Abstract
Background and Objectives
Remdesivir is an antiviral drug used to treat coronavirus disease 2019 (COVID-19) with a relatively obscure cardiac effect profile. Previous studies have reported bradycardia associated with remdesivir, but few have examined its clinical characteristics. The objective of this study was to investigate remdesivir associated bradycardia and its associated clinical characteristics and outcomes.
Methods
This is a single-institution retrospective study that investigated bradycardia in 600 patients who received remdesivir for treatment of COVID-19. A total of 375 patients were included in the study after screening for other known causes of bradycardia (atrioventricular [AV] nodal blockers). All patients were analyzed for episodes of bradycardia from when remdesivir was initiated up to 5 days after completion, a time frame based on the drug’s putative elimination half-life. Univariate and multivariate statistical tests were conducted to analyze the data.
Results
The mean age of the sample was 56.63 ± 13.23 years. Of patients who met inclusion criteria, 49% were found to have bradycardia within 5 days of remdesivir administration. Compared to the cohort without a documented bradycardic episode, patients with bradycardia were significantly more likely to experience inpatient mortality (22% vs 12%, p = 0.01). The patients with bradycardia were found to have marginally higher serum D-dimer levels (5.2 vs 3.4 µg/mL, p = 0.05) and were more likely to undergo endotracheal intubation (28% vs 14%, p = 0.008). Male sex, hyperlipidemia, and bradycardia within 5 days of completing remdesivir were significant predictors of inpatient mortality. No significant differences in length of stay were found.
Conclusions
Bradycardia that occurs during or shortly after remdesivir treatment in COVID-19 patients may be associated with an increased rate of in-hospital mortality. However, COVID-19 and its cardiac complications cannot be excluded as potential contributors of bradycardia in the present study. Future studies are needed to further delineate the cardiac characteristics of COVID-19 and remdesivir.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-022-01187-x.
Key Points
| Despite being a common treatment for severe coronavirus disease 2019 (COVID-19), the cardiac profile of remdesivir and its associated clinical outcomes are not well characterized. |
| This retrospective study reviewed remdesivir-associated bradycardia based on the drug’s elimination half-life and found an increased risk of in-hospital mortality. |
| The results highlight the need to evaluate the cardiac-related adverse events of remdesivir treatment. |
Background
Remdesivir is an antiviral prodrug of a nucleotide analog and currently a mainstay therapy in the treatment of coronavirus disease 2019 (COVID-19) [1]. Although the drug has been studied extensively in clinical trials, the cardiac side effect profile of remdesivir is not well studied [2]. One such phenomenon is remdesivir-associated bradycardia (RAB). Touafchia et al. [3] found an increased risk of reporting bradycardia following the use of remdesivir compared to other drugs, a finding consistent with a number of case reports and observational studies [3–7]. RAB is fairly common, with a reported incidence of 21–60% [3, 4, 7]. One proposed mechanism of RAB refers to remdesivir’s active metabolite, GS-441524, a nucleotide triphosphate derivative similar in structure to adenosine [8]. Adenosine is known to exert a negative chronotropic and dromotropic effect by slowing sinoatrial automaticity [9]; GS-441524 may lead to RAB via a similar mechanism to adenosine. Remdesivir antiviral therapy has offered a potential decrease in recovery time for COVID-19 patients, with an acceptable side effect profile [14]. However, despite its wide utilization, there remains a paucity of data regarding the cardiac-related clinical outcomes of remdesivir. RAB appears to occur at a disproportionate rate when compared to other pharmaceuticals administered to patients with COVID-19 [3], but only a few studies have appreciated the clinically relevant outcomes that result from the bradycardia [4, 7].
Likewise, the association between COVID-19 and sinus bradycardia is well documented in the literature [10, 11]. Bradycardia in the context of COVID-19 may be associated with increased rates of mortality [12]. Furthermore, patients with severe disease may be more prone to incident bradycardia [12, 13]. The potential etiology for COVID-19 arrhythmogenicity is manifold and includes myocardial insults (infarction, myocarditis), hypoxic injury, a systemic inflammatory response, autonomic disturbance, and electrolyte abnormalities [10]. It is not entirely clear whether drugs used in the treatment of COVID-19 that have QT interval-prolonging properties or certain drug–drug interactions also induce bradycardia [10].
Given the current widespread use of remdesivir and the comparatively modest attention placed on its potential cardiac pharmacodynamic properties, a better understanding of the clinical implications of RAB is warranted. The overarching goal of this study was to investigate RAB and its associated clinical characteristics and outcomes.
Methods
Patient Selection Criteria
Following University Medical Center of Southern Nevada institutional review board approval, a retrospective audit was performed on 600 consecutive patients at a single institution who were evaluated at hospital admission for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as defined by the COVID-19 treatment guidelines panel of the National Institute of Health, identified via polymerase chain reaction (PCR) between March 1, 2020 and March 31, 2021. Exclusion criteria included patients who tested negative for SARS-CoV-2, were less than 18 years old, were found to be pregnant, had less than 2 days length of stay (LOS), had severe hepatic dysfunction (AST or ALT > 5 times the upper limit of normal), or had evidence of renal impairment (glomerular filtration rate < 30 mL/min) (see the electronic supplementary material, Supplementary Material A) [15]. Patients who received atrioventricular (AV) nodal agents, a known cause of bradycardia, on admission or within 5 days of completing remdesivir treatment were also excluded [16]. Only patients who received a full 5-day course of remdesivir (200-mg intravenous [IV] loading dose, 100-mg IV daily maintenance dose for 4 days) were included in the present analysis [15].
Remdesivir’s active form, GS-441524, has a half-life of 20–27 h [1]. Over 95% elimination of the active metabolite would be expected approximately 5 days following completion of IV infusion of the drug [1, 17]. This study utilized a therapeutic window based on the drug’s elimination half-life, which was defined as when remdesivir was initiated up to 5 days after completion of a full course [15]. Bradycardia that was recorded during this window was considered RAB+, while bradycardia that occurred outside of this window was determined as RAB−. Two physician reviewers independently verified all inclusion and exclusion criteria (Online Resource 1, see the electronic supplementary material).
Definition of Bradycardia
For the purposes of this study, an episode of bradycardia (EB) was defined as two consecutive episodes of sustained heart rate (HR) less than 60 bpm at least 4 h apart throughout a patient’s admission. EBs were then classified based on whether they presented as single (i.e., one isolated instance of EB as defined above) or multiple events. Multi-event EBs were further defined as episodic (two episodes of EB separated by a normal HR), continuous (at least three consecutive episodes of sustained HR < 60 bpm), or both. An EB was determined to meet “symptomatic bradycardia” if systolic blood pressure (BP) was < 90 mmHg, diastolic BP was < 60 mmHg, or if patients showed clinical symptoms related to bradycardia as identified in their chart. Patients who experienced an EB during the drug effect window (DEW) were stratified into RAB+ (documented EB within DEW) and RAB− (no EB within DEW), which were verified by two separate reviewers using a Microsoft Excel macro [18].
Data Collection
Clinical data for all included patients was abstracted by a team of physician reviewers and medical trainees using both physician-facing medical record export (when available) and manual collection. Variables included demographics (age, race/ethnicity, sex, body mass index); LOS; mortality; baseline vitals from admission (HR, temperature, oxygen saturation); inflammatory biochemical markers (white blood cell count, ferritin, C-reactive protein [CRP], D-dimer) and highest level of oxygenation (i.e., bilevel positive airway pressure [BiPAP], endotracheal intubation) between admission and 5 days after completing remdesivir; past medical history (including prior arrhythmia, thyroid disease, and sleep apnea); tobacco use; electrocardiographic (ECG) data; and cardiac studies (echocardiogram, wire pacing, and cardiac catheterization). Data cleaning was performed to eliminate errors of transcription and implausible values.
Sample Size Justification
Due to the lack of existing literature on this topic area, we relied on the conventional method of power analysis. G power software (version 3.1) was used to perform a priori power analysis [19–21]. The a priori power analysis was conducted to ascertain the required sample size for a test with a predetermined alpha and beta (power) level. Power was ascertained separately for t, chi-square, and multiple logistic regression by using Cohen’s effect size conventions (effect size = 0.5 for t tests; effect size = 0.3 for chi-square test) [22]. For the logistic regression analysis, we used the formula proposed by Green (223, N ≥ 50 + 8m, where m corresponds to the number of predictors) [23]. The total number of predictors was 11, according to which, N = 138 was deemed appropriate. The total sample size estimated with a power of 0.95 was 210 and 220 for t test and chi-square test, respectively. The sample size with the greatest value (N = 220) was considered appropriate since it satisfies the minimum requirement of all the statistical tests used. The sample used in this study was relatively larger to allow subgroup comparisons.
Data Analysis
The primary outcomes of the study were rates of inpatient mortality and hospital LOS. Patients who underwent inpatient mortality were not included in the LOS analysis. Data were first cleaned and re-coded for running analytical operations. All statistical assumptions, including the normality, homogeneity of variance, and multicollinearity were accessed. Box plots were visually inspected to identify outliers in the data. Depending on their distribution (normal or not), quantitative variables were expressed as means ± standard deviations (SDs), and then compared with an independent samples t test/Welch two-sample t test or as medians (25th–75th percentiles) and then compared with a Mann-Whitney U test. Kruskal-Wallis test was used to compare medians of more than two groups. Qualitative variables were expressed as counts (percentages) and then compared with the chi-square analysis. Adjusted standardized residuals greater than 2 were considered significant cells for contingency tables larger than 2 × 2 chi-square analysis. In univariate statistics, 95% confidence intervals (CIs) of proportions were calculated using normal approximation to the binomial distribution. Two multivariate logistic regression models were fit to generate adjusted odds ratios for inpatient mortality and intubation as outcomes. Estimates of parameters were obtained through the maximum likelihood estimation method with 95% Wald’s confidence limits for the logistic model. The final model was selected based upon the Akaike information criterion (AIC) and the Schwarz criterion (SC) [24]. For regression analyses, polytomous categorical variables were dummy coded to calculate accurate parameters. All tests were two-sided, and a p value of < 0.05 was considered significant. The Statistical Package for Social Sciences for Windows, version 27.0 (SPSS, Chicago, IL, USA), and Statistical Analysis System (SAS 9.4) were used to analyze the data.
Results
A total of 375 patients were included in the analysis. The mean age of the sample was 56.63 ± 13.23 years. Over 60% of the sample were males, with the remaining 40% being females (Table 1). The white and non-white racial groups were nearly equally split. The mean body mass index of the sample population was 32.8 ± 8.57 kg/m2, and nearly 60% of the patients were obese. Approximately half of the sample (45.1%) had type 2 diabetes mellitus, and 42.7% had a medical history of hypertension. Hyperlipidemia was found among 31% of the cases (Table 1). As indicated in Supplementary Material B (see the electronic supplementary material), the minimum and maximum HR was 50.59 ± 12.8 and 112.91 ± 20.5 bpm, respectively. The mean levels of ferritin, CRP, low-density lipoprotein, and D-dimer were 948.64 ± 183.07 µg/L, 123.88 ± 63.5 mg/L, 492.26 ± 218.0 µL, and 5.06 ± 3.80 µg/mL, respectively. Clinical data were missing for certain variables during the retrospective abstraction process, as noted in Table 1, which did not exceed 0.5% of patients per variable.
Table 1.
Baseline demographic, behavioral, medical, clinical, and cardiac characteristics of study population at time of admission (N = 375)
| Variable | Categories | Overall sample, n (%) | Patients with bradycardia within 5 days of remdesivir, n (%) | P value | |
|---|---|---|---|---|---|
| Yes 182 (48.5) |
No 193 (51.5) |
||||
| Age (mean ± SD) | – | 56.63 ± 13.23 | 56.74 ± 12.85 | 56.53 ± 13.62 | 0.8 |
| Sex | Female | 145 (38.7) | 69 (37.9) | 76 (39.4) | 0.7 |
| Male | 230 (61.3) | 113 (62.1) | 117 (60.6) | ||
| Race/ethnicity | White | 71 (18.9) | 33 (18.6) | 38 (20.8) | 0.3 |
| Non-white | 75 (20.0) | 32 (18.1) | 43 (23.5) | ||
| Hispanic | 214 (57.1) | 112 (63.3) | 102 (55.7) | ||
| Body mass index in kg/m2 (mean ± SD) | – | 32.8 ± 8.57 | 32.7 ± 7.57 | 32.8 ± 9.43 | 0.9 |
| Body mass index categories | Underweight | 1 (3.0) | 0 (0.0) | 1 (0.5) | 0.6 |
| Normal weight | 69 (18.4) | 29 (15.9) | 40 (20.7) | ||
| Overweight | 86 (22.9) | 46 (25.3) | 40 (20.7) | ||
| Obesity class 1 | 94 (25.1) | 48 (26.4) | 46 (23.8) | ||
| Obesity class II | 57 (15.2) | 28 (15.4) | 29 (15.0) | ||
| Obesity class III | 68 (18.1) | 31 (17.0) | 37 (19.2) | ||
| History of tobacco use | Current | 16 (4.3) | 7 (3.8) | 9 (4.7) | 0.2 |
| Former | 41 (10.9) | 25 (13.7) | 16 (8.3) | ||
| Never | 318 (84.8) | 150 (82.4) | 168 (87.0) | ||
| History of drug abuse | Yes | 12 (3.2) | 6 (3.3) | 6 (3.1) | 0.9 |
| No | 363 (96.8) | 176 (96.7) | 187 (96.9) | ||
| Type 2 diabetes mellitus | Yes | 169 (45.1) | 82 (45.1) | 87 (45.3) | 0.9 |
| No | 205 (54.7) | 100 (54.9) | 105 (54.7) | ||
| Hypertension | Yes | 160 (42.7) | 79 (43.4) | 81 (42.2) | 0.8 |
| No | 214 (57.1) | 103 (56.6) | 111 (57.8) | ||
| Hyperlipidemia | Yes | 115 (30.7) | 60 (33.0) | 50 (28.6) | 0.4 |
| No | 259 (69.1) | 122 (67.0) | 137 (71.4) | ||
| Hypothyroidism | Yes | 28 (7.5) | 16 (8.8) | 12 (6.2) | 0.3 |
| No | 347 (92.5) | 166 (91.2) | 181 (93.8) | ||
| Prior arrhythmia | Yes | 5 (1.3) | 1 (0.5) | 4 (2.1) | 0.2 |
| No | 369 (98.4) | 181 (99.5) | 188 (97.9) | ||
| Coronary artery disease | Yes | 14 (3.7) | 7 (3.8) | 7 (3.6) | 0.9 |
| No | 360 (96.0) | 175 (96.2) | 185 (96.4) | ||
| Obstructive sleep apnea | Yes | 17 (4.5) | 9 (4.9) | 8 (4.1) | 0.7 |
| No | 358 (95.5) | 173 (95.1) | 185 (95.9) | ||
Some percentages may not add up to 100% due to missing information
P values < 0.05 are considered statistically significant
As shown in Supplementary Material B, the RAB+ group had a lower mean minimum HR than the RAB− group, with a statistically significant mean difference (44.78 ± 10.66 vs 57.52 ± 9.43 bpm, p < 0.001). Likewise, the mean maximum HR was lower among patients with bradycardia than with patients without bradycardia (108.24 ± 22.60 vs 113.20 ± 18.47, p = 0.02). The mean values of minimum body temperature were also statistically different. The differences between mean values of D-dimer levels were marginally significant, with the RAB+ group having the value of 5.19 ± 5.76 µg/mL compared with 3.38 ± 2.31 µg/mL among patients without an EB (p = 0.05, Table 2). Patients with an EB had lower mean HR on ECG as compared to RAB− patients, with a statistically significant mean difference (70.81 ± 23.41 vs 91.49 ± 23.41, p < 0.001, Table 2). Among bradycardic patients, a significantly larger proportion underwent intubation as opposed to non-bradycardic patients (27.5% vs 14.0%, p = 0.008). Among RAB+ patients (n = 182), 148 (81.3%) had the multiple events of bradycardia and only 18.7% had the single event (Table 3). The mean days of bradycardia were 3.51 ± 2.28. The mean systolic and diastolic BP values of this group were 107.57 ± 17.82 mmHg and 54.12 ± 11.5 mmHg, respectively. The PR, QRS, and QTc intervals were 149.75 ± 20.8 ms, 98.19 ± 13.60 ms, and 435.16 ± 26.54 ms, respectively. Among RAB+ patients, 17.6% of patients had hypoxia (Table 3). A significantly larger proportion of the RAB+ group had inpatient mortality as compared to the RAB− group (22.0% vs 12.4%, p = 0.01). However, no significant differences in the median length of hospital stay were observed. Upon analyzing the subgroups of patients with a documented EB by characteristics of bradycardia, no significant differences were noted for inpatient mortality (Table 4). The median LOS did not differ significantly by the bradycardia characteristics (Table 5).
Table 2.
Comparing clinical characteristics among groups with or without bradycardia after initiation of remdesivir (N = 375)
| Variable | Patients with bradycardia within 5 days of remdesivir, mean ± SD | P value | |
|---|---|---|---|
| Yes | No | ||
| Number of patients, n (%) | 182 (48.5) | 193 (51.5) | – |
| Minimum HR (bpm) | 44.78 ± 10.66 | 57.52 ± 9.43 | < 0.001 |
| Maximum HR (bpm) | 108.24 ± 22.60 | 113.20 ± 18.47 | 0.02 |
| Minimum temperature (Celsius) | 35.96 ± 0.72 | 36.14 ± 0.36 | 0.003 |
| Maximum temperature (Celsius) | 37.85 ± 0.93 | 37.85 ± 0.93 | 0.5 |
| Minimum SpO2 (%) | 82.31 ± 10.90 | 82.89 ± 12.25 | 0.6 |
| Maximum SpO2 (%) | 99.27 ± 1.132 | 99.26 ± 1.025 | 0.9 |
| WBC (cu/mm3) | 12.88 ± 0.57 | 13.24 ± 1.10 | 0.6 |
| Ferritin (µg/L) | 806.56 ± 114.91 | 825.98 ± 182.25 | 0.8 |
| CRP (mg/L) | 105.94 ± 67.01 | 106.36 ± 65.22 | 0.9 |
| D-dimer (µg/mL) | 5.19 ± 5.76 | 3.38 ± 2.31 | 0.05a |
| LDL (µL) | 494.37 ± 191.21 | 458.92 ± 205.29 | 0.1 |
| HR on ECG (bpm) | 70.81 ± 23.41 | 91.49 ± 23.41 | < 0.001 |
| PR interval (ms) | 149.56 ± 22.67 | 150.78 ± 27.61 | 0.7 |
| QRS interval (ms) | 97.92 ± 14.32 | 94.21 ± 17.47 | 0.1 |
| QTc (ms) | 436.55 ± 28.65 | 445.24 ± 30.68 | 0.06 |
| Supplemental O2 method | 0.008 | ||
| BiPAP, n (%) | 1 (0.5) | 1 (0.5) | |
| HFNC, n (%) | 65 (35.7) | 70 (36.3) | |
| Intubation, n (%) | 50 (27.5) | 27 (14.0) | |
| NC, n (%) | 63 (34.6) | 94 (48.7) | |
| None, n (%) | 3 (1.6) | 1 (0.5) | |
BiPAP bilevel positive airway pressure, CRP C-reactive protein, ECG electrocardiogram, HFNC high-flow nasal cannula, HR heart rate, LDL low-density lipoprotein, NC nasal cannula, WBC white blood count, SpO2 oxygen saturation
P values < 0.05 are considered statistically significant and are bolded in the table
Table 3.
ECG and bradycardia characteristics of group with remdesivir associated bradycardia (N = 182)
| Variable | Value | 95% CI |
|---|---|---|
| Single event of bradycardia, n (%) | 34 (18.7) | 13.30–25.11 |
| Multiple events of bradycardia, n (%) | 148 (81.3) | 74.8–86.70 |
| Diastolic BP (mmHg) | 54.12 ± 11.5 | 51.35–56.88 |
| Systolic BP (mmHg) | 107.57 ± 17.82 | 103.28–111.85 |
| Pulse pressure (mmHg) | 53.45 ± 20.96 | 48.41–58.59 |
| HR on ECG | 66.61 ± 18.8 | 62.08–71.14 |
| PR interval (ms) | 149.75 ± 20.8 | 144.73–154.77 |
| QRS interval (ms) | 98.19 ± 13.60 | 94.92–101.45 |
| QTc (ms) | 435.16 ± 26.54 | 428.78–441.54 |
| Hypoxia during bradycardia, n (%) | ||
| Yes | 32 (17.6) | 12.4–23.9 |
| No | 150 (82.4) | 76.10–87.65 |
Values are expressed as mean ± SD unless specified otherwise
BP blood pressure, CI confidence interval, ECG electrocardiogram, HR heart rate
Table 4.
Comparing inpatient mortality among subgroups by the quality and type of bradycardia among patients who developed it within 5 days of remdesivir (N = 182)
| Variable | Inpatient mortality, n (%) | P value | |
|---|---|---|---|
| Yes | No | ||
| Single event of bradycardia | 7 (17.5) | 27 (19.0) | 0.8 |
| Multiple events of bradycardia | 33 (82.5) | 115 (81.0) | |
| Episodic | 4 (12.1) | 18 (15.7) | 0.7 |
| Continuous | 6 (18.2) | 15 (13.0) | |
| Both | 23 (69.7) | 82 (71.3) | |
| Symptomatic bradycardia | |||
| Yes | 27 (67.5) | 74 (52.1) | 0.08 |
| No | 13 (32.5) | 68 (47.9) | |
Table 5.
Comparing length of hospital stay among subgroups by the quality and type of bradycardia among patients who developed it within 5 days of remdesivir (N = 182)
| Variable | Category | Length of hospital staya | P value | |
|---|---|---|---|---|
| Median | 25th, 75th percentile | |||
| Bradycardia frequency | Single events | 8.0 | 5, 16 | 0.8 |
| Multiple events | 10.00 | 8, 22 | ||
| Multi-event bradycardia characteristics | Episodic | 10.00 | 8, 16.75 | 0.06 |
| Continuous | 18.00 | 6, 30.00 | ||
| Both | 10.00 | 8, 20.25 | ||
| Symptomatic bradycardia | Yes | 11.50 | 8, 22 | 0.3 |
| Yes | No | 10.00 | 6.25, 17 | |
aPatients who underwent inpatient mortality were not included in the length of hospital stay calculations
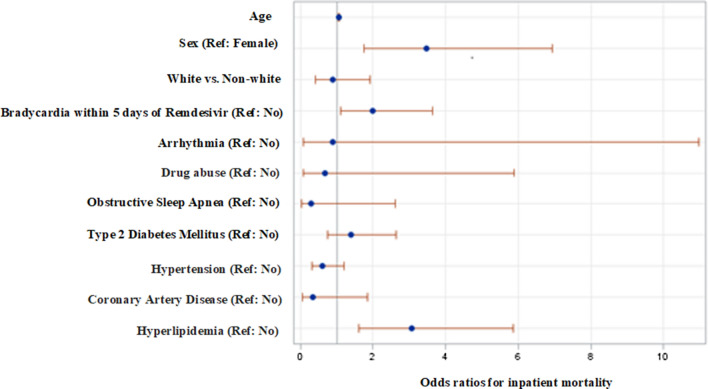
As shown in Fig. 1 and Table 6, the sex of the patient, hyperlipidemia, and bradycardia within 5 days of completing remdesivir treatment were significant predictors of inpatient mortality. Males had the higher odds (adjusted odds ratio = 3.39, 95% CI 1.69–6.76) of inpatient mortality compared with their female counterparts. Patients who developed bradycardia within 5 days of remdesivir were nearly two times more likely to die as opposed to those who did not develop bradycardia (adjusted odds ratio = 1.93, 95% CI 1.058–3.537). Hyperlipidemic patients had the higher odds of inpatient mortality as compared to non-hyperlipidemic patients (adjusted odds ratio = 3.27, 95% CI 1.69–6.30; Fig. 1; Table 6).
Fig. 1.
Forest plot displaying odds ratios and 95% Wald confidence intervals for inpatient mortality
Table 6.
Odds ratio estimates for inpatient mortality
| Variable | Odds ratio | 95% CI | |
|---|---|---|---|
| LCL | UCL | ||
| Age | 1.054 | 1.028 | 1.082 |
| Sex (Ref. female) | 3.470 | 1.739 | 6.925 |
| White vs non-white | 0.896 | 0.421 | 1.905 |
| Bradycardia within 5 days of remdesivir (Ref. no) | 1.997 | 1.096 | 3.641 |
| Arrhythmia (Ref. no) | 0.896 | 0.073 | 10.969 |
| Drug abuse (Ref. no) | 0.678 | 0.078 | 5.877 |
| Obstructive sleep apnea (Ref. no) | 0.297 | 0.034 | 2.613 |
| Type 2 diabetes mellitus (Ref. no) | 1.393 | 0.737 | 2.635 |
| Hypertension (Ref. no) | 0.615 | 0.312 | 1.209 |
| Coronary artery disease (Ref. no) | 0.333 | 0.060 | 1.833 |
| Hyperlipidemia (Ref. no) | 3.072 | 1.610 | 5.862 |
CI confidence interval, Ref. reference
Discussion
Our study focused primarily on the clinical ramifications of RAB. This study defined RAB as any bradycardic episode (i.e., EB) that occurred during the DEW, defined as the period between initiation to up to 5 days after completion of a full course of remdesivir. Twenty-two percent of RAB+ patients met in-hospital mortality, which is a 1.8-fold increase in all-cause mortality compared to RAB− patients. This is consistent with one study that observed RAB-related death in up to 17% of patients given remdesivir [3]. After adjusting for other potential causes of bradycardia, RAB+ patients experienced inpatient death nearly twice more than RAB− patients, suggesting that any documented EB during DEW predicted mortality.
Previous investigations have identified a relationship between bradycardia and COVID-19 [25, 26]. Potential mechanisms include aggravation of pre-existing heart conduction disorders during acute illness, pulmonary injury leading to hypoxia and secondary bradycardia, as well as myocardial damage resulting from inflammatory storm [27, 28]. Mol et al. [29] have proposed viewing bradycardia as a marker of COVID-19-induced inflammation. Another possible explanation is that bradycardia stems from carotid and aortic body-mediated sympathetic inhibition due to proximal aortic dilation as a result of increasing respiratory distress [29]. In this theory, COVID-driven hypoxia modulates respiratory effort and increases negative intrathoracic pressures, which transmit through the pericardial cavity and increase left ventricular afterload, which is then sensed by the carotid and aortic bodies [30].
Although previous COVID-19 studies have found associations between bradycardia and mortality [25, 26, 31, 32], the literature is conflicting. Several cases have observed RAB followed by a return to normal HR range following withdrawal of remdesivir therapy [33, 34]. One subgroup analysis in a small prospective study identified sinus bradycardia in one in five remdesivir-treated patients [7]. No differences in mortality or intensive care admission rate were found, although the limited sample size may have restricted the analysis. Another large multicenter investigation did not find a significant relationship between remdesivir and bradycardia [32]. In both studies, bradycardic episodes were measured throughout the entirety of a patient’s hospital course, which may introduce a confounding effect. Randomized clinical trials have reported serious cardiac-related adverse events (e.g., myocardial infarction, arrhythmia), but bradycardia has not been noted specifically [14]. No studies to date have analyzed RAB in relation to remdesivir’s elimination half-life. This study is the first to analyze RAB that occurred specifically within a predefined DEW. EBs occurring outside the DEW cannot be attributed to remdesivir given > 95% clearance of the active metabolites; bradycardia documented outside of the DEW may be related to other agents or to COVID-19 itself.
Our study also attempted to delineate EB characteristics amongst patients who experienced RAB, in order to identify markers that may correlate with poor clinical outcomes. This included EB patterns, the DEW, and sequelae attributable to a bradycardic episode (i.e., hypotension, clinical symptoms). Subgroup analysis of RAB+ cohort showed a 15% increase in death relative to the non-symptomatic counterparts, but this was not a statistically significant finding. Likewise, median LOS appeared longer in patients with a “continuous” EB pattern, but the finding was not quite significant.
Biological sex was one factor that significantly predicted mortality in this study, with males being 3.4 times more likely to experience in-hospital mortality compared to females. A substantial number of epidemiologic studies worldwide have found higher fatality rates of men over women [35–37], with males also being more likely to experience complications [35, 38–43]. This trend appears to persist in the United States even after adjusting for age, race, and comorbidities [43]. One proposed theory suggests that this dichotomy may be explained by sex-associated differences in gene expression and regulation of the angiotensin-converting enzyme 2 (ACE2) receptor and TMPRSS2, cell surface proteins that mediate the binding of SARS-CoV-2 and promote cell entry [35, 44]. More robust inflammatory and immune responses in females, as well as the potentially protective properties of estrogen in dampening the harmful effects of COVID-19, may also play a significant role in sex-specific differences [35, 44, 45]. Future studies are warranted to further elucidate the precise underlying mechanisms to account for these biological sex-related differences.
Multiple cardiovascular disease risk factors (hypertension, diabetes mellitus) and other conditions (obstructive sleep apnea, hypothyroidism) were analyzed that may have portended a greater likelihood of bradyarrhythmia [41, 46]. In our analysis, only dyslipidemia was found to have a significant association. The relationship between dyslipidemia and COVID-19 disease has been studied extensively [47, 48]. Our study found that a prior diagnosis of dyslipidemia rendered patients a 3.3-fold greater likelihood of experiencing death during hospital admission, consistent with other studies in the literature [41, 48]. A pooled cohort analysis of 28 studies by Liu and colleagues found that dyslipidemia independently predicted both a greater degree of COVID-19 disease severity along with an increased risk of mortality. One hypothesis suggests that the innate response to SARS-CoV-2 infection may directly alter serum high-density lipoprotein (HDL), which may lead to a diminished anti-inflammatory response and resultant lung inflammation in severe COVID-19 disease [49]. Another suggested mechanism involves viral-led organ damage secondary to overactivation of the innate immune system and increased oxidized phospholipids [49]. Only 23 patients in this study were found to have abnormalities via echocardiogram; none underwent interventional cardiac studies such as temporary cardiac pacing or catheterization.
LOS was of primary interest, but no meaningful differences were appreciated. Compared to placebo, a number of randomized controlled trials have observed a reduction in LOS and increased hospital discharge rates in patients receiving remdesivir [14, 50, 51]. However, this potential benefit has been challenged in follow-up studies [52–55] under the pretense that completing the full therapeutic course may be taking precedence over earlier discharges in otherwise recovering patients [56–59]. In relation to bradycardia, one study observed extended hospital stays in COVID-19 patients with bradycardia [58], while another found similar LOS between RAB and non-RAB patients [7]. Although a subgroup analysis revealed that RAB+ patients who had more than two consecutive EBs remained more than a week longer before discharge, this finding did not reach statistical significance.
Of all the laboratory biomarkers analyzed, only D-dimer was found to be marginally significant with RAB+ patients compared to RAB− patients, a finding supported in one other study [7]. D-dimer is a degradation product formed by the breakdown of fibrin cross-links in a clot [60]. Severe disease course and mortality have been strongly associated with the thrombotic complications of COVID-19, which is theorized to result from SARS-CoV-2 endothelial cell invasion and the downstream immune and inflammatory responses [61, 62]. The relationship between mild versus severe COVID-19 cohorts and D-dimer levels has prompted investigations into D-dimer as a predictive biomarker for disease severity and survival [41, 62–65]. One multicenter laboratory analysis identified 1.5 µg/mL as an optimal cutoff when obtained at hospital admission to accurately determine an increased risk of inpatient mortality in COVID-19 patients [66]. However, recent studies have questioned the true predictive utility of D-dimer in the inpatient setting [67].
A significant percentage (28%) of RAB+ patients underwent endotracheal intubation for invasive mechanical ventilation, which is double the number of RAB− patients and appears consistent with at least one other study focused on COVID-related bradycardia [58]. Furthermore, hypoxia occurred in nearly one in five RAB+ patients during an EB. In contrast, the RAB− population was 15% more likely to utilize non-invasive measures (i.e., nasal cannula) for respiratory support. Our results suggest that patients with any documented EB receive close monitoring in anticipation of requiring more invasive modalities of respiratory support. Although prior COVID-19 studies have identified risk factors for intubation such as sex and body mass index [42, 43], this is the first study to the authors’ knowledge where bradycardia may be attributable to invasive mechanical support. Given the multiple risks associated with intubation [68], future studies are warranted to investigate this finding further.
The presumed mechanism by which RAB occurs is via the adenosine moiety on remdesivir’s active metabolite, GS-441524 [3, 8, 69–71]. Adenosine is an antiarrhythmic agent known to have negative chronotropic and dromotropic effects via a left ventricular vagal reflex resulting in sinus bradycardia [9, 11]. It is used in the treatment of AV nodal reentrant tachycardias, given its inhibitory effects on the AV node [9, 72]. Although plausible, our findings entertain the possibility of RAB being influenced by a combination of both severity of illness and the adenosine moiety of GS-441524. RAB+ patients were found to be associated with a greater propensity for inpatient mortality, elevated inflammatory markers such as D-dimer, and higher rates of invasive mechanical ventilation (p = 0.008), suggesting that EBs amongst RAB+ patients may be related to the severity of illness. Furthermore, the incidence of EBs amongst patients receiving remdesivir appeared to be higher compared to rates of bradycardia in studies of patients with COVID-19 in the literature [25, 26, 31, 32]. Further comparative studies are needed to tease out the exact etiology of RAB.
With regard to the ECG findings, all of which were obtained during remdesivir’s DEW, there were no appreciable differences between patients who experienced bradycardia and those who did not. We expected the ECG effects of remdesivir to manifest uniformly amongst both the RAB+ and RAB− groups. Previous reports that observed RAB noted variations in the QTc interval, specifically in patients who experienced bradycardia [5, 6]. Although there were differences in QTc between the RAB+ and RAB− cohorts, the findings demonstrated no statistical or clinical significance (p = 0.06). Further studies comparing ECG results between patients who received remdesivir and those who did not may be beneficial.
There are a number of limitations to this study. The retrospective nature makes it prone to potential selection biases, and certain variables were missing clinical data, although this was never more than 0.5% per variable. All patients in this study met criteria for severe COVID-19, which has been purported to causes bradycardia in and of itself. As such, treatment with remdesivir cannot exclusively explain the incidence of bradycardia and RAB-related mortality, and a follow-up comparison study with matched controls would be of benefit. There may be a residual confounding effect secondary to variables that were left unmeasured (e.g., when bradycardia occurred in a patient’s hospital course, how many experienced bradycardia after completion of remdesivir, concomitant therapies). The study population predominantly lacked medical and cardiovascular risk factors, which weakens the formal predictors of mortality analysis. The relatively large study size and roughly equal representation of sex and ethnic backgrounds are a strength in our study and improve the generalizability of our findings. However, future prospective and randomized studies are needed to further elucidate the mechanisms of RAB and better understand its associated clinical implications.
Conclusions
Patients with COVID-19 who experienced bradycardia shortly after completing a course of remdesivir had higher rates of associated death, increased serum D-dimer levels, and a greater likelihood of requiring endotracheal intubation. Sex, hyperlipidemia, and any observed EB during administration of remdesivir up to 5 days after completion predicted inpatient mortality. The exact etiology of RAB and its associated clinical outcomes remains speculative, and future comparison trials are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Shabada Asad, M.D. for assisting with project design and for providing crucial feedback on clinical questions.
Declarations
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by AS, JB, SM, KL, NH, UP, CC, KI, and AG. Data analysis was performed by KB, AS, and JB. The first draft of the manuscript was written by ASr and JB, and all authors commented on previous versions of the manuscript. DH, JD, and CA provided supervision. All authors read and approved the final manuscript.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the institutional review board (IRB) of the University Medical Center of Southern Nevada, who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the University Medical Center of Southern Nevada.
Conflicts of interest
The authors declare no conflicts of interests.
Funding
This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
Consent to participate
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
- 1.National Center for Biotechnology Information. PubChem Compound Summary for CID 121304016, Remdesivir. 2022; https://pubchem.ncbi.nlm.nih.gov/compound/Remdesivir. Accessed 10 Apr 2022.
- 2.Nabati M, Parsaee H. Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review. Cardiovasc Toxicol. 2022;22:268–272. doi: 10.1007/s12012-021-09703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touafchia A, Bagheri H, Carrié D, Durrieu G, Sommet A, Chouchana L, Montastruc F. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clin Microbiol Infect. 2021;27:791.e5–791.e8. doi: 10.1016/j.cmi.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallotto C, Suardi LR, Gabbuti A, Esperti S, Mecocci L, Blanc P. Potential remdesivir-related transient bradycardia in patients with coronavirus disease 2019 (COVID-19) J Med Virol. 2021;93:2631–2634. doi: 10.1002/jmv.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubitosa JC, Kakar P, Gerula C, Nossa H, Finkel D, Wong K, Khatri M, Ali H. Marked sinus bradycardia associated with remdesivir in COVID-19: a case and literature review. JACC Case Rep. 2020;2:2260–2264. doi: 10.1016/j.jaccas.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta AK, Parker BM, Priyadarshi V, Parker J. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus. 2020;12:e11132. doi: 10.7759/cureus.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attena E, Albani S, Maraolo AE, Mollica M, De Rosa A, Pisapia R, Fiorentino G, Parrella R, Severino S, Russo V. Remdesivir-induced bradycardia in COVID-19: a single center prospective study. Circ Arrhythm Electrophysiol. 2021;14:e009811. doi: 10.1161/CIRCEP.121.009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelleg A, Belhassen B. The mechanism of the negative chronotropic and dromotropic actions of adenosine 5’-triphosphate in the heart: an update. J Cardiovasc Pharmacol. 2010;56:106–109. doi: 10.1097/fjc.0b013e3181e0f8b2. [DOI] [PubMed] [Google Scholar]
- 10.Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451–460. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douedi S, Mararenko A, Alshami A, Al-Azzawi M, Ajam F, Patel S, Douedi H, Calderon D. COVID-19 induced bradyarrhythmia and relative bradycardia: an overview. J Arrhythm. 2021;37:888–892. doi: 10.1002/joa3.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in patients With COVID-19: a calm before the storm? Cureus. 2020 doi: 10.7759/cureus.8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capoferri G, Osthoff M, Egli A, Stoeckle M, Bassetti S. Relative bradycardia in patients with COVID-19. Clin Microbiol Infect. 2021;27:295–296. doi: 10.1016/j.cmi.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-D, Ruiz-Palacios G, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study GM Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aleem A, Kothadia JP. Remdesivir. [Updated 2022 May 11]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022 Jan. Available from https://www.ncbi.nlm.nih.gov/books/NBK563261/
- 16.Tisdale J, Chung M, Campbell K, Hammadah M, Joglar J, Leclerc J, Rajagopalan B. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214–e233. doi: 10.1161/CIR.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 17.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauzon J, Murphy C, Wahi-Gururaj S. Using macros in microsoft excel to facilitate cleaning of research data. J Community Hosp Intern Med Perspect. 2021;11:653–657. doi: 10.1080/20009666.2021.1954282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenth RV. Some practical guidelines for effective sample size determination. Am Stat. 2001;55:187–193. doi: 10.1198/000313001317098149. [DOI] [Google Scholar]
- 20.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/brm.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 22.Lachenbruch PA, Cohen J. Statistical power analysis for the behavioral sciences, vol 84. 2. Routledge; 1989. p. 1096. [Google Scholar]
- 23.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res. 1991;26:499–510. doi: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 24.Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–445. doi: 10.1007/bf02353864. [DOI] [PubMed] [Google Scholar]
- 25.Antwi-Amoabeng D, Beutler BD, Singh S, Taha M, Ghuman J, Hanfy A, Manasewitsch NT, Ulanja MB, Ghuman J, Awad M, Gullapalli N, Gbadebo TD. Association between electrocardiographic features and mortality in COVID-19 patients. Ann Noninvasive Electrocardiol. 2021;26:e12833. doi: 10.1111/anec.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Arcuri C, Chaudhuri S, Gupta R, Aseri M, Barve P, Shah S. A novel study on SARS-COV-2 virus associated bradycardia as a predictor of mortality-retrospective multicenter analysis. Clin Cardiol. 2021;44:857–862. doi: 10.1002/clc.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. Cardiovasc Electrophys. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinitz JS, Goyal R, Harding M, Veseli G, Gruberg L, Jadonath R, Maccaro P, Gandotra P, Ong L, Epstein LM. Bradyarrhythmias in patients with COVID-19: marker of poor prognosis? Pacing Clin Electrophysiol. 2020;43:1199–1204. doi: 10.1111/pace.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mol MBA, Strous MTA, van Frits HMO, Vogelaar FJ, Barten DG, Farchi M, Foudraine NA, Gidron Y. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS ONE. 2021;16:e0258841. doi: 10.1371/journal.pone.0258841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur Respir J. 2012;41:1439–1451. doi: 10.1183/09031936.00128412. [DOI] [PubMed] [Google Scholar]
- 31.Chalkias A, Pantazopoulos I, Papagiannakis N, Skoulakis A, Laou E, Kolonia K, Ntalarizou N, Tourlakopoulos K, Pagonis A, Kampolis C, De Guadiana Romualdo LG, Ragias D, Eugen-Olsen J, Gourgoulianis K, Arnaoutoglou E, Investigators S Sinus bradycardia is associated with poor outcome in critically ill patients with COVID-19 due to the B.1.1.7 Lineage. Toxic Rep. 2021;8:1394–1398. doi: 10.1016/j.toxrep.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umeh C, Watanabe K, Tuscher L, Ranchithan S, Gupta R. Comparison of clinical characteristics and outcomes of COVID-19 between young and older patients: a multicenter, retrospective cohort study. Cureus. 2022;14:e21785. doi: 10.7759/cureus.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory GE, Gregory HM, Liaqat H, Ghaly MM, Johnson-Pich K. Remdesivir-associated sinus arrest in COVID-19: a potential indication for close cardiac monitoring. Cureus. 2022;14:e22328. doi: 10.7759/cureus.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvaraj V, Bavishi C, Patel S, Dapaah-Afriyie K. Complete heart block associated with remdesivir in COVID-19: a case report. Eur Heart J Case Rep. 2021;5:ytab200. doi: 10.1093/ehjcr/ytab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramírez-Soto MC, Ortega-Cáceres G, Arroyo-Hernández H. Sex differences in COVID-19 fatality rate and risk of death: an analysis in 73 countries, 2020–2021. Infec Med. 2021;29:402–407. doi: 10.53854/liim-2903-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortunato F, Martinelli D, Lo Caputo S, Santantonio T, Dattoli V, Lopalco PL, Prato R. Sex and gender differences in COVID-19: an Italian local register-based study. BMJ Open. 2021;11:e051506. doi: 10.1136/bmjopen-2021-051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mensah AA, Lacy J, Stowe J, Seghezzo G, Sachdeva R, Simmons R, Bukasa A, O’Boyle S, Andrews N, Ramsay M, Campbell H, Brown K. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. 2022;84:542–550. doi: 10.1016/j.jinf.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, Liu S, Yang J-K. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozenberg S, Vandromme J, Martin C. Are we equal in adversity? Does Covid-19 affect women and men differently? Maturitas. 2020;138:62–68. doi: 10.1016/j.maturitas.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Cid C, Catalano HN, Agarwal A, Foroutan F, Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE. 2020;15:e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, Southern WN, Mantzoros CS. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen NT, Chinn J, De Ferrante M, Kirby KA, Hohmann SF, Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE. 2021;16:e0254066. doi: 10.1371/journal.pone.0254066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciarambino T, Para O, Giordano M. Immune system and COVID-19 by sex differences and age. Womens Health (Lond). 2021;17:17455065211022262. doi: 10.1177/17455065211022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, Vázquez-Cárdenas P, Cruz-Ramos M, Palacios-Gonzalez B, Gomez-Quiroz L, Martínez-Nava GA. NLRP3 inflammasome: the stormy link between obesity and COVID-19. Front Immunol. 2020;11:570251. doi: 10.3389/fimmu.2020.570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Pan Y, Yin Y, Chen W, Li X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): a meta-analysis. Virol J. 2021;18:157. doi: 10.1186/s12985-021-01604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorokin AV, Karathanasis SK, Yang Z-H, Freeman L, Kotani K, Remaley AT. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34:9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abd-Elsalam S, Salama M, Soliman S, Naguib AM, Ibrahim IS, Torky M, Abd EG, Samir M, Abdul-Baki E, Elhendawy M. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am J Trop Med Hyg. 2021;106:886–890. doi: 10.4269/ajtmh.21-0606. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Al-Abdouh A, Bizanti A, Barbarawi M, Jabri A, Kumar A, Fashanu OE, Khan SU, Zhao D, Antar AAR, Michos ED. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Contemp Clin Trials. 2021;101:106272. doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S, WHO Solidarity Trial Consortium Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali K, Azher T, Baqi M, Binnie A, Borgia S, Carrier FM, Cavayas YA, Chagnon N, Cheng MP, Conly J, Costiniuk C, Daley P, Daneman N, Douglas J, Downey C, Duan E, Duceppe E, Durand M, English S, Farjou G, Fera E, Fontela P, Fowler R, Fralick M, Geagea A, Grant J, Harrison LB, Havey T, Hoang H, Kelly LE, Keynan Y, Khwaja K, Klein G, Klein M, Kolan C, Kronfli N, Lamontagne F, Lau R, Fralick M, Lee TC, Lee N, Lim R, Longo S, Lostun A, MacIntyre E, Malhamé I, Mangof K, McGuinty M, Mergler S, Munan MP, Murthy S, O’Neil C, Ovakim D, Papenburg J, Parhar K, Parvathy SN, Patel C, Perez-Patrigeon S, Pinto R, Rajakumaran S, Rishu A, Roba-Oshin M, Rushton M, Saleem M, Salvadori M, Scherr K, Schwartz K, Semret M, Silverman M, Singh A, Sligl W, Smith S, Somayaji R, Tan DHS, Tobin S, Todd M, Tran T-V, Tremblay A, Tsang J, Turgeon A, Vakil E, Weatherald J, Yansouni C, Zarychanski R, Canadian Treatments for C-19 (CATCO), Association of Medical Microbiology and Infectious Disease Canada (AMMI). Clinical Research Network and the Canadian Critical Care TG Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–E251. doi: 10.1503/cmaj.211698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaikh Q, Sarfaraz S, Rahim A, Hussain M, Shah R, Soomro S. Effect of Remdesivir on mortality and length of stay in hospitalized COVID-19 patients: a single center study. Pak J Med Sci. 2022;38:405–410. doi: 10.12669/pjms.38.ICON-2022.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohl ME, Miller DR, Lund BC, Kobayashi T, Richardson Miell K, Beck BF, Alexander B, Crothers K, Mary SVS. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4:e2114741. doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang S-C, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM, Investigators G-U-540-5774 Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaka AS, MacDonald R, Greer N, Vela K, Duan-Porter W, Obley A, Wilt TJ. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2021;174:663–672. doi: 10.7326/M20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson MR, Bach PB, Baldwin MR. Hospital length of stay for patients with severe COVID-19: implications for Remdesivir’s value. Pharmacoecon. 2021;5:129–131. doi: 10.1007/s41669-020-00243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umeh C, Giberson C, Kumar S, Aseri M, Barve P. A multicenter retrospective analysis on the etiology of bradycardia in COVID-19 patients. Cureus. 2022;14:e21294. doi: 10.7759/cureus.21294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 61.McFadyen JD, Stevens H, Peter K. The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Z, Yang KY, Huang Y, Lui KO. Endothelial contribution to COVID-19: an update on mechanisms and therapeutic implications. J Mol Cell Cardiol. 2022;164:69–82. doi: 10.1016/j.yjmcc.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- 64.Han H, Yang L, Liu R, Liu F, Wu K, Li J, Liu X, Zhu C. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 65.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poudel A, Poudel Y, Adhikari A, Aryal BB, Dangol D, Bajracharya T, Maharjan A, Gautam R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE. 2021;16:e0256744. doi: 10.1371/journal.pone.0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cidade JP, Coelho L, Costa V, Morais R, Moniz P, Morais L, Fidalgo P, Tralhão A, Paulino C, Nora D, Valerio B, Mendes V, Tapadinhas C, Póvoa P. Predictive value of D-dimer in the clinical outcome of severe COVID19 patients: are we giving it too much credit? Clin Appl Thromb Hemost. 2022;28:10760296221079612. doi: 10.1177/10760296221079612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tikka T, Hilmi OJ. Upper airway tract complications of endotracheal intubation. Br J Hosp Med (Lond) 2019;80:441–447. doi: 10.12968/hmed.2019.80.8.441. [DOI] [PubMed] [Google Scholar]
- 69.Hbf878. Chemical structure of Remdesivir [Image]. Wikimedia Commons. 2020; https://commons.wikimedia.org/wiki/File:Remdesivir.svg. Accessed 15 May 2022.
- 70.Anypodetos. Chemical structure of GS-441524 [Image]. Wikimedia Commons. 2020; https://commons.wikimedia.org/wiki/File:GS-441524_skeletal.svg. Accessed 15 May 2022.
- 71.NEUROtiker. Structure of adenosine [Image]. Wikimedia Commons. 2022; https://commons.wikimedia.org/wiki/File:Adenosin.svg. Accessed 15 May 2022.
- 72.Jalife J, Stevenson WG. Zipes and Jalife’s cardiac electrophysiology: from cell to bedside. Elsevier; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.