PURPOSE
The development of the selective RET inhibitors selpercatinib and pralsetinib has revolutionized the treatment of metastatic progressive RET-mutant medullary thyroid carcinoma (MTC) and other RET-driven cancers, given their more favorable side-effect profile. The aim of this study is to investigate the mechanisms of selpercatinib-induced thyroid dysfunction in athyreotic patients with RET-mutant MTC and in patients with RET-mutant non–small-cell lung cancer (NSCLC) who had a functional thyroid.
MATERIALS AND METHODS
Thyroid hormone levels were evaluated in an observational cohort of five athyreotic patients with MTC and 30 patients with NSCLC before and after initiation of selpercatinib. In vitro experiments to identify the mechanism of selpercatinib-induced thyroid dysfunction were conducted in cells expressing endogenous D1, D2, and D3 iodothyronine deiodinases.
RESULTS
Upon initiating treatment with selpercatinib, athyreotic patients developed clinical hypothyroidism with approximately 60% lower T3 levels despite adequate levothyroxine supplementation, whereas in patients with NSCLC, who retain a normal thyroid, selpercatinib resulted in a more attenuated reduction in serum T3, which was dose-dependent. We conducted studies in cells endogenously expressing either D1, D2, or D3, the three iodothyronine deiodinases. Selpercatinib inhibited D2-mediated T3 production in MSTO-211 cells by 50%. A modest repression of D2 mRNA was present in human thyroid cancer TT cells that express RET, but not in the MSTO-211 cells that do not. No effect of the drug was observed on D1 (activating deiodinase) or D3 (inactivating deiodinase). Thus, a nontranscriptional effect of selpercatinib on D2 activity is the most plausible explanation for the low T3 levels.
CONCLUSION
An off-target effect of selpercatinib on D2-mediated T3 production leads to clinical hypothyroidism, primarily in levothyroxine-treated athyreotic patients. Liothyronine supplementation was needed to achieve normal T3 levels and restore clinical euthyroidism.
INTRODUCTION
Activating receptor tyrosine kinase RET gene alterations have been identified as oncogenic drivers in multiple malignancies. RET gene rearrangements leading to the illegitimate expression of its kinase domain are oncogenic drivers in papillary thyroid cancer and non–small-cell lung cancer (NSCLC), among others. Activating somatic and germline point mutations of RET are the main drivers of sporadic and familial medullary thyroid carcinoma (MTC) syndromes, respectively.1
CONTEXT
Key Objective
We found that the selective RET-inhibitor selpercatinib caused a sharp decrease in serum T3 levels immediately after initiation of the drug. Here, we investigate the mechanisms underlying this observation in athyreotic patients with RET-mutant medullary thyroid carcinoma and in patients with RET fusion–positive lung cancer who had a functional thyroid.
Knowledge Generated
Treatment with selpercatinib inhibited T3 production in a dose-dependent fashion (up to 50%) in MSTO-211 cells, which endogenously express the type 2 iodothyronine deiodinase (D2) that converts T4 to T3. A modest repression of D2 mRNA was only found in human thyroid cancer TT cells that express RET, suggesting a predominant nontranscriptional effect of selpercatinib on D2 activity. No effect of the drug was observed on the other deiodinases, D1 or D3.
Relevance
Liothyronine supplementation may be needed to restore normal T3 levels and resolve symptoms of hypothyroidism in levothyroxine-treated athyreotic patients receiving selpercatinib.
Many multikinase inhibitors have activity against RET. Among them, vandetanib and cabozantinib are approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of advanced MTC. As a result of the nonselective nature of these drugs (they also inhibit VEGFR2, among other kinases) and their narrow therapeutic window, patients develop adverse events including hypertension, rash, and diarrhea that often require dose reductions, limiting their inhibitory effect on RET kinase activity. In 2020, the US Food and Drug Administration approved selpercatinib (LOXO‐292) and pralsetinib (BLU667) for the treatment of adult patients with metastatic RET fusion–positive NSCLC and patients age 12 years and older with advanced or metastatic RET-mutant MTC or RET fusion–positive thyroid cancer who require systemic therapy,2,3 thus revolutionizing the treatment of RET-mutant malignancies.
The clinical trials of selpercatinib and pralsetinib reported only grade 1 or 2 adverse events, with only 1.7%4,5 and 2.9%6,7 of patients needing treatment discontinuation, respectively. This compares with the 21% and 8% drug discontinuation rate with vandetanib and cabozantinib, respectively. Despite the good tolerability of the new selective RET inhibitors, the full extent of potential off-target effects is unknown.
Here, we show that an important off-target effect of selpercatinib is inhibition of extrathyroidal T3 production through decreased activity of the type 2 iodothyronine deiodinase (D2). After identifying an index case, we expanded these observations to a series of levothyroxine (LT4)-supplemented thyroidectomized patients treated with selpercatinib that required addition of liothyronine to sustain their serum T3 levels within the normal reference range and render them clinically and biochemically euthyroid, and contrasted these findings to selpercatinib-treated patients with NSCLC, who had a normal thyroid.
MATERIALS AND METHODS
Patients with MTC and NSCLC receiving selpercatinib as part of their clinical treatment at Memorial Sloan Kettering Cancer Center had thyroid function tests before and after initiation of the drug. This study was approved by the Memorial Sloan Kettering Institutional Review Board (IRB #17-256).
Deiodinase Studies
All experiments with animals were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Deiodinase 1.
Alpha mouse liver cells (AML-12) were grown as described8 in 6-well collagen I–coated plates and William's medium E plus primary hepatocyte maintenance supplements (GIBCO) containing 10 nM T3. For the assay, 125I-T4 (106 cpm; 100 µCi/mmol; Perkin Elmer Co, Waltham, MA) was added to 70%-80% confluent cells. Twenty-four hours later, 150 µl of medium was mixed 1:1 volume of ice-cold methanol. The supernatant was then mixed with 300 µl of 0.02 M ammonium acetate plus 4% methanol plus 4% PE buffer (0.1 M phosphate-buffered saline [PBS], 1 mM ethylenediaminetetraacetic acid), and applied to the ultra performance liquid chromatography column. The iodothyronine fractions were identified through an in-tandem flow scintillator detector. In some assays, cells were preincubated for 24 hours with 100 nM selpercatinib (LOXO-292; Selleck Co, Houston, TX), and control cells were incubated with the same volume of vehicle (dimethyl sulphoxide [DMSO]). The results were expressed as fractional conversion/hours/mg of protein.
Deiodinase 2.
Mesothelioma cells (MSTO-211) were grown as described9 in 6-well plates and RPMI supplemented with 10% fetal bovine serum. For the assay, 125I-T4 (106 cpm) was added to 70%-80% confluent cells, and the fetal bovine serum was reduced to 1%.10 Twenty-four hours later, 150 µL of medium was collected and studied as above. In some assays, cells were preincubated for 24 hours with 5-500 nM selpercatinib, 5-50 nM pralsetinib (BLU-667), 250 nM cabozantinib (BMS-907351; Selleck Co), or 20 mM N-acetyl-cysteine amide (NACA; Tochris, Minneapolis, MN), and control cells incubated with the same volume of vehicle only (DMSO).
MSTO-211 cells were washed twice with PBS, harvested, sonicated in 0.25 M sucrose in PE buffer, and processed for D2 assay.10 Essentially, 100 µg protein sonicate was incubated at 37°C in the presence of 10 mM dithiothreitol, 0.25 M sucrose, and 1μM 125I-T4 (approximately 250,000 cpm) for 3 hours. Reaction was stopped by moving the tubes to ice-cold water and addition of 1:1 volume of ice-cold methanol, and subsequently processed as described for the D1 assay. In some assays, reactions occurred in the presence of 1-100 nM selpercatinib, and control tubes were incubated with the same volume of vehicle only (DMSO).
Deiodinase 3.
Embryonic day 16 C57BL/6 WT mice were harvested from pregnant dams and processed for isolation of cortical neurons as previously described.11 Cells were grown for 5 days in full neurobasal media. For the assay, 125I-T3 (106 cpm) was added to the maintenance medium and 24 hours later, 150 µL of medium was collected and studied as above. In some assays, cells were preincubated for 24 hours with 100 nM selpercatinib, and control cells were incubated with the same volume of vehicle only (DMSO).
Real time–Quantitative Polymerase Chain Reaction
After incubations, MSTO cells or human thyroid cancer TT cells (MTC cell line with a RET-C634W mutation) were washed twice with PBS, harvested, and total RNA was extracted with a RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer's protocol. DNA contaminants were digested with DNase I (Ambion, Austin, TX). Total RNA (1.0 µg) was reverse-transcribed with the High-Capacity complementary DNA Reverse Transcription Kit (Roche, Basel, Switzerland), and 10 ng complementary DNA was used in each reaction. Deiodinase expression was analyzed using specific primers (IDT Technologies, Coralville, IA) using Sybr Fast Universal PCR Master Mix (Thermo Fisher Scientific, Waltham, MA). Expression of β-actin was used as a housekeeping gene. Reactions were assayed on StepOne Applied Biosystems Real-Time PCR instrument (Applied Biosystems, Waltham, MA). The primer sequences for DIO2 were forward: 5′-GCTGCTGTTGAGCCGCTC-3′, reverse: 5′-GCTCAGGGCTGGCAAAGT-3′. Primer sequences for RET were RET-1 forward: 5′-CTCCCTTCCACATGGATTGA, RET-1 reverse: 5′-TCAGCTCTCGTGAGTGGTACA, RET-2 forward: 5′-CCGTGAAGATGCTGAAAGAGA, RET-2 reverse: 5′-CCTGCTTCAGGACGTTGAA.
Thyroid Function Tests
Thyroid stimulating hormone (TSH), free T4, total T3, and free T3 were measured using the ARCHITECT assays (Abbott Laboratories, Abbott Park, IL), which are chemiluminescent microparticle immunoassays. Reverse T3 was measured at Mayo Clinic Laboratories with a chemiluminescent immunoassay. The normal ranges for these hormones were TSH: 0.35-4.94 mIU/L; free T4: 0.7-1.50 ng/dL; total T3 90-168 ng/dL; free T3 2.3-4.2 pg/mL; and reverse T3 10-24 ng/dL.
RESULTS
Case Reports
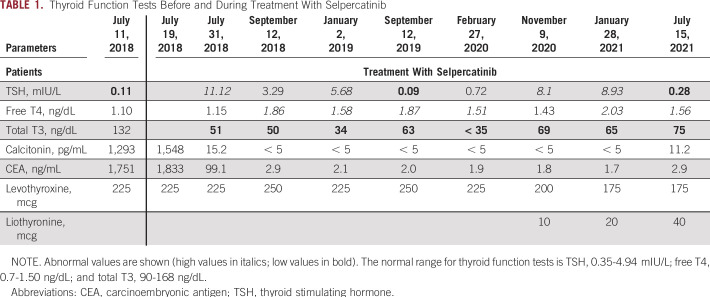
The index case is a 29-year-old man who underwent a total thyroidectomy and bilateral central compartment dissection in 2002 at age 10 years for a multifocal MTC. He is a carrier of germline heterozygous RET-M918T mutation. In 2016, he was found to have widespread bone metastases in the context of a right hip fracture. In 2017, he received vandetanib, followed by cabozantinib because of progression of disease. In 2018, he was briefly treated with pembrolizumab, radiation therapy, and denosumab. The patient's calcitonin and carcinoembryonic antigen (CEA) were 1,548 pg/mL and 1,833 ng/mL, respectively. Within two months of treatment with selpercatinib, the patient's calcitonin became undetectable at < 5 pg/mL, and CEA 2.9 ng/mL (Table 1). The calcitonin and CEA levels remained undetectable with a complete structural response by RECIST criteria on cross-sectional imaging studies. Before receiving selpercatinib, thyroid function tests showed that he was slightly over-replaced (TSH = 0.11 mIU/L) while on 225 mcg LT4. After starting selpercatinib, T3 levels were noted to be consistently low, with TSH levels rising intermittently into the hypothyroid range despite levothyroxine doses ranging between 225 and 250 mcg PO daily. Addition of 10-40 mcg liothyronine PO daily in combination with 175 mcg/day of LT4 eventually restored euthyroidism.
TABLE 1.
Thyroid Function Tests Before and During Treatment With Selpercatinib
Five additional patients treated with selpercatinib while on thyroid hormone replacement therapy were identified (Table 2). All had normal T3 levels before initiation of selpercatinib and reduced total and free T3 levels as early as one month after initiation of the drug. Reverse T3 levels were measured in a subset of patients and were consistently elevated (Table 2). Patient 1 is of interest because he did not have a thyroidectomy, as he presented with unresectable disease. He only required a low dose of LT4 and, despite a functional thyroid gland, his T3 levels also decreased, although to a lesser degree.
TABLE 2.
Thyroid Function Tests Before and During Treatment With Selpercatinib in Patients With Levothyroxine Replacement Therapy
We had a total of six patients with metastatic RET fusion–positive papillary thyroid cancer thyroidectomized and treated with selpercatinib. All six of them developed low total T3 levels immediately after starting selpercatinib.
To investigate the effects of selpercatinib on patients who had not had a thyroidectomy, we measured thyroid function tests in patients with NSCLC harboring RET kinase fusions. In these patients, there was a decrease in total T3 levels after initiation of selpercatinib, but not as marked as that seen in thyroidectomized patients. The mean difference in T3 levels before and after selpercatinib was 41 ng/dL among patients with an intact thyroid (n = 30; 45% decrease from baseline), whereas it was 65.4 ng/dL among thyroidectomized patients (n = 5; 63% decrease from baseline; P = .07). TSH levels rose to an average of 4.8 mIU/L in patients with NSCLC compared with 10.9 mIU/L in thyroidectomized patients, P = .06. Only 6 of 30 patients with NSCLC required levothyroxine replacement with a maximum dose of 50 mcg daily. The effects of selpercatinib in patients with NSCLC was dose-dependent: up to 60-80 mg twice a day did not lower total T3 levels significantly, whereas doses of 120 mg twice a day and higher resulted in substantial declines in total T3 to levels below the lower limit of the reference range (Table 3).
TABLE 3.
Total T3 Levels With Increasing Doses of Selpercatinib
Selpercatinib Decreases D2-Mediated T3 Production
To define the mechanism through which treatment with selpercatinib reduces serum T3 levels, we next investigated whether deiodinases were involved. We first tested whether T3 production via D2 was affected, knowing that this is the main source of circulating T3 in levothyroxine-maintained hypothyroid individuals.12 For these experiments, we selected MSTO-211 cells that express D2 endogenously, but not the other deiodinases.9 In the control conditions, incubation with 125I-T4 for 24 hours led to the production of about 1.6 pmol T3/hours/mg protein. However, exposure to 50-500 nM selpercatinib reduced T3 production in a dose-dependent fashion, down to 50% of baseline values (Fig 1A). To find out if this effect of selpercatinib extends to other members of this family of Ret kinase inhibitors, we next studied T3 production in MSTO-211 cells in the presence of 50 nM pralsetinib or 250 nM cabozantinib, but only pralsetinib decreased T3 production by about 10% (Fig 1B).
FIG 1.

(A) Effects of selpercatinib on D2-mediated T3 production in MSTO cells. Media samples were collected after 24 hours. (B) Same as in (A), except that MSTO cells were treated with the RET kinase inhibitors pralsetinib and cabozantinib. (C) Effects of selpercatinib on DIO2 mRNA levels in MSTO cells. (D) RET-1 and (E) RET-2 mRNA levels in MSTO and TT cells. (F) Same as in (C), but in TT cells. (G) Effects of selpercatinib on T4 deiodination in MSTO cell sonicates. (H) Same as in (A), except that NACA was added. (I) Effects of selpercatinib on D1-mediated T3 production in AML12 cells. (J) Effects of selpercatinib on D3-mediated T3 inactivation in primary neuronal cultures. Values are mean ± SD of 5-9 independent wells; *P < .05, **P < .01, ***P < .001; ****P < .0001 versus vehicle. DIO2/B-ACTIN, type 2 iodothyronine deiodinase/beta-actin; MSTO cells, mesothelioma cells; NACA, N-acetyl-cysteine amide; ns, not significant; PR, pralsetinib; SE, selpercatinib; TT, human thyroid cancer TT cells.
Next, we tested whether selpercatinib affected DIO2 expression. We measured DIO2 mRNA levels in MSTO-211 cells during treatment with 25-100 nM selpercatinib, but no effects were observed (Fig 1C). Selpercatinib inhibits RET kinase activity, but MSTO-211 cells do not express RET (Figs 1D and 1E). Thus, we also tested TT cells, which express low levels of DIO2 but do express RET, which is constitutively activated in these MTC cells. In this setting, treatment with 5-50 nM selpercatinib decreased DIO2 mRNA levels by 60% (Fig 1F).
Because there is a robust inhibition of selpercatinib on D2-mediated T3 production in MSTO-211 cells, which do not express RET, it is likely that this is occurring through an off-target effect. To explore a direct interference of selpercatinib on the D2 enzyme, we tested whether in vitro incubation of MSTO-211 cell sonicates with 1-100 nM selpercatinib inhibited D2 activity. No interference in T3 production was observed (Fig 1G).
Thus, selpercatinib inhibits D2-mediated T3 production in intact MSTO-211 cells but does not inhibit D2 enzymatic activity in cell sonicates. These results could reflect an interference of selpercatinib with D2's endogenous cofactor (when assayed in vitro, an excess of the artificial cofactor dithiothreitol is used). To test this possibility, we attempted to rescue the inhibitory effect of selpercatinib on D2-mediated T3 production by incubating MSTO-211 cells with 20 mM NACA, which failed to prevent the inhibition of T3 production by selpercatinib (Fig 1H).
We also explored the possibility that selpercatinib inhibited T3 production via D1. This was done by studying D1-mediated T3 production in AML12 liver cells, which only express D1, in the presence of 100 nM selpercatinib. However, no effects were observed (Fig 1I). Similarly, we tested whether selpercatinib could be shortening T3 half-life by accelerating its clearance via D3. This was tested by studying D3-mediated T3 inactivation in primary neuronal cultures in the presence of 100 nM selpercatinib. Again, no effects of the drug were observed (Fig 1J).
DISCUSSION
The development of novel RET-selective inhibitors represents a major step forward in the treatment of patients with advanced RET fusion–positive NSCLCs and thyroid cancers with activating RET mutations. Their impressive response rate and durable activity against RET-mutant tumors coupled with their beneficial safety profile make these selective inhibitors an attractive therapeutic option. The randomized phase III LIBRETTO-431 (ClinicalTrials.gov identifier: NCT04194944) and LIBRETTO-531 (ClinicalTrials.gov identifier: NCT04211337) trials are further evaluating the efficacy of selpercatinib in patients with RET fusion–positive NSCLC and treatment-naive RET-mutant MTC, respectively. The phase II LIBRETTO-321 trial (ClinicalTrials.gov identifier: NCT04280081) is underway in China evaluating the efficacy of selpercatinib in patients with advanced solid tumors including RET fusion–positive solid tumors, MTC, and other tumors with RET activation. LIBRETTO-121 (ClinicalTrials.gov identifier: NCT03899792) is an ongoing multicenter phase I/II dose escalation trial in patients age 6 months to 21 years with advanced, RET-altered solid and CNS tumors. These trials will hopefully shed light on the full therapeutic potential of these drugs, spectrum of side effects, and off-target effects not recognized to date.
In this paper, we describe the inhibitory effect of selpercatinib on D2-mediated T3 production, which in athyreotic patients required the addition of liothyronine to supplement levothyroxine replacement to sustain normal T3 levels and relieve symptoms of hypothyroidism (Table 1). At the same time, treatment with selpercatinib in patients with a functioning thyroid gland resulted in a more modest reduction of serum T3, because any decrease in T4 deiodination is likely compensated through TSH stimulation of thyroidal T3 biosynthesis and secretion. All patients treated with selpercatinib exhibited an elevation in serum TSH levels in the presence of relatively higher serum T4 levels (Tables 1 and 2), but the degree of TSH elevation in patients with a functioning thyroid was less pronounced. Although this could be the result of falling serum T3 levels, it could also reflect the role played by D2 in the T4-mediated TSH feedback mechanism. D2 is highly expressed in the median eminence13,14 and thyrocytes,15 both structures where T4 is converted to T3 to inhibit secretion of TRH and TSH, respectively. We observed a dose-response effect of selpercatinib on T3 reduction (Table 3), which provides strong evidence of the effect of the drug on the type 2 iodothyronine deiodinase.
In thyroidectomized patients maintained on LT4, D1- and D2-mediated deiodination of T4 are the only sources of T3, which is the biologically active thyroid hormone. D2 accounts for approximately 80% of the T3 production. This is based on evidence from LT4-treated athyreotic patients treated with propylthiouracil, which selectively inhibits D1, and which only reduced serum T3 levels by about 20%.12 Therefore, the magnitude of the drop in serum T3 levels observed in the selpercatinib-treated cases was suggestive of a D2 involvement.
The studies performed in intact D2-expressing cells conclusively show that selpercatinib inhibits D2-mediated T3 production (Figs 1A and 1B). We saw a modest inhibition of D2 in vitro with pralsetinib as well (Fig 1B); however, there were no patients at our institution treated with pralsetinib, so we cannot confirm whether this drug resulted in decreased T3 levels in clinical practice. A transcriptional repression of D2 was documented in TT cells that express RET (Fig 1F), but not in the MSTO-211 cells that lack significant amounts of RET (Fig 1C). Thus, it is conceivable that in cells that express RET and D2, a transcriptional effect might contribute to repression of T3 production. However, only a limited set of cell types and tissues express the RET receptor,16 so it is unlikely that on-target effects of selpercatinib on RET kinase activity explain the low T3 levels observed in patients.
Hence, the predominant effect on T3 production seems to be nontranscriptional, given the approximately 50% drop observed in MSTO-211 cells exposed to selpercatinib (Fig 1A). Different compounds have been shown to nonselectively inhibit the D2 pathway, ie, iopanoic acid, gold, and amiodarone, through an allosteric effect on the enzyme.17,18 This was not the case with selpercatinib, as its addition at high concentrations in the D2 assay (performed in cell sonicates) did not inhibit T3 production (Fig 1G).
The nature of the D2-mediated deiodination reaction involves oxidation of the active center followed by a sequential reduction by a cofactor needed for the next round of deiodination. Thus, it is conceivable that selpercatinib interferes with cofactor/s required for reactivation of the enzyme and not the enzyme's catalytic mechanism per se. We would not see this in cell sonicates because of the vast excess of dithiothreitol, the artificial cofactor used to sustain the reaction, but we would see this in intact cells, which rely on the constant regeneration of an endogenous cofactor. Although we failed to rescue the selpercatinib-mediated inhibition of T3 production with the antioxidant NACA (Fig 1H), we cannot exclude this possibility because the endogenous D2 cofactor is not known.
The increase in rT3 seen in a subset of our patients could be mediated by three independent mechanisms: inhibition of the D1 pathway (D1 is the main enzyme involved in rT3 clearance), acceleration of the D3 pathway (D3 converts T4 to rT3), and/or elevation of T4 serum levels. The latter seems more logical, given that selpercatinib did not interfere with D1 or D3 activities, and FT4 levels remained at the upper limit or above the normal range.
To our knowledge, the effect of tyrosine kinase inhibitors on peripheral metabolism of thyroid hormones was first proposed by de Groot et al19 in eight athyreotic patients with MTC receiving imatinib. He observed significant elevations of TSH with symptoms of hypothyroidism that did not reverse with increased levothyroxine (LT4) therapy. The authors hypothesized that an enhanced T4 and T3 clearance was the most likely cause of this thyroid dysfunction. Adulrahman et al20 conducted a prospective study on 21 athyreotic patients with non-MTC who received sorafenib for 26 weeks and observed higher requirements for LT4 and significantly decreased T3:T4, T3:rT3, and T4:rT3 ratios, consistent with D3 induction. Braun et al21 demonstrated that tyrosine kinase inhibitors inhibited MCT8-dependent T3 and T4 uptake leading to partial inhibition of pituitary and hypothalamic thyroid hormone feedback. Kappers et al22 also documented decreased T3:rT3 ratios in 15 patients after 4 weeks of sunitinib therapy. Moreover, they found that rats exposed to sunitinib had increased hepatic D3 activity as well as inhibition of D1, with corresponding decreases in T3 and T4 levels. To our knowledge, D3 activity has not been reported in MTC. In addition, the timeline does not support this possibility, given that at baseline, the total and free T3 concentrations in our patients were in the normal range. The drop in T3 serum levels was only observed within 10 days of initiating selpercatinib in athyreotic patients, which persisted despite varying doses of LT4 replacement (Table 1) and normal levels of T4 (Table 2). In addition, we found no effect of selpercatinib on D3 activity in D3-expressing cells (Fig 1J).
To our knowledge, D2 inhibition has not been previously implicated as an off-target effect of any other kinase inhibitor. This is clinically consequential, as liothyronine supplementation may be needed to achieve normal T3 levels and treat hypothyroid symptoms in athyreotic patients receiving this drug.
Eric Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis
Research Funding: Plexxikon (Inst), Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst)
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Ipsen, Bridge Medicines, Syros Pharmaceuticals, Amgen, Jazz Pharmaceuticals, Epizyme, Syros Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck
Research Funding: Merck (Inst), Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, TP Therapeutics, Verastem, Ignyta/Genetech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, RV More
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Bio
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Merus, Boehringer Ingelheim
Antonio C. Bianco
Consulting or Advisory Role: AbbVie, Allergan, Syntonics, Sention, Thyron
James A. Fagin
Honoraria: Eisai Europe
Consulting or Advisory Role: Loxo
Patents, Royalties, Other Intellectual Property: 3. MSK Ref. SK 2014-024-03 Title: Treatment of H-RAS-Driven Tumors. Application Number: 15/305,788, Anti-TSHR Multispecific Antibodies and Uses Thereof. Provisional patent (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by grant P30-CA008748 (Craig Thompson PI).
L.B. and F.S.-L. are both first authors.
AUTHOR CONTRIBUTIONS
Conception and design: Laura Boucai, Antonio C. Bianco, James A. Fagin
Financial support: Laura Boucai, Antonio C. Bianco, James A. Fagin
Administrative support: Laura Boucai
Provision of study materials or patients: Laura Boucai, Eric Sherman, Charles M. Rudin, Alexander Drilon, James A. Fagin
Collection and assembly of data: Laura Boucai, Federico Salas-Lucia, Gnana P. Krishnamoorthy, Eric Sherman, Charles M. Rudin, Alexander Drilon
Data analysis and interpretation: Laura Boucai, Federico Salas-Lucia, Gnana P. Krishnamoorthy, Alexander Drilon, Antonio C. Bianco, James A. Fagin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric Sherman
Consulting or Advisory Role: Eisai, UpToDate, Lilly, Blueprint Medicines, Exelixis
Research Funding: Plexxikon (Inst), Regeneron (Inst), Lilly (Inst), HUTCHMED (Inst), Novartis (Inst)
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Ipsen, Bridge Medicines, Syros Pharmaceuticals, Amgen, Jazz Pharmaceuticals, Epizyme, Syros Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck
Research Funding: Merck (Inst), Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Alexander Drilon
Stock and Other Ownership Interests: Treeline Biosciences
Honoraria: Pfizer, Loxo/Bayer/Lilly, IASLC, Helsinn Therapeutics, BeiGene, Remedica, TP Therapeutics, Verastem, Ignyta/Genetech/Roche, AstraZeneca, Liberum, Lungevity, NIH, PER, OncLive/MJH Life Sciences, Clinical Care Options/NCCN, Lung Cancer Research Foundation, Associazione Italiana Oncologia Toracica (AIOT), Chugai Pharma, Sirio Libanes Hospital, Answers in CME, Research to Practice, RV More
Consulting or Advisory Role: Ignyta, Loxo, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Takeda/Millennium, BerGenBio, MORE Health, Lilly, AbbVie, 14ner Oncology/Elevation Oncology, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Repare Therapeutics, Melendi, Archer, Nuvalent, Inc, Janssen, Amgen, Merus, Axis Pharma, Medscape, Liberum, Med Learning, PeerView, EPG Health, Journal of the National Comprehensive Cancer Network, Ology Medical Education, Clinical Care Options, touchIME, Entos, Prelude Therapeutics, Applied Pharmaceutical Science, Treeline Bio
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology, Merus, Boehringer Ingelheim
Antonio C. Bianco
Consulting or Advisory Role: AbbVie, Allergan, Syntonics, Sention, Thyron
James A. Fagin
Honoraria: Eisai Europe
Consulting or Advisory Role: Loxo
Patents, Royalties, Other Intellectual Property: 3. MSK Ref. SK 2014-024-03 Title: Treatment of H-RAS-Driven Tumors. Application Number: 15/305,788, Anti-TSHR Multispecific Antibodies and Uses Thereof. Provisional patent (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Fagin JA, Wells SA., Jr Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:2307. doi: 10.1056/NEJMc1613118. [DOI] [PubMed] [Google Scholar]
- 2. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 4. Drilon A. Registrational results of LIBRETTO-001: A phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol. 2019;14:S6–S7. [Google Scholar]
- 5. Wirth L. Registrational results of LOXO-292 in patients with RET-altered thyroid cancers. Ann Oncol. 2019;30 suppl 5; abstr V933. [Google Scholar]
- 6. Taylor MH, Gainor JF, I-Nan Hu M, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC) J Clin Oncol. 2019;37 suppl 15; abstr 6018. [Google Scholar]
- 7. Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients with advanced RET-Fusion+Non-small cell lung cancer. J Clin Oncol. 2019;37 suppl; abstr 9008. [Google Scholar]
- 8. Fernandes GW, Bocco BM, Fonseca TL, et al. The FoxO1-inducible transcriptional repressor Zfp125 causes hepatic steatosis and hypercholesterolemia. Cell Rep. 2018;22:523–534. doi: 10.1016/j.celrep.2017.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curcio C, Baqui MM, Salvatore D, et al. The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J Biol Chem. 2001;276:30183–30187. doi: 10.1074/jbc.C100325200. [DOI] [PubMed] [Google Scholar]
- 10. Bianco AC, Anderson G, Forrest D, et al. American Thyroid Association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. doi: 10.1089/thy.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jo S, Kallo I, Bardoczi Z, et al. Neuronal hypoxia induces hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J Neurosci. 2012;32:8491–8500. doi: 10.1523/JNEUROSCI.6514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geffner DL, Azukizawa M, Hershman JM. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975;55:224–229. doi: 10.1172/JCI107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tu HM, Kim SW, Salvatore D, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 14. Guadano-Ferraz A, Obregon MJ, St Germain DL, et al. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA. 1997;94:10391–10396. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christoffolete MA, Ribeiro R, Singru P, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743. doi: 10.1210/en.2005-1300. [DOI] [PubMed] [Google Scholar]
- 16.The Human Protein Atlas https://www.proteinatlas.org/ENSG00000165731-RET/tissue
- 17. Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 18. Rosene ML, Wittmann G, Arrojo e Drigo R, et al. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology. 2010;151:5961–5970. doi: 10.1210/en.2010-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Groot JW, Zonnenberg BA, Plukker JT, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther. 2005;78:433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 20. Abdulrahman RM, Verloop H, Hoftijzer H, et al. Sorafenib-induced hypothyroidism is associated with increased type 3 deiodination. J Clin Endocrinol Metab. 2010;95:3758–3762. doi: 10.1210/jc.2009-2507. [DOI] [PubMed] [Google Scholar]
- 21. Braun D, Kim TD, le Coutre P, et al. Tyrosine kinase inhibitors noncompetitively inhibit MCT8-mediated iodothyronine transport. J Clin Endocrinol Metab. 2012;97:E100–E105. doi: 10.1210/jc.2011-1837. [DOI] [PubMed] [Google Scholar]
- 22. Kappers MH, van Esch JH, Smedts FM, et al. Sunitinib-induced hypothyroidism is due to induction of type 3 deiodinase activity and thyroidal capillary regression. J Clin Endocrinol Metab. 2011;96:3087–3094. doi: 10.1210/jc.2011-1172. [DOI] [PubMed] [Google Scholar]