoscore and prognosis among clinical risk groups with data from another cohort of stage III colon cancers from the N0147 trial (n = 1,532) where TIL densities had been quantified.10 Consistent with data for Immunoscore, the poorest DFS was found for tumors that were clinically high-risk/TIL-Low compared with all other groups (log-rank P < .0001; Appendix Fig A1A). Data from this cohort also supported the observation that differences in DFS between clinically low-risk/TIL-Low tumors and those that were clinically high-risk/TIL-High did not differ significantly (P = .156), and this was especially evident among pMMR tumors (HR, 1.03 [95% CI, 0.68 to 1.55]; P = .890; Appendix Fig A1B).
DISCUSSION
In this study, we found that Immunoscore can refine patient prognosis within clinically low-risk and high-risk stage III colon cancers treated with adjuvant FOLFOX chemotherapy. Patients with Immunoscore-Low versus Immunoscore-High tumors had a significantly higher likelihood of relapse and death in both clinical risk groups. The poorest DFS was observed among patients with clinically high-risk/Immunoscore-Low tumors with a 5-year DFS rate of 55.3%. Importantly, 40% of all high-risk tumors were classified as Immunoscore-Low. The poor outcome of this Immunoscore-defined subset occurred despite all patients receiving 6 months of adjuvant FOLFOX, which underscores the need for novel treatment strategies for these patients. Conversely, the most favorable DFS rate was observed in clinically low-risk/Immunoscore-High tumors with a 5-year DFS rate of 91.8%. DFS rates of the poorest and most favorable groups remained similar when the cohort was restricted to pMMR tumors. Further support for our findings is derived from multivariable models in the overall cohort where the addition of Immunoscore was shown to significantly enhance prognostication compared with a model containing only clinical risk groups and other covariates. Furthermore, multivariable models generated in clinically low-risk and high-risk groups also demonstrated that the addition of Immunoscore can improve survival prediction. In an exploratory analysis, we examined a separate cohort of patients from the N0147 trial where results for TIL densities11 and DFS by clinical risk group were found to be concordant with those obtained for Immunoscore.
We made the important observation that patients with clinically low-risk/Immunoscore-Low tumors had DFS rates that did not differ significantly compared with those with clinically high-risk/Immunoscore-High tumors, and this result was maintained among pMMR cancers. This finding was also supported in a separate cohort of N0147 tumors where differences in DFS between low-risk/TIL-Low tumors compared with those that were high-risk/TIL-High were not statistically different. We regard our finding for Immunoscore and risk group to suggest the hypothesis that high-risk/Immunoscore-High tumors may warrant a similar duration of adjuvant treatment as is recommended for clinically low-risk stage III tumors. Further study, however, is needed to test this important hypothesis. Since all patients in our study received 6 months of adjuvant FOLFOX, it is unknown whether the most favorable prognostic group (low-risk/Immunoscore-High) received significant benefit from adjuvant treatment or whether this outcome (5-year DFS of 91.8%) would have been seen in the absence of treatment. Although we were unable to examine the predictive potential of Immunoscore in our study cohort, analysis of Immunoscore in patients with stage III colon cancer treated in the IDEA France PRODIGE-Gercor study found that patients with Immunoscore-Intermediate + Immunoscore-High tumors showed significantly greater benefit from 6 months versus 3 months of oxaliplatin-based chemotherapy than did Immunoscore-Low tumors.3 These data, although provocative, await validation in independent patient cohorts, and as of this writing, the predictive utility of Immunoscore, if any, awaits further study.
Among clinically low-risk tumors, a statistically significant interaction was observed for MMR status with DFS. In an analysis of pooled data from patients treated with fluoropyrimidine plus oxaliplatin in adjuvant trials that included N0147, an association of deficient MMR status with better DFS was limited to low-risk stage III colon cancers.12 In our study, MMR status was significantly associated with DFS in a multivariable model in low-risk patients. However, this association was no longer significant when Immunoscore was added to the model, indicating that Immunoscore provides information beyond that provided by MMR status.13
Strengths of our study include our clinical trial cohort with mature patient survival data, data on MMR status and limited molecular markers, and a separate cohort from N0147 that enabled an exploratory analysis. In the separate cohort, TIL densities had been manually quantified by light microscopy11 using methodology that has not been standardized to ensure reproducibility, whereas Immunoscore is determined using a centralized and standardized testing platform and has been clinically validated.2,3 Immunoscore has been incorporated into the 2020 ESMO Clinical Practice Guidelines for localized colon cancer to refine prognosis in conjunction with TNM staging.5 Study limitations include the fact that all patients received adjuvant FOLFOX for 6 months such that we were unable to address the predictive utility of Immunoscore for chemotherapy outcomes. In summary, we demonstrate that Immunoscore can enhance patient prognostication beyond clinical risk categorization that is currently used to guide the recommended duration of adjuvant chemotherapy in patients with stage III colon cancer. Further study of Immunoscore is warranted to determine its potential to inform decision making for adjuvant chemotherapy. Specifically, it will be important to determine whether high-risk and Immunoscore-High tumors can receive a similar duration of adjuvant chemotherapy as is recommended for clinically low-risk stage III colon cancers.
APPENDIX
TABLE A1.
Comparison of Clinicopathologic Features in the Study Cohort Versus Other Cases in the FOLFOX Arm of the Parent Study
TABLE A2.
Multivariable Analysis of Clinical Risk Group and Other Covariates With Disease-Free Survival in Immunoscore-High and Immunoscore-Low Tumors
FIG A1.
KM plots of 5-year disease-free survival rates by clinical risk group/TIL density in patients with stage III colon cancer treated with adjuvant infusional fluorouracil, leucovorin, and oxaliplatin. Risk group is categorized as low-risk (T1-3 N1) or high-risk (T4 and/or N2), and TIL densities are dichotomized as low or high (high/high, high/low, low/high, and low/low). Multivariable HRs and 95% CIs are shown. Results are shown for the (A) overall cohort and (B) study cohort restricted to cancers showing proficient mismatch repair. Est, estimate; HR, hazard ratio; KM, Kaplan-Meier; TIL, tumor-infiltrating lymphocyte.
Frank A. Sinicrope
Stock and Other Ownership Interests: Illumina
Honoraria: Targeted Oncology
Consulting or Advisory Role: Guardant Health
Research Funding: Ventana Medical Systems (Inst)
Patents, Royalties, Other Intellectual Property: Patent royalty related to immune markers in colon cancer. Patent jointly held between myself and Roche/Ventana Medical Systems (Inst)
Travel, Accommodations, Expenses: Guardant Health
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Aurelie Catteau
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Graham M. Poage
Employment: Biotheranostics, HalioDx, Veracyte
Stock and Other Ownership Interests: Biotheranostics
Bernhard Mlecnik
Patents, Royalties, Other Intellectual Property: INSERM
Al B. Benson
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma, Bristol Myers Squibb, Samsung Bioepis, Pfizer, HalioDx, AbbVie, Janssen Oncology, Natera, Apexigen, Artemida Pharma, Xencor, TheraBionic, Mirati Therapeutics, Boston Scientific, HUTCHMED
Research Funding: Infinity Pharmaceuticals (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Rafael Pharmaceuticals (Inst), MedImmune (Inst), Xencor (Inst), Astellas Pharma (Inst), Amgen (Inst), SynCoreBio (Inst), Elevar Therapeutics (Inst), Tyme Inc (Inst), ST Pharm (Inst), ITM Solucin (Inst)
Sharlene Gill
Honoraria: Amgen, Taiho Oncology, Eisai
Consulting or Advisory Role: Taiho Pharmaceutical, Amgen, Roche Canada, Eisai, Ipsen, Pfizer, Merck, Bristol Myers Squibb Canada, Bayer
Research Funding: Taiho Pharmaceutical (Inst)
Richard M. Goldberg
Stock and Other Ownership Interests: Advanced Chemotherapy Technologies
Consulting or Advisory Role: Taiho Pharmaceutical, AstraZeneca, Bayer, G1 Therapeutics, Compass Therapeutics, UpToDate, Eisai/H3 Biomedicine, Sorrento Therapeutics, Adaptimmune, IQVIA, GlaxoSmithKline, Merck
Expert Testimony: Taiho Pharmaceutical, Genentech/Roche
Morton S. Kahlenberg
Employment: Tenet Healthcare
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, BioNTech, Gilead Sciences
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Nektar (Inst)
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Esperas Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jerome Galon
Employment: Veracyte
Leadership: HalioDx
Stock and Other Ownership Interests: Veracyte
Consulting or Advisory Role: Northwest Biotherapeutics, CatalYm, Lunaphore Technologies, IO Biotech
Research Funding: Ultivue (Inst), Akoya Biosciences (Inst), ImCheck therapeutics (Inst), AstraZeneca (Inst), NanoString Technologies (Inst)
Patents, Royalties, Other Intellectual Property: INSERM (French NIH)
Travel, Accommodations, Expenses: Veracyte
No other potential conflicts of interest were reported.
SUPPORT
Study investigators received grant support from the US National Cancer Institute (R01 CA210509; to F.A.S.) and from the French National Institute of Health and Medical Research (INSERM; to J.G.). Support for the N0147 trial was provided by Eli Lilly and Company, Pfizer, and Bristol Myers Squibb.
AUTHOR CONTRIBUTIONS
Conception and design: Frank A. Sinicrope, Jerome Galon
Financial support: Frank A. Sinicrope, Aurelie Catteau
Administrative support: Frank A. Sinicrope
Provision of study materials or patients: Frank A. Sinicrope, Al B. Benson, Sharlene Gill, Richard M. Goldberg, Morton S. Kahlenberg, Suresh G. Nair, Anthony F. Shields, Steven R. Alberts
Collection and assembly of data: Frank A. Sinicrope, Thomas C. Smyrk, Steven R. Alberts
Data analysis and interpretation: Frank A. Sinicrope, Qian Shi, Aurelie Catteau, Graham M. Poage, Tyler J. Zemla, Jerome Galon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Frank A. Sinicrope
Stock and Other Ownership Interests: Illumina
Honoraria: Targeted Oncology
Consulting or Advisory Role: Guardant Health
Research Funding: Ventana Medical Systems (Inst)
Patents, Royalties, Other Intellectual Property: Patent royalty related to immune markers in colon cancer. Patent jointly held between myself and Roche/Ventana Medical Systems (Inst)
Travel, Accommodations, Expenses: Guardant Health
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Aurelie Catteau
Employment: Veracyte
Stock and Other Ownership Interests: Veracyte
Travel, Accommodations, Expenses: Veracyte
Graham M. Poage
Employment: Biotheranostics, HalioDx, Veracyte
Stock and Other Ownership Interests: Biotheranostics
Bernhard Mlecnik
Patents, Royalties, Other Intellectual Property: INSERM
Al B. Benson
Consulting or Advisory Role: Merck Sharp & Dohme, Array BioPharma, Bristol Myers Squibb, Samsung Bioepis, Pfizer, HalioDx, AbbVie, Janssen Oncology, Natera, Apexigen, Artemida Pharma, Xencor, TheraBionic, Mirati Therapeutics, Boston Scientific, HUTCHMED
Research Funding: Infinity Pharmaceuticals (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Rafael Pharmaceuticals (Inst), MedImmune (Inst), Xencor (Inst), Astellas Pharma (Inst), Amgen (Inst), SynCoreBio (Inst), Elevar Therapeutics (Inst), Tyme Inc (Inst), ST Pharm (Inst), ITM Solucin (Inst)
Sharlene Gill
Honoraria: Amgen, Taiho Oncology, Eisai
Consulting or Advisory Role: Taiho Pharmaceutical, Amgen, Roche Canada, Eisai, Ipsen, Pfizer, Merck, Bristol Myers Squibb Canada, Bayer
Research Funding: Taiho Pharmaceutical (Inst)
Richard M. Goldberg
Stock and Other Ownership Interests: Advanced Chemotherapy Technologies
Consulting or Advisory Role: Taiho Pharmaceutical, AstraZeneca, Bayer, G1 Therapeutics, Compass Therapeutics, UpToDate, Eisai/H3 Biomedicine, Sorrento Therapeutics, Adaptimmune, IQVIA, GlaxoSmithKline, Merck
Expert Testimony: Taiho Pharmaceutical, Genentech/Roche
Morton S. Kahlenberg
Employment: Tenet Healthcare
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, BioNTech, Gilead Sciences
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Nektar (Inst)
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Esperas Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jerome Galon
Employment: Veracyte
Leadership: HalioDx
Stock and Other Ownership Interests: Veracyte
Consulting or Advisory Role: Northwest Biotherapeutics, CatalYm, Lunaphore Technologies, IO Biotech
Research Funding: Ultivue (Inst), Akoya Biosciences (Inst), ImCheck therapeutics (Inst), AstraZeneca (Inst), NanoString Technologies (Inst)
Patents, Royalties, Other Intellectual Property: INSERM (French NIH)
Travel, Accommodations, Expenses: Veracyte
No other potential conflicts of interest were reported.
REFERENCES
- 1. Mlecnik B, Bifulco C, Bindea G, et al. Multicenter International Society for Immunotherapy of Cancer study of the consensus Immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;38:3638–3651. doi: 10.1200/JCO.19.03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 3. Pages F, Andre T, Taieb J, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. doi: 10.1016/j.annonc.2020.03.310. [DOI] [PubMed] [Google Scholar]
- 4. Sinicrope FA, Shi Q, Hermitte F, et al. Contribution of Immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:pkaa023. doi: 10.1093/jncics/pkaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argiles G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 6. Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378:1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angell H, Galon J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 9. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10. Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H, Sha D, Foster NR, et al. Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance) Ann Oncol. 2020;31:487–494. doi: 10.1016/j.annonc.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen R, Taieb J, Fiskum J, et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol. 2021;39:642–651. doi: 10.1200/JCO.20.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]